Abstract
The Western blot (WB) assay is the most widely accepted confirmatory assay for the detection of antibodies to human immunodeficiency virus type 1 (HIV-1). However, indeterminate WB reactivity to HIV-1 proteins may occur in individuals who do not appear to be infected with HIV. The profiles of WB reactivity among Ethiopians are hardly known. Here, we describe the profiles of indeterminate WB reactivity in Ethiopians with discordant screening assays. Between 1996 and 2000, a total of 12,124 specimens were tested for HIV-1 antibodies. Overall, 1,437 (11.9%) were positive for HIV-1 antibody. Ninety-one (≈0.8%) gave equivocal results because of discordant results among the various screening assays and indeterminate WB profiles by the American Red Cross (ARC) criteria. Most (30.4%) of these indeterminate WB results were due to p24 reactivity. However, 12 samples (13.2%) displayed reactivity to p24 and gp41 or to p24 and gp120/160 proteins (positive by Centers for Disease Control and Prevention [CDC] criteria). Only two samples (2.2%) were reactive to both env glycoproteins gp41 and gp120/160 (positive by the World Health Organization [WHO] criteria). Of 31 WB assays initially indeterminate by the ARC criteria and with follow-up samples, 29 (93.5%) became negative when retested subsequently while 2 (6.5%) remained indeterminate for more than a year and were thus considered negative. Using CDC and WHO criteria, 6 (19.4%) and 2 (6.5%), respectively, of these WB assays would have been considered falsely positive. In addition, 17 indeterminate samples were negative when assessed by a nucleic acid-based amplification assay for HIV-1 viremia. In general, there was 97.8% concordance between the ARC and WHO criteria and 85.7% concordance between the ARC and CDC criteria for an indeterminate WB result. The ARC criteria best met the specified objectives for diagnosis in our setting.
Although the Western blot (WB) assay is probably the most widely accepted confirmatory assay for the detection of antibodies to human immunodeficiency virus type 1 (HIV-1), indeterminate WB profiles in HIV-uninfected subjects are frequent and are as high as 23 to 53% in some African populations (5, 8). The type of HIV-1 predominantly circulating in Ethiopia is subtype C (1, 2, 7). The performance of WB assays in identifying samples from individuals infected with HIV-1 subtype C has not been widely investigated. The frequency and profiles of indeterminate WB reactivity to HIV-1 proteins among the Ethiopian population have not been systematically investigated. In this report we describe the profiles of indeterminate WB reactivity in blood samples found to be reactive by rapid testing and/or enzyme-linked immunosorbent assay (ELISA) screenings.
MATERIALS AND METHODS
Study population.
The samples evaluated in this study were obtained from participants in a long-term cohort study of the progression of HIV-1 infection in Ethiopia (13, 14) and several other studies. These studies are being conducted by the Ethio-Netherlands AIDS Research Project (ENARP), Ethiopian Health and Nutrition Research Institute (EHNRI), Addis Ababa, Ethiopia. Informed consent was obtained from ENARP study participants, and the study protocols were reviewed and approved by institutional and national ethical clearance committees.
Laboratory methods.
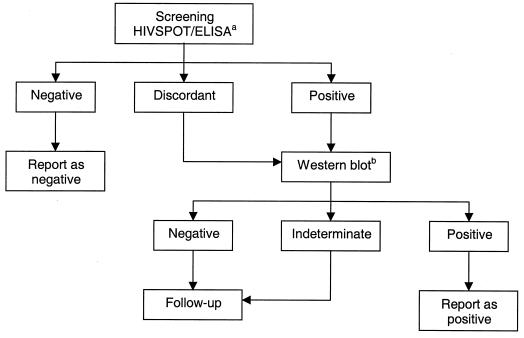
HIV screening for samples from ENARP cohorts was performed by a rapid test (HIVSPOT; Genelabs Diagnostics, Singapore) and ELISA (Vironostika-HIV Uni-Form II plus O; Organon Teknika, Boxtel, The Netherlands). Specimens giving discordant results or those testing reactive by both rapid test and ELISA were then confirmed by WB (HIV Blot 2.2; Genelabs Diagnostics). The algorithm for HIV testing is shown in Fig. 1. HIV screening for samples from other studies was initially performed with an ELISA (Vironostika-HIV Uni-Form II plus O). Specimens testing reactive were then confirmed by another methodologically different ELISA (HIV 1+2; Murex Biotech Ltd., Dartford, United Kingdom). Only discrepant results were then evaluated by WB (HIV Blot 2.2; Genelabs Diagnostics). In HIVSPOT, the capture HIV proteins are the recombinant protein of HIV-1, corresponding to a region overlapping the junction between the gp120 and gp41 fragment of the env protein, plus a highly purified peptide which corresponds to a region of the envelope transmembrane protein of HIV-1. In Vironostika ELISA, the HIV antigens are a mixture of HIV-1 p24, HIV-1 gp160, HIV-1 ANT70 peptide, and HIV-2 env peptide (amino acids 592 to 603). In Murex ELISA, the HIV antigens comprise the highly purified immunodominant antigens of the core and envelope proteins of HIV-1 and an immunodominant peptide of the HIV-2 envelope. In our laboratory, a WB test is interpreted as positive for HIV-1 antibodies according to the American Red Cross (ARC) criteria, requiring the presence of at least three bands, one from each gene product group of gag, pol, and env (4). Three observers read all the WB results independently. Also, we compared the performance of the ARC criteria with those of the World Health Organization (WHO) (a specimen is interpreted as positive when there is reactivity to at least two env bands [16]) and of the Centers for Disease Control and Prevention (CDC) (a specimen is interpreted as positive when there is reactivity to any two of p24, gp41, or gp120/160 bands [3]). The HIV-1 viral load in plasma was assessed by using nucleic acid-based amplification assay (NASBA; Organon Teknika). All assays were done as specified by the manufacturers.
FIG. 1.
HIV testing algorithm of ENARP laboratory for cohort samples. aELISA, Vironostika-HIV Uni-Form II plus O; bWestern blot test results interpreted according to the ARC criteria, requiring the presence of at least three bands, one from each gene product group of gag, pol, and env (4).
Statistical analysis.
Factors associated with false-positive HIVSPOT and ELISA and indeterminate WB test results were examined by univariate analysis (chi-square and t test where appropriate) and multivariate analysis (logistic regression). The statistical analysis was performed using the STATA statistical package (Intercooled Stata 6.0; Stata Corp., College Station, Tex.).
RESULTS
Between 1996 and 2000, a total of 12,124 samples were tested for HIV-1 antibodies. A total of 1,437 plasma specimens (11.9%) were HIV-1 antibody positive. Of these, 91 (≈0.8%) gave equivocal results, with discordant serological data and indeterminate WB profiles.
The WB reactivities measured on 91 indeterminate test results are summarized in Table 1. Most of the indeterminate WB results were due to antibodies against the HIV-1 core antigen p24 (30.4%). In addition, reactivities to pol p51 (13.9%), pol p66 (14.6%), and env gp41 (15.2%) antigens were frequent. Isolated p17 reactivity was observed rarely (only 2.3% of the samples). A total of 12 samples (13.3%) displayed reactivity to two of the three proteins p24, gp41, and gp120/160 and thus can be considered positive by CDC criteria (3). Only two samples (2.2%) were found to be reactive to both env glycoproteins gp41 and gp120/160. These samples (also positive by the CDC criteria) can be considered as positive by the WHO criteria (16). Combined reactivities to pol (p51 or p66) and env or core (p24) were not frequent (6.6%). Other infrequent WB profiles were a combination of p17 and p24 (2.3%), p17 and pol proteins p51 and/or p66 (6.7%), or p17 and gp41 (4.5%). In general, there was 97.8% concordance between the ARC and WHO criteria and 85.7% between the ARC and CDC criteria for an indeterminate result.
TABLE 1.
Profile of indeterminate WB reactivity to HIV-1 proteins in HIV uninfected Ethiopians
| Band profile | No. (%) of indeterminate resultsa |
|---|---|
| env | |
| gp120/160 | 12 (7.6) |
| gp41 | 24 (15.2) |
| pol | |
| p66 | 23 (14.6) |
| p51 | 22 (13.9) |
| p31 | 4 (2.5) |
| gag | |
| p55 | 10 (6.3) |
| p24 | 48 (30.4) |
| p17 | 15 (9.5) |
The total number of samples with indeterminate WB test results is 91. Therefore, some samples had band profiles in overlapping categories (see the text).
Among 1,475 HIV-negative cohort participants, 31 (2.1%) had at least one indeterminate WB test result during follow-up, of which 19 (61%) were related to false-positive ELISA results only, 11 (36%) were related to false-positive HIVSPOT test results only, and 1 (3%) was related to both (Table 1). Most of the initially indeterminate WB assays (30 of 31 [97%]), including samples considered positive by the CDC criteria (6 of 6), were negative when retested. One subject with initial indeterminate WB profile was negative throughout 30 months of follow-up, at which time he seroconverted. Only two samples remained persistently indeterminate (as long as 18 and 48 months, respectively) without developing any WB reactivity that indicated seroprogression. In addition, 17 indeterminate WB assays, including the two samples that persistently gave indeterminate WB profiles, were assessed by NASBA for HIV-1 viremia. Plasma HIV-1 viremia was not detected in any of the above specimens. None of the indeterminate WB test results turned out to be a preseroconversion sample.
In univariate analysis, the proportion of cohort participants with false-positive HIVSPOT test results was higher among heavy smokers (10 or more cigarettes per day) than among light smokers or nonsmokers (5.6 and 0.6%, respectively; P < 0.001). Also, hemoglobin levels were lower in those with false-positive HIVSPOT test results compared to others (14.1 and 15.3 g/dl, respectively; P = 0.009). In multivariate analysis, false-positive HIVSPOT test results were independently associated with cigarette smoking and lower hemoglobin levels whereas false-positive ELISA results were independently associated with male gender and lower hemoglobin levels (Table 2; marginal associations, P = 0.08 and 0.06, respectively). Samples that are viscous or that contain a precipitate may form a residue and can interfere with the assay (M. Moore, personal communication). It appears possible, therefore, that alterations of blood viscosity linked to smoking (6) may lead to the false-positive HIVSPOT test results that we observed in this study. Of note, one male participant had a transient false-positive HIVSPOT test result at a visit when he was diagnosed with Taenia saginata infection. We did not find any association between false-positive test results and other intestinal parasitic infections, symptomatic medical conditions (e.g., tuberculosis), or high-risk sexual behavior (e.g., frequent contacts with sex workers).
TABLE 2.
Factors associated with false-positive HIVSPOT and ELISA and indeterminate WB test results from cohort participants
| HIV test status and variables | ORa (95% CI) |
|---|---|
| False-positive HIVSPOTb (n = 12/1,475 [0.8%]) | |
| Smoking (cigarettes per day) | |
| <10 | 1.0 |
| >10 | 10.4 (2.67, 40.4) |
| Per increase of 1 g of hemoglobin/dl | 0.75 (0.61, 0.92) |
| False-positive ELISAb (n = 20/1,475 [1.4%]) | |
| Male gender | |
| No | 1.0 |
| Yes | 4.31 (0.85, 21.9) |
| Per increase of 1 g of hemoglobin/dl | 0.82 (0.66, 1.01) |
OR, odds ratio.
False-positive HIVSPOT: specimens tested initially as positive by HIVSPOT but negative by ELISA and indeterminate by WB; false-positive ELISA: specimens tested initially as positive by ELISA but negative by HIVSPOT and indeterminate by WB. All were then subsequently negative by all parameters.
The performance of the screening assays is summarized in Table 3. Based on the results at intake of the cohort, the sensitivity of ELISA was 100% (one-sided 97.5% confidence interval [CI], 97.6 to 100.0), with a specificity of 99.0% (95% CI, 98.3 to 99.4%), a positive predictive value of 90.9% (95% CI, 85.4 to 94.8%), and a negative predictive value of 100% (one-sided 97.5% CI, 99.7 to 100.0%). The sensitivity of the HIVSPOT was 97.4% (95% CI, 93.4 to 99.3%), with a specificity of 99.5% (95% CI, 99.0 to 99.8%), a positive predictive value of 95.5% (95% CI, 90.9 to 98.2%), and a negative predictive value of 99.7% (95% CI, 99.3 to 99.9%).
TABLE 3.
| Assay type | Sensitivity (%) | Specificity (%) | PPVc | NPVc |
|---|---|---|---|---|
| ELISA | 100 (97.6–100.0)d | 99.0 (98.3–99.4) | 90.9 (85.4–94.8) | 100.0 (99.7–100.0)d |
| HIVSPOT | 97.4 (93.4–99.3) | 99.5 (99.0–99.8) | 95.5 (90.9–98.2) | 99.7 (99.3–99.9) |
All WB-negative results, or not done (when the test results of the two screening assays were negative), or indeterminate results were considered HIV negative, and all WB-positive results were considered HIV positive.
Data are presented as percentages (95% CI or one-sided 97.5% CI).
PPV, positive predictive value; NPV, negative predictive value.
One-sided 97.5% CI.
DISCUSSION
The results of this study indicate that indeterminate WB reactivity to HIV-1 proteins using the ARC criteria may occur among Ethiopians who do not otherwise appear to be infected with HIV-1. In most cases, the indeterminate WB results in this population were due to reactivities to p24 antigens, although reactivities to other HIV-1 antigens were also present. None of the indeterminate WB test results turned out to be a preseroconversion sample.
In our setting, using negative HIV-1 serology at follow-up visits as a “gold standard” for the subjects being uninfected with HIV-1, the CDC criteria would have inappropriately classified 12 ARC-indeterminate samples (13.2%) as HIV-1 seropositive and the WHO criteria would have misclassified 2 samples (2.2%). The most strict interpretation criteria set by ARC proved to be the most accurate in the Ethiopian context. Thus, because of the reported relatively high frequency of the indeterminate WB profile in apparently healthy HIV-seronegative Africans (5, 8), it could be concluded that, in general, stricter criteria may be required for interpretation of WB test results on African samples.
Many initially indeterminate results that subsequently become negative or remain indeterminate are probably the result of nonspecific reactions between antibodies to residual cell components on the WB strips or are due to the presence of hypergammaglobulinemia or of cross-reacting antibodies to some parasites or are due to infection with an unknown but related retrovirus (4, 15). HIV-1 vaccine trials are under way in many developing countries. One of the major practical obstacles for HIV-1 vaccine trials is the development of tests that discriminate between vaccinees and those who are truly infected. This is particularly relevant for those who will receive candidate vaccines that elicit antibodies to a limited number of HIV-1 proteins. The WB profiles of such vaccinees will have to be studied and put in the context of the indeterminate WB profiles, as described in this paper. In the Ugandan vaccine trial currently under way, infection with helminths was associated with indeterminate WB bands to HIV-1 glycoproteins, thus necessitating deworming of volunteers before vaccination (R. Mugerewa, personal communication). These findings indicate that it is important to assess the background WB profile of HIV-uninfected individuals in a given community before embarking on vaccine trials. However, in the present study we did not find any association between false-positive test results and other intestinal parasitic infections. However, one male cohort participant had a transient false-positive HIVSPOT test result at a visit when he was diagnosed with T. saginata infection. A high rate of indeterminate WB profiles in Africans might be the result of dysregulated immune status background, which is commonly observed in these populations (10, 12). The underlying reason(s) for such high background immune activation status remains to be determined (10).
The interpretation of indeterminate WB test results, in particular those with discordant results in two screening assays, like rapid tests and ELISA, is a challenge to laboratories in the developing or least developed countries. An important implication of this is how to address these issues with respect to counseling. Although such problems may not be frequent (<1%), counseling should be tailored differently for individuals with equivocal HIV-1 test results. Follow-up of such cases, in combination with an offer to obtain a repeat blood sample for retesting, is one of the most important measures. This would be a more practical approach than to use alternative laboratory tests, such as determination of plasma viral load, to help clarify such cases (9, 11; this study). Viral load determinations are still largely not available in many developing countries where HIV-1 infection is also highly prevalent.
New HIV-1 subtypes and circulating recombinant forms of HIV-1 are being discovered regularly. Thus, the performance of WB assays in correctly identifying samples from individuals infected with these viruses needs constant attention. In conclusion, each laboratory must adopt the criteria that best meet specified objectives for diagnosis. In the Ethiopian setting, it is recommended to opt for the most stringent criteria of interpretation of the WB, namely, by ARC. It may well be that these criteria prove optimal for other African contexts too.
Acknowledgments
ENARP is a collaborative research project among EHNRI, the Amsterdam Municipal Health Service, the Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, and the Academic Medical Centre of the University of Amsterdam. ENARP is a bilateral project financially supported The Netherlands Ministry of Foreign Affairs and the Ethiopian Ministry of Health.
We thank the study participants for their kind collaboration.
REFERENCES
- 1.Abebe, A., C. L. Kuiken, J. Goudsmit, M. Valk, T. Messele, T. Sahlu, H. Yeneneh, A. Fontanet, F. D. Wolf, and T. F. Rinke de Wit. 1997. HIV type 1 subtype C in Addis Ababa, Ethiopia. AIDS Res. Hum. Retrovirol. 13:1071–1075. [DOI] [PubMed] [Google Scholar]
- 2.Abebe, A., G. Pollakis, A. L. Fontanet, B. Fisseha, B. Tegbaru, A. Kliphuis, G. Tesfaye, H. Negassa, M. Cornelissen, J. Goudsmit, and T. F. Rinke de Wit. 2000. Identification of a genetic subcluster of HIV type 1 subtype C (C’) widespread in Ethiopia. AIDS Res. Hum. Retrovirol. 16:1909–1914. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. 1989. Interpretation and use of the Western blot assay for serodiagnosis of human immunodeficiency virus type 1 infection. Morb. Mortal. Wkly. Rep. 38:1. [Google Scholar]
- 4.Constantine, N. T., J. D. Callahan, and D. M. Watts. Retroviral testing: essentials for quality control and laboratory diagnosis. CRC Press, Inc., Boca Raton, Fla.
- 5.Downing, R. G., R. A. Otten, E. Marum, B. Biryahwaho, M. G. Alwano-Edgeyu, S. D. K. Sempala, C. A. Fridlund, T. J. Dondero, C. Campbell, and M. A. Rayfield. 1998. Optimizing the delivery of HIV counseling and testing services: the Uganda experience using rapid HIV antibody testing algorithms. J. Acquired Immune Defic. Syndr. Hum. Retrovirol. 18:384–388. [DOI] [PubMed] [Google Scholar]
- 6.Ernst, E. 1995. Hemorrhological consequences of chronic cigarette smoking. J. Cardiovasc. Risk 2:435–439. [DOI] [PubMed] [Google Scholar]
- 7.Hussein, M., A. Abebe, G. Pollakis, M. Brouwer, B. Petros, A. L. Fontanet, and T. F. Rinke de Wit. 2000. HIV-1 subtype C in commercial sex workers in Addis Ababa, Ethiopia. J. Acquired Immune Defic. Syndr. Hum. Retrovirol. 23:120–1127. [DOI] [PubMed] [Google Scholar]
- 8.Kaleebu, P. 1999. Application of HIV diagnostic paradigms in HIV vaccine trials: Ugandan experience, p.19–20. In HIV diagnostics and genetic variation in the context of HIV vaccine studies. Proc. Uganda Vaccine Workshop.
- 9.Kleinman, S., M. P. Busch, L. Hall, R. Thomson, S. Glynn, D. Gallahan, H. E. Ownby, and A. E. Williams. 1998. False-positive HIV-1 test results in a low-risk screening setting of voluntary blood donation: Retrovirus Epidemiology Donor Study. JAMA 280:1080–1085. [DOI] [PubMed] [Google Scholar]
- 10.Messele, T., M. Abdulkadir, A. L. Fontanet, B. Petros, D. Hamann, M. Koot, M. T. L. Roos, P. T. A. Schellenkens, F. Miedema, and T. F. Rinke de Wit. 1999. Reduced naive and increased activated CD4 and CD8 cells in healthy adult Ethiopians compared with their Dutch counterparts. Clin. Exp. Immunol. 115:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mylonakis, E., M. Paliou, T. C. Greenbough, T. P. Flaningan, N. L. Letvin, and J. D. Rich. 2000. Report of false-positive HIV test result and the potential use of additional tests in establishing HIV serostatus. Arch. Intern. Med. 160:2386–2388. [DOI] [PubMed] [Google Scholar]
- 12.Rizzardini, G., S. Piconi, S. Ruzante, M. L. Fusi, M. Lukwiya, S. Declich, M. Tamburini, M. L. Villa, M. Fabiani, F. Milazzo, and M. Clerici. 1996. Immunological activation markers in the serum of African and European HIV seropositive and seronegative individuals. AIDS 10:1535–1542. [DOI] [PubMed] [Google Scholar]
- 13.Sahlu, T., A. Fontanet, T. Rinke de Wit, T. Messele, R. Doorly, H. Yeneneh, P. Bindels, and R. Coutinho. 1998. Identification of a site for a cohort study of natural history of HIV infection in Ethiopia. J. Acquired Immune Defic. Syndr. Hum. Retrovirol. 17:149–155. [DOI] [PubMed] [Google Scholar]
- 14.Sahlu, T., E. Kassa, T. Agonafer, A. Tsegaye, T. Rinke de Wit, H. Gebremariam, R. Doorly, I. Spijkerman, H. Yeneneh, R. A. Coutinho, and A. Fontanet. 1998. Sexual behaviors, perception of risk of HIV infection, and factors associated with attending HIV post-test counseling in Ethiopia. AIDS 13:1263–1272. [DOI] [PubMed] [Google Scholar]
- 15.Watt, G., P. Chanbancherd, and A. E. Brown. 2000. Human immunodeficiency virus type 1 test results in patients with malaria and dengue infections. Clin. Infect. Dis. 30:819. [DOI] [PubMed] [Google Scholar]
- 16.WHO Collaborating Group on Western Blotting. 1990. Proposed WHO criteria for interpreting results from Western blot assays for HIV-1, HIV-2 and HTLV-I/HTLV-II. Wkly. Epidemiol. Rec. 37:281–283. [PubMed] [Google Scholar]