Abstract
Few studies have analyzed the immune response to Helicobacter pylori CagA and urease antigens across age groups in the same population. The aim of this study was to analyze the serologic immunoglobulin G (IgG) response to CagA and urease proteins in children and adults with gastrointestinal symptoms and belonging to the same population and similar socioeconomic levels. The serologic response was studied in 352 children and 293 adults with gastrointestinal symptoms. IgG antibodies against CagA and urease were tested by enzyme-linked immunosorbent assay methods using highly purified recombinant antigens. H. pylori infection was defined as a positive result in a serologic assay using whole-cell H. pylori extracts as the antigen. We found, in H. pylori-positive children, a seroprevalence of 46.9% to CagA and 16.2% to urease, whereas in H. pylori-positive adults, a seroprevalence of 78.9% to CagA and 59% to urease was found. In children, the magnitude of the response to CagA was significantly higher and the response to urease was significantly lower than those in adults. The kinetics of serologic response to CagA and to urease across age groups was contrastably different. Whereas CagA is a strong immunogen, urease is a poor immunogen during natural infection. These differences in the humoral response may be important for the short-term or long-term outcome of the infection. These results add to our knowledge of the epidemiology of H. pylori infection.
At present, it is well accepted that infection with Helicobacter pylori may lead to duodenal and gastric ulcers, mucosa-associated gastric lymphoma, and distal gastric cancer in humans (5). The infection is usually acquired during childhood, although expression of disease does not occur in most cases until adulthood. The presence of the organism causes gastric inflammation in all individuals; with time, this chronic inflammation may lead to injury of the gastric mucosa (23). Inflammatory mediators, such as interleukin-8 and interleukin-1, alter physiologic functions, such as gastric acid secretion. The infection during childhood is often transitory, and spontaneous eradication can be seen (27). In contrast, this phenomenon is rarely observed in adults. The reasons for this difference are unclear, but the nature of the inflammatory and immune responses may partially explain the phenomenon. In particular, the immune response to some antigens in H. pylori infection in children may differ from the response observed in adults. It has been reported that after infection, children mount an immune serologic response primarily to low-molecular-weight antigens (24); this contrasts with the serologic response observed in adults, in whom antibodies against both low- and high-molecular-weight proteins are observed. These results suggest that during the acute phase of the infection, the host responds mainly to low-molecular-weight antigens, and that it may take months or even years to mount a detectable serologic response to other antigens (19). These differences in the humoral response to H. pylori infection may be important for the short-term outcome of infection in children, such as spontaneous eradication, or the long-term outcome in adults, such as development of gastrointestinal diseases.
There are a rather large number of reports studying the humoral immune response to H. pylori whole-cell extract or to a pool of semipurified antigen preparations in individuals of different ages in the same populations (3, 12, 15, 20, 21, 24, 25, 26, 28, 29). These studies have been useful for learning the prevalence of the infection across age groups; however, few studies have analyzed the humoral response to purified antigens in individuals of all ages in the same population. In particular, there are few studies (2, 4, 6) that have determined the natural immune response to urease, a prominent vaccine candidate that has been studied in the animal model (7). The aim of this study was to analyze the serologic immunoglobulin G (IgG) response to two of the most important antigens of H. pylori, CagA and urease proteins, in symptomatic children and adults of the same population and similar socioeconomic levels. These antigens were chosen because CagA is a marker for the presence of the cag pathogenicity island and urease is an enzyme responsible for counteracting gastric acidity, allowing H. pylori colonization of the gastric mucosa.
MATERIALS AND METHODS
Patients.
The pediatric population studied included 352 children who were seen at the Gastroenterology Unit of the Pediatric Hospital (Centro Medico Nacional SXXI-IMSS) because of gastric symptoms; of this group, 242 had recurrent abdominal pain (based on Appley’s criteria), 89 presented with nonulcer dyspepsia (NUD) (abdominal discomfort), and in 21 children the clinical diagnosis was unknown. The adult population consisted of 293 persons seen at the Gastroenterology Unit at the General Hospital of the Centro Medico Nacional Siglo XXI-IMSS; of this group, 143 had peptic ulcer disease (PUD) and 150 presented with NUD. A blood sample was obtained from each patient, and the serum was separated from the cells by centrifugation and stored at −20°C until it was used. All of the patients lived in Mexico City and belonged to a low-to-middle socioeconomic group. The Ethics Committee at the Centro Mé dico Nacional Siglo XXI-IMSS approved this study.
Definition of H. pylori infection.
H. pylori infection was determined by detection of IgG-specific antibodies to H. pylori antigens by enzyme-linked immunosorbent assay (ELISA), previously validated for use in children and adults in Mexico (2). In brief, a pool of whole-cell antigen preparation was obtained from sonicated preparations of three strains of H. pylori. Serum samples were diluted 1:1,000, and 100-μl aliquots were plated. Next, a 1:1,000 dilution of anti-human IgG monoclonal antibodies conjugated to alkaline phosphatase (Southern Biotech, Birmingham, Ala.) was applied. A 1-mg/ml solution of p-nitrophenylphosphate was used as a substrate, and absorbance was read at 405 nm.
ELISA for IgG anti-urease.
IgG anti-urease was determined by ELISA, previously validated in our laboratory as described elsewhere (2). The urease antigen preparation (kindly supplied by Acambis, Inc., Cambridge, Mass.) was a recombinant urease apoenzyme antigenically similar to native urease with an identical large molecular size and particulate structure (16). Briefly, plates were coated with 0.5 μg of urease/well, and serum samples were added at 1:400 dilution. Next, monoclonal antibodies for anti-human IgG conjugated to alkaline phosphatase, diluted 1:1,000, were added; p-nitrophenylphosphate was used as a substrate, and absorbance was read at 405 nm.
ELISA for IgG anti -CagA.
We used ELISA, previously validated in our laboratory, to determine IgG anti-CagA (2). In brief, 0.1 μg of a recombinant CagA peptide antigen (molecular mass, 60 KDa) (Acambis, Inc.)/well was used to coat microtiter plates, and serum at 1:200 dilution was added. Alkaline phosphatase-conjugated anti-human IgG monoclonal antibodies diluted 1:1,000 were used, and a 1-mg/ml solution of p-nitrophenylphosphate was used as a substrate. Absorbance was read at 405 nm.
Interpretation of ELISAs.
The result for each serum sample was expressed as the ratio of the optical density value of the sample to the threshold value and was expressed in ELISA units, as previously reported (2). Accordingly, serum samples with ELISA units of >1.0 were considered seropositive. Each serum sample was tested in duplicate.
Culture from biopsies.
Antral and corpus biopsy specimens were taken from both children and adults. The biopsy specimens were homogenized and inoculated onto selective Columbia agar plates supplemented with 7% sheep blood (10). The plates were incubated at 37°C in an atmosphere of 9% CO2 for 10 days; colonies with a morphology suggestive of H. pylori were confirmed with Gram stain, urease, catalase, and oxidase tests.
UBT.
The urea breath test (UBT) was used as an internal control for the serological assay and was applied to some of the children and adults studied. The patients fasted for at least 6 h before the study. For children, we used a test kit from Isomed (Madrid, Spain) with [13C[urea as a substrate and followed the manufacturer’s instructions (31). Samples were analyzed in a mass spectrometer (ABCA; Europe Scientific), and the cutoff value was established at 3.5δ according to the method of Logan et al. (17). In adults, the UBT was performed using [14C]urea capsules (Tri-Med, Charlottesville, Va.).
Statistical analyses.
Differences in the frequency of immune response between children and adults according to clinical diagnosis and between anti-CagA-positive and -negative patients were studied using the chi square test with Yates’ correction. Differences in the magnitude of the immune response between the different groups were studied using the Student t test.
RESULTS
There were 645 subjects studied, including 352 children with a mean age of 9.5 ± 3.9 years and 293 adults with a mean age of 48.1 ± 15.9 years. H. pylori infection was detected by serology in 130 (36.9%) children, while among those infected, 61 (46.9%) had antibodies against CagA and 21 (16.2%) had antibodies against urease. In contrast, 261 (89.1%) of 293 adults were infected based on serology; among these, 206 (78.9%) had antibodies against CagA and 154 (59%) had antibodies against urease.
Clinical diagnoses were available for 331 children and for all 293 adults; response to whole-cell antigen, CagA, and urease in these patients is described in Table 1. In children, H. pylori infection was similar in both patients with recurrent abdominal pain (RAP) and patients with NUD; however, response to CagA was significantly higher in children with NUD. In adults, H. pylori infections determined by serology were similar in patients with PUD and with NUD; responses to CagA and urease were not significantly different in these two groups.
TABLE 1.
Serum IgG responses to whole-cell extract, CagA, and urease in children and adults according to clinical diagnosis
| Age group and diagnosis | No. of patients studied | No. (%) positive for IgG antibodies againsta:
|
||
|---|---|---|---|---|
| Whole cell | CagA | Urease | ||
| Children | ||||
| RAP | 242 | 86 (35.5) | 40 (46.5)* | 9 (10.5)** |
| NUD | 89 | 31 (34.8) | 21 (67.7) | 8 (25.8) |
| Adults | ||||
| PUD | 143 | 129 (89.6) | 108 (83.7)*** | 72 (55.8) |
| NUD | 150 | 133 (86.7) | 99 (74.4) | 83 (62.4) |
*, P = 0.04, RAP versus NUD; **, P = 0.07, RAP versus NUD; ***, P = 0.07, PUD versus NUD.
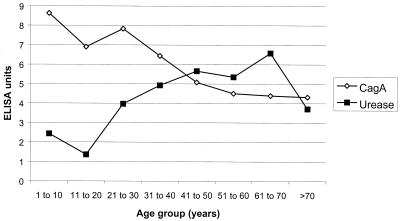
The magnitudes of the IgG responses to these antigens in infected children and adults are described in Table 2. IgG anti-CagA was significantly higher and IgG anti-urease was significantly lower in children than in adults. To further characterize this contrasting response, its magnitude was analyzed in different age groups (Fig. 1). It is important to mention that the highest response to CagA was observed in children (>7 ELISA units); response decreased steadily associated with age (to <5 ELISA units). In contrast, the magnitude of the response to urease was low in children (<3 ELISA units), subsequently increasing in young adults (>5 ELISA units) and decreasing in older individuals (<4 ELISA units).
TABLE 2.
Magnitudes of IgG responses to CagA and urease in children and adultsa
| Age group | Magnitude of IgG response (ELISA units)
|
|||
|---|---|---|---|---|
| No. of patients | CagA | No. of patients | Urease | |
| Children | 53 | 10.39 ± 6.94** | 12 | 2.44 ± 1.42** |
| Adults | 208 | 6.94 ± 5.4 | 155 | 6.10 ± 3.28 |
All patients were seropositive for H. pylori infection by serology; **, P < 0.001, children versus adults.
FIG. 1.
Magnitudes (in ELISA units) of the serologic IgG responses to CagA and urease in H. pylori-infected patients according to age.
Because of the difference in results observed between the responses to CagA and urease, we compared the responses to urease in patients positive or negative for CagA antibodies (Table 3). Seropositivity to urease was higher in patients negative for CagA in both children and adults, but the difference was significant only in adult patients, although the magnitude of the anti-urease response in CagA-positive children and adults was not significantly different from that in individuals negative for anti-CagA antibodies.
TABLE 3.
Frequency and magnitude of IgG anti-urease response in patients infected with H. pylori with or without antibodies against CagAa
| Age group and anti-CagA status | n | Response to urease
|
|
|---|---|---|---|
| No. (%) seropositive | Magnitude (ELISA units) | ||
| Children | |||
| Anti-CagA positive | 62 | 8 (12.9) | 3.3 ± 1.6 |
| Anti-CagA negative | 68 | 13 (19.1) | 2.3 ± 1.1 |
| Adults | |||
| Anti-CagA positive | 208 | 114 (54.8)** | 5.9 ± 3.3 |
| Anti-CagA negative | 52 | 38 (73.1) | 6.3 ± 3.3 |
Infection was defined serologically, with a whole-cell antigen ELISA; * *, P < 0.01, anti-CagA positive versus anti-CagA negative.
DISCUSSION
As expected, using the serologic IgG response to whole-cell antigens as the criterion to define infection, H. pylori infection was more frequent in adults than in children. Also, the frequency of the response to both CagA and urease antigens was significantly lower in children than in adults. When the clinical diagnosis was considered, children with NUD had a higher response to CagA and urease than children with RAP. It is probable that children with RAP represent patients with an acute infection, whereas children with NUD represent patients with a more prolonged infection, which explains the higher IgG response to H. pylori antigen preparations. In a previous report, we found similar results (2). An alternative hypothesis is that the poor immune response to H. pylori antigens in children with RAP may suggest that H. pylori has no association with RAP, as suggested by others (27).
In a study of schoolchildren in Estonia (29), a population with a high prevalence of H. pylori infection similar to that reported in our country (26), 46% of the infected children were seropositive to CagA. It should be noted that the criterion used for H. pylori infection in that study was exclusively serologic. When we used the same serologic criterion, a similar seropositivity to CagA (45%) was found. Interestingly, in a previous study of the same Estonian population (28), a higher seropositivity to CagA was found in adults (63%), also in accordance with our results, suggesting that H. pylori cagA-positive strains are more prevalent in adults than in children in both countries. In contrast, a study of symptomatic Japanese children (12) reported a seropositivity of >80% to CagA. However, >60% of these children had PUD, indicating that the criterion used in the selection of patients was different from that used in our study. It should also be noted that in Japan, the prevalence of cagA-positive H. pylori strains is very high, nearly 100%; in contrast to the results in our population, there was no difference between children and adults (12).
Other studies have also reported the seroprevalence to CagA in children when invasive tests are used to diagnose H. pylori infection. In France (24), in children positive by culture and histology, antibodies against CagA were detected in 43% of the infected children; in Israel (30), among children with H. pylori-positive culture and histology test results, 43% were seropositive for CagA. However, in Finnish children (14), seropositivity to CagA was 69% in patients positive by culture. In Italy (18), in a study using a rapid urease test and histology for H. pylori diagnosis, seropositivity to CagA in children was 43%. These values probably represent the actual prevalence of cagA-positive strains colonizing children in those populations, although the age of the patient may also influence the rate of seropositivity. In fact, in children H. pylori-positive by culture and histology in Brazil (25), response to CagA was 66% in those without ulcers; however, this seropositivity increased with age, from 12% in 2- to 6-year-old patients to 88% in those >12 years of age. These results agree with our findings and further suggest that the prevalence of cagA-positive strains has decreased in younger generations. In a recent report (9), we compared the presence of the cagA gene in strains isolated from children and adults and found that 47% of H. pylori isolates from children had the cagA gene, in contrast to 90% in H. pylori isolates from adults; thus, cagA genotyping agrees with serologic detection of CagA protein expression (46.9%). There is, however, a proportion of children with serology positive for H. pylori as the only evidence for infection; these are probably children colonized with cagA-negative H. pylori strains, which further suggests the disappearance of cagA-positive strains in the younger generations.
An alternative explanation is that immune response to CagA requires more time than response to other antigens, such as in those studies that demonstrated that in children the initial response is mounted mainly against low-molecular-weight proteins and that it may take months to see a response to high-molecular-weight proteins, including CagA (24, 25). Another possibility is the spontaneous eradication of H. pylori in children (1, 13, 22); these children would be seropositive for H. pylori infection but negative for immune response to CagA.
An intriguing finding was that the magnitude of the IgG response to CagA was higher in children and that it decreased with age; this result contrasted with the magnitude of the response to urease, which increased steadily with age and reached a maximum in patients >60 years of age. These results suggest that the kinetics of the immune responses to CagA and urease are contrastably different. The contrasting responses to CagA and to urease are further documented with our finding that the serologic response to urease is significantly higher in both children and adults seronegative for CagA. These observations are difficult to interpret; one possibility would be that cagA-negative strains express more urease, offering a higher antigenic challenge to the gastric mucosa.
In the present study, we found that serologic IgG response to urease is low in children (16%) and that this response increases with age, although only 60% of adults developed IgG anti-urease antibodies. The magnitude of the serologic response also increased with age. These results suggest either that during a natural infection urease is a weak immunogen or that expression of this antigen is gradually increased during the infection and that years of colonization are required to induce an immune response. In previous studies, we have reported similar results in both symptomatic patients and a community-based population (2, 15). The results of the present study further document our previous findings. In a recent study of young adults (average age, 26 years) (8), no serologic IgG response to urease was detected in patients with gastritis. These results are in accordance with our findings of a low response to urease in young adults.
Several authors have suggested that urease is highly immunogenic and have even proposed its use for diagnosis of the infection (4, 11). However, the majority of these studies have used partially purified urease, which probably contains other more potent antigens, such as heat shock proteins (6). A possible explanation for these results may be related to the type of antigen used in the serologic assays. In all our studies (2), we have used a highly purified recombinant antigen that is devoid of other H. pylori antigens. In addition, we have previously shown that there is a good correlation between the recognition of recombinant urease in ELISA and the recognition of native urease in Western blot analysis (2); this suggests that recombinant urease is a good antigen for the study of the IgG response to urease.
In conclusion, our results demonstrate that there are differences between the serologic responses to CagA and urease in children and adults. The low response observed in children may confirm the gradual decline in colonization with the most virulent H. pylori strains in the community, particularly in younger generations. The observation that children with RAP had a lower IgG response to CagA and urease than children with NUD suggests that RAP may not be associated with H. pylori infection. The kinetics of response to CagA and urease are contrastably different: in the natural infection, apparently CagA is a strong immunogen and urease is a poor immunogen.
Acknowledgments
This work was supported by the Coordinación de Investigación Médica, IMSS, and by Conacyt (Mexico) grant 28040.
REFERENCES
- 1.Ashorn, M., M. Maki, M. Uhari, H. K. Akerblom, J. Viikari, and A. Miettinen. 1995. Helicobacter pylori infection in Finnish children and adolescents. Scand. J. Gastroenterol. 30:876–879. [DOI] [PubMed] [Google Scholar]
- 2.Camorlinga-Ponce, M., J. Torres, G. Pérez-Pérez, Y. Leal-Herrera, B. Gonzalez-Ortiz, A. Madrazo-de la Garza, A. Gómez, and O. Muñoz. 1998. Validation of a serologic test for the diagnosis of Helicobacter pylori infection and the immune response to urease and CagA in children. Am. J. Gastroenterol. 93:1264–1270. [DOI] [PubMed]
- 3.Caswall, T. H., H.-O. Nilsson, M. Bergström, P. Aleljung, T. Wadström, A. K. Dahlström, M. J. Albert, and S. A. Sarker. 1999. Evaluation of serology, 13C-urea breath test, and polymerase chain reaction of stool samples to detect Helicobacter pylori in Bangladeshi children. J. Pediatr. Gastroenterol. Nutr. 28:31–36. [DOI] [PubMed] [Google Scholar]
- 4.Dent, J. G., C. A. M. McNulty, J. S. Uff, M. W. L. Gear, and S. P. Wilkinson. 1988. Campylobacter pylori urease: a new serologic test. Lancet i:1002. [DOI] [PubMed]
- 5.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997.Helicobacter pylori. Microbiol. Rev. 10:720–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans, D. J., D. G. Evans, L. Engstrand, and D. Y. Graham. 1992. Urease associated heat shock protein of Helicobacter pylori. Infect. Immun. 60:2125–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrero, R. L., J. M. Thiberge, M. Huerre, and A. Labigne. 1994. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect. Immun. 62:4981–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Futagami, S., H. Takahashi, Y. Norose, and M. Kobayashi. 1998. Systemic and local immune responses against Helicobacter pylori urease in patients with chronic gastritis: distinct IgA and IgG productive sites. Gut 43:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Valencia, G., J. C. Atherton, O. Muñoz, M. Dehesa, A. Madrazo-de la Garza, and J. Torres. 2000. Helicobacter pylori vacA and cagA genotypes in Mexican adults and children. J. Infect. Dis. 182:1450–1454. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin, C. S., E. D. Blincow, J. R. Warren, T. E. Waters, C. R. Sanderson, and L. Easton. 1985. Evaluation of cultural techniques for isolation of Campylobacter pyloridis from endoscopic biopsies of the gastric mucosa. J. Clin. Pathol. 38:1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson, F. D. 1989. Evaluation of three Helicobacter pylori antigen preparations for screening sera from patients undergoing endoscopy. J. Clin. Pathol. 42:723–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato, S., T. Sugiyama, M. Kudo, K. Ohnuma, K. Ozawa, K. Iinuma, M. Asaka, and M. J. Blaser. 2000. CagA antibodies in Japanese children with nodular gastritis or peptic ulcer disease. J. Clin. Microbiol. 38:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein, P. D., R. H. Gilman, R. León-Barua, F. Díaz, E. O. Smith, and D. Y. Graham. 1994. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. Am. J. Gastroenterol. 89:2196–2200. [PubMed] [Google Scholar]
- 14.Kolho, K. L., R. Karttunen, P. Heikkila, H. Lindahl, and H. Rautelin. 1999. Gastric inflammation is enhanced in children with CagA-positive Helicobacter pylori infection. Pediatr. Infect. Dis. J. 18:337–341. [DOI] [PubMed] [Google Scholar]
- 15.Leal-Herrera, Y., J. Torres, G. Pérez-Pérez, A. Gómez, T. Monath, R. Tapia-Conyer, and O. Muñoz. 1999. Serologic IgG response to urease in Helicobacter pylori infected persons from Mexico. Am. J. Trop. Med. Hyg. 60:587–592. [DOI] [PubMed] [Google Scholar]
- 16.Lee, C. K., R. Weltzin, W. D. Thomas, H. Kleanthous, T. H. Ermak, G. Soman, J. E. Hill, S. K. Ackerman, and T. P. Monath. 1995. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J. Infect. Dis. 172:161–172. [DOI] [PubMed] [Google Scholar]
- 17.Logan, R. P. H., S. Dill, F. E. Bauer, M. M. Walker, A. M. Hirschl, P. A. Gummett, et al. 1991. The European 13C-urea breath test for the detection of Helicobacter pylori. Eur. J. Gastroenterol. Hepatol. 3:915–921. [Google Scholar]
- 18.Luzza, F., A. Contaldo, M. Imeneo, M. Mancuso, L. Pensabene, L. Giancotti, A. M. La Vecchia, M. C. Costa, P. Strisciuglio, C. Docimo, F. Pallone, and S. Guandalini. 1999. Testing for serum IgG antibodies to Helicobacter pylori cytotoxin-associated protein detects children with higher grades of gastric inflammation. J. Pediatr. Gastroenterol. Nutr. 29:302–307. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell, H. M., S. L. Hazell, T. Kolesnikow, J. Mitchell, and D. Frommer. 1996. Antigen recognition during progression from acute to chronic infection with a cagA-positive strain of Helicobacter pylori. Infect. Immun. 64:1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell, H. M., S. L. Hazell, T. D. Bohane, P. Hu, M. Chen, and Y. Y. Li. 1999. The prevalence of antibody to CagA in children is not a marker for specific disease. J. Pediatr. Gastroenterol. Nutr. 28:71–75. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira, A. M. R., G. A. Rocha, D. M. M. Queiroz, E. N. Mendes, A. S. T. Carvalho, T. C. A. Ferrari, and A. M. M. F. Nogueira. 1999. Evaluation of enzyme-linked inmmunosorbent assay for the diagnosis of Helicobacter pylori infection in children from different age groups with and without duodenal ulcer. J. Pediatr. Gastroenterol. Nutr. 28:132–134. [DOI] [PubMed] [Google Scholar]
- 22.Perri, F., M. Pastore, R. Clemente, V. Festa, M. Quitadamo, G. Niro, P. Conoscitore, P. Rutgeersts, and A. Andriulli. 1998. Helicobacter pylori infection may undergo spontaneous eradication in children: a 2-year follow-up study. J. Pediatr. Gastroenterol. Nutr. 27:181–183. [DOI] [PubMed] [Google Scholar]
- 23.Peterson, W. L. 1991. Helicobacter pylori and peptic ulcer disease. N. Engl. J. Med. 324:1043–1048. [DOI] [PubMed] [Google Scholar]
- 24.Raymond, J., C. Sauvestre, N. Kalach, M. Bergeret, and C. Dupont. 2000. Immunoblotting and serology for diagnosis of Helicobacter pylori infection in children. Pediatr. Infect. Dis. J. 19:118–121. [DOI] [PubMed] [Google Scholar]
- 25.Rocha, G. A., A. M. R. Oliveira, D. M. M. Queiroz, A. S. T. Carvalho, and A. M. M. F. Nogueira. 2000. Immunoblot analysis of humoral immune response to Helicobacter pylori in children with and without duodenal ulcer. J. Clin. Microbiol. 38:1777–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres, J., Y. Leal-Herrera, G. Pérez-Pérez, A. Gómez, M. Camorlinga-Ponce, R. Cedillo-Ribera, R. Tapia-Conyer, and O. Muñoz. 1998. A community-based seroepidemiological study of Helicobacter pylori infection in Mexico. J. Infect. Dis. 178:1089–1094. [DOI] [PubMed] [Google Scholar]
- 27.Torres, J., G. Pérez-Pérez, K. J. Goodman, J. C. Atherton, B. D. Gold, P. R. Harris, A. Madrazo-de la Garza, J. Guarner, and O. Muñoz. 2000. A comprehensive review of the natural history of Helicobacter pylori infection in children. Arch. Med. Res. 31:431–469. [DOI] [PubMed] [Google Scholar]
- 28.Vorobjova, T., I. Nilsson, K. Kull, H.-I. Maaroos, A. Covacci, T. Wädstrom, and R. Uibo. 1998. CagA protein seropositivity in a random sample of adult population and gastric cancer patients in Estonia. Eur. J. Gastroenterol. Hepatol. 10:41–46. [DOI] [PubMed] [Google Scholar]
- 29.Vorobjova, T., H. Grunberg, M. Oona, H.-I. Maaroos, I. Nilsson, T. Wadström, A. Covacci, and R. Uibo. 2000. Seropositivity to Helicobacter pylori and CagA protein in schoolchildren of different ages living in urban and rural areas in southern Estonia. Eur. J. Gastroenterol. Hepatol. 12:97–101. [DOI] [PubMed] [Google Scholar]
- 30.Yahav, J., A. Fradkin, B. Weisselberg, A. Diver-Haver, H. Shmuely, and A. Jonas. 2000. Relevance of CagA positivity to clinical course of Helicobacter pylori infection in children. J. Clin. Microbiol. 38:3534–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yañez, P., A. Madrazo-de la Garza, G. Pérez-Pérez, L. Cabrera, O. Muñoz, and J. Torres. 2000. Comparison of invasive and noninvasive methods for the diagnosis and evaluation of eradication of Helicobacter pylori infection in children. Arch. Med. Res. 31:415–421. [DOI] [PubMed] [Google Scholar]