Abstract
Anionic peptides (APs) are small anionic antimicrobial peptides composed of 7 aspartic acid residues and are produced in the lungs of humans, sheep, and cattle. Although expression by epithelial cells of some antimicrobial peptides (e.g., β-defensins) of humans and ruminants is increased in response to acute infection, AP expression is not increased during acute infection, which suggests that the expression of the latter peptide is constitutive. In this study, the degree of AP expression during the progression (acute, subacute, and chronic) of bronchopneumonia was determined. Mannheimia (Pasteurella) haemolytica, a known inducer of bovineβ-defensins, was inoculated intrabronchially with a fiber-optic bronchoscope in nine 3-month-old sheep, and tissues were collected at 1, 15, and 45 days postinoculation (p.i.); nine control animals received pyrogen-free saline by the same procedure and were killed at the same time points. In the acute group (1 day p.i.), the lungs had lesions typical of bronchopneumonia and the distribution and intensity of AP immunoreactivity (AP-IR) were similar to those of previous studies (minimal intensity and distribution of AP-IR in bronchiolar epithelial cells). In the subacute group (15 days p.i.), there was prominent hyperplasia of bronchiolar and alveolar epithelial cells, and the chronic group (45 days p.i.) had yet more pronounced hyperplasia. In the subacute and chronic groups, the intensity and distribution of AP-IR in the cytoplasm of hyperplastic bronchiolar and type II alveolar cells were significantly increased compared to those of saline-inoculated and contralateral (noninoculated) lung lobes. Although AP expression appears constitutive, the constitutive production of AP is higher in hyperplastic, less differentiated cells than in fully differentiated, mature cells of the respiratory airways. The increased intensity and distribution of AP-IR in immature (hyperplastic) epithelial cells may be a mechanism by which production of a noninducible antimicrobial is increased temporarily during lesion progression and repair. This increased production of AP by hyperplastic cells may protect the lung against further infection until new, fully differentiated epithelial cells are capable of expressing their own inducible array of antimicrobial peptides.
Antimicrobial peptides serve as a part of the innate immune system of respiratory epithelia, potentially reducing bacterial numbers nonspecifically and therefore limiting bacterial colonization (9, 11, 13). Some antimicrobial peptides are upregulated in response to lipopolysaccharide and other mediators of inflammation (9, 10, 14, 18, 19, 21), while others are produced constitutively (9, 13). Anionic antimicrobial peptides (APs) are small homopolymeric peptides of 7 aspartic acid residues that have been detected in the lungs and bronchoalveolar lavage fluid of humans, sheep, and cattle (5–8). An AP has potent antimicrobial activity but is distinct from many antimicrobial peptides for the following reasons: it is smaller in size, it has an anionic charge due to its being a septamer of aspartate, it has a requirement for zinc as a cofactor for bactericidal activity, and it is present in pulmonary surfactants (3, 5, 7). AP may be a product of posttransitional cleavage of a larger peptide, such as a zymogen (7); however, the identity of the mature protein product from which AP is cleaved has not as yet been determined.
Expression of some antimicrobial peptides by respiratory epithelia such as human β-defensin 2 (20) and bovine β-defensins (lingual antimicrobial peptides and tracheal antimicrobial peptides) (10, 18, 19, 21) increases in the presence of pulmonary infection and/or inflammation; however, expression of these peptides in neonates tends to be limited. Although expression of AP occurs in neonates, AP expression is likely constitutive, because AP distribution and intensity are not increased in the lungs of adult humans with pneumonia, children with or without cystic fibrosis, or cattle with acute pneumonia caused by Mannheimia haemolytica (6, 8). It is our hypothesis that AP expression may increase during chronic phases of pneumonia in immature, regenerative epithelial cells during the repair of lung injury. Increased expression of a constitutively expressed antimicrobial peptide such as AP may protect the lung from reinfection during repair and allow time for the cells to mature in order to express inducible forms of antimicrobial peptides such as the β-defensins. In this study, we assess the distribution of AP expression in acute (day 1), subacute (day 15), and chronic (day 45) lesions of M. haemolytica infection in the lungs of sheep. M. haemolytica is a known inducer in respiratory epithelia of bovine β -defensins (lingual antimicrobial peptides and tracheal antimicrobial peptides) (21), whose presence can result in chronic, regenerative lesions in the lung characterized by hyperplasia of alveolar and bronchiolar epithelial cells (1, 12, 15, 17).
MATERIALS AND METHODS
Experimental animals.
Eighteen 3-month-old sheep of both sexes and mixed breeds were obtained from Laboratory Animal Resources, Iowa State University. The animals were maintained in isolation rooms in accordance with the procedures approved by the American Association of Laboratory Animal Care. After 24 h, the animals were randomly assigned to two groups; one group was administered doses of M. (Pasteurella) haemolytica, and the other was administered saline. Subsequently, these groups were subdivided according to the time of sacrifice at 1, 15, and 45 days postinoculation (p.i.). This arrangement resulted in six groups, with each group containing three animals.
Inoculation.
M. haemolytica (strain 82/25; originally recovered from a natural case of pneumonic pasteurellosis in sheep) was grown in tryptose broth medium to 10 CFU/ml (2,4). An inoculum of either bacterial broth or pyrogen-free saline (5 ml) was deposited into the right cranial lobe bronchus with a fiber-optic bronchoscope as described previously (2, 4, 16, 17).
Collection of lung samples.
Animals were euthanized with an intravenous overdose of sodium pentobarbital. Lung tissues were collected from the site of inoculum deposition in the right lungs and additionally from the equivalent site in the noninoculated (left) lungs from those animals that received the bacterial inoculum. Two samples of lung per site were collected. Lung tissues were fixed by immersion in 10% neutral buffered formalin (48 h at room temperature).
Immunohistochemistry.
Slides were stained for AP-IR by a previously described procedure (6, 8). Briefly, slides were deparaffinized and subjected to antigen retrieval (microwaved to boiling, simmered for 5 min at near-boiling temperature in citrate buffer [pH 6.0], and then cooled in a −20°C freezer for 15 min and returned to room temperature). The slides were washed in TNTB buffer (0.05 M Tris, 0.85% NaCl, 0.05% Tween 20, 1% bovine serum albumin) and then incubated with the mouse anti-AP immunoglobulin G (IgG) 1G9-1C2 at a 1:1,000 dilution overnight in a humidified chamber at 4°C. 1G9-1C2 was produced in CF1 mice by using the synthetic peptide H-DDDDDDD-OH conjugated to keyhole limpet hemocyanin (5, 7). The following morning, the slides were warmed to room temperature, rinsed with TNTB buffer, and incubated for 30 min at room temperature with biotinylated goat anti-mouse IgG at a 1:400 dilution (Vector, Burlingame, Calif.). The slides were rinsed in BioGenex wash buffer, and then supersensitive alkaline phosphatase-conjugated streptavidin (BioGenex, San Ramon, Calif.) was applied for 30 min. The slides were rinsed and then exposed to chromogen (Vector Red) for 10 to 18 min, rinsed, counterstained, and coverslipped. Specificities of antibody affinities with tissue sections were determined by preincubating the primary antibody, 1G9-1C2, with differing concentrations of up to 0.5 mM synthetic AP (H-DDDDDDD-OH). Inhibition of 100% of the AP-IR was observed after 1G9-1C2 was preincubated for 45 min with 25 μg of the synthetic target peptide/ml. Other included slides that were not incubated with primary antibody or nonspecific mouse IgG served as negative controls. In all control sections, there was no immunoreactivity except in occasional regions of lungs with small foci of necrotic debris.
Scoring of AP immunohistochemistry.
The cellular distribution and intensity of AP-IR were assessed and characterized by light microscopy. The distributions of staining of epithelial cells of airways (bronchi and bronchioles) and alveoli were scored on a subjective scale from five random fields (×40 magnification) in two lung sections, where 0 indicated no AP-IR, 1 indicated that<10% of epithelial cells had AP-IR, 2 indicated that 10 to 30% of epithelial cells had AP-IR, 3 indicated that 30 to 60% of epithelial cells had AP-IR, and 4 indicated that 60 to 90% of epithelial cells had AP-IR. The intensities of AP-IR in epithelial cells were also scored with a subjective scale where 0 indicated no AP-IR, 1 indicated nuclear staining only; 2 indicated multifocal, mild cytoplasmic, and diffuse nuclear staining; 3 indicated diffuse, light red cytoplasmic AP-IR, with or without nuclear staining; and 4 indicated diffuse, bright red cytoplasmic AP-IR.
Specificity of AP monoclonal antibody. (i) Assessment of antibody specificity.
Antibody specificity was previously assessed by a competitive enzyme-linked immunosorbent assay (5, 7). Briefly, bovine serum albumin-DDDDDDD-OH conjugate (50 ng of conjugate/well) was used as the adsorbed antigen and differing graded concentrations (1.0 to 0.002 mM) of H-DDDDDDD-OH, H-GADDDDD-OH, H-VDDDDK-OH, H-TQDDGGK-OH, H-GGEEK-OH, and H-SGSGSGS-OH were used as the competitive antigens in the presence of 1G1-1C2. 1G1-1C2 was specific for peptides with C-terminal Asp residues. The antibody recognized H-GADDDDD-OH nearly as well as H-DDDDDDD-OH. However, H-VDDDDK-OH, H-TQDDGGK-OH, H-GGEEK-OH, and H-SGSGSGS-OH were not recognized.
(ii) Identification of antibody epitopes.
The epitope binding site of the monoclonal (1G9-1C2) antibody was previously determined in great detail (5, 7). Thirty peptides (7 residues each) corresponding to a single residue frameshift of the sequence were synthesized and then screened simultaneously on a derivatized cellulose sheet (SPOTs; Genosys, The Woodlands, Tex.). Antibody 1G9-1C2 recognized epitopes that contained greater than 2 C-terminal Asp (D) residues.
Statistical analysis.
The results of the subjectively scored slide assays were analyzed by the Kruskal-Wallis test. All calculations were performed with SAS Institute (Cary, N.C.) statistical software. Results were classified as significant when P was <0.05.
RESULTS
Gross lesions.
Animals that received the bacterial inoculum had lesions during the acute, subacute, and chronic stages typical of those caused by M. haemolytica pneumonia. Noninoculated (left) lungs and lungs from all lambs inoculated with pyrogen-free saline (control) lacked gross lesions.
Microscopic lesions.
The lungs of animals in the acute group (1 day p.i.) that received bacterial broth had extensive areas of edema, hemorrhage, infiltrates of neutrophils, and necrosis typical of M. haemolytica pneumonia (1, 12, 17).
In the subacute group (15 days p.i.), histologic lesions of lung lobes that had received bacterial inoculation were characterized by (i) large foci of caseous necrotic and pyogranulomatous chronically dilated bronchi surrounded by lobular, suppurative bronchial cells indicative of bronchopneumonia; (ii) fibrous connective tissue in alveolar septa; (iii) a marked proliferation of large, cuboidal epithelial cells in bronchioles; and (iv) alveoli and infiltrates of lymphocytes and plasma cells in large bronchi. There was an underlying pattern of mild interstitial pneumonia and peribronchiolar lymphoid hyperplasia in all sections of lung examined.
The sections of lungs from the chronic (45 days p.i.) group had differing degrees of chronic changes, including alveolar and bronchiolar fibrosis and proliferation of bronchiolar epithelia, accompanied by a marked infiltration of lymphocytes and plasma cells. In one animal, small airways were surrounded by fibrous connective tissue that sometimes encroached on tortuous bronchioles or even formed organized masses inside lumena (bronchiolitis fibrosa obliterans). The alveolar and bronchiolar lumina contained occasional clusters of neutrophils and cell debris.
The lungs of control animals lacked microscopic lesions. However, three animals had mild hyperplasia of peribronchiolar lymphoid tissue, with no other significant changes.
AP-IR.
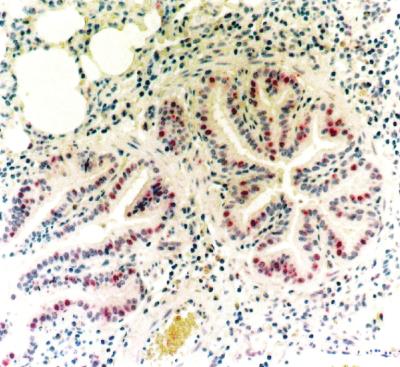
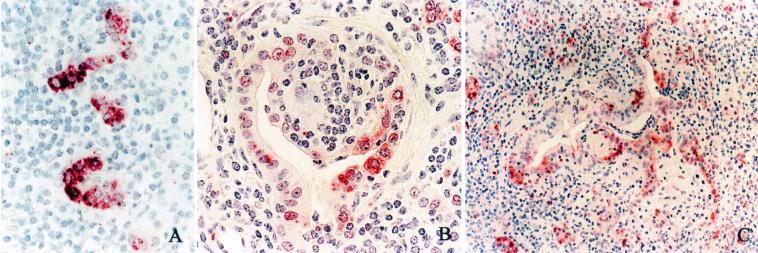
AP-IR was mild in bronchioles of the lung lobes contralateral to inoculated lobes and in the bronchial and bronchiolar epithelial cells of lambs inoculated with saline (Fig. 1 and Tables 1 and 2). AP-IR in lambs from the acute group (1 day p.i.) was similar to that described previously for acute pneumonia of cattle and sheep (A. J. Fales-Williams, K. A. Brogden, E. Huffman, J. M. Gallup, and M. R. Ackermann, unpublished data). Lung sections from the lambs from the subacute group (15 days p.i.) had diffuse AP-IR in hyperplastic bronchiolar epithelial cells lining alveoli and bronchioles in areas of inflammation. AP-IR was also present in individual bronchial and bronchiolar epithelial cells adjacent to the foci of peribronchial lymphoid hyperplasia. In the remaining, noninflamed areas of the lungs, AP-IR was similar to that of the control sections (contralateral and saline-inoculated lungs). AP-IR was present in the cuboidal bronchiolar epithelia in regions of bronchioles exhibiting early fibrosis (Fig. 2A), and prominent AP-IR was present in hyperplastic cells of bronchioles and alveoli (Fig. 2B). In the lungs of the lambs in the chronic group (45 days p.i.), AP-IR was intense in the cytoplasm of the bronchiolar epithelial cells in the foci of bronchiolitis fibrosa obliterans, as well as in cuboidal-to-columnar epithelial cells throughout the foci of lobular bronchopneumonia in one lamb with these histologic changes (Fig. 2C). The intensity of AP-IR in hyperplastic bronchiolar epithelial cells was greater in the chronic group (45 days p.i.) than the subacute pneumonia group (15 days p.i.). In sections of the contralateral lung lobes from infected animals, there were no foci of epithelial hyperplasia, other than multifocal epithelial hyperplasia adjacent to the foci of lymphoid hyperplasia. In both the subacute and chronic pneumonia lesions, there was no AP-IR in hyperplastic bronchiolar epithelia when the tissues were incubated with nonspecific mouse IgG. Although there were distinct visual differences in the intensity of AP-IR in the acute group compared to those of the subacute and chronic groups, there were no statistical differences in the subjective scores as determined by the Kruskal-Wallis test; however, the distribution and intensity of AP-IR were significantly increased in areas of epithelial cell hyperplasia compared to those of areas lacking hyperplastic cells (Table 3). In addition, in all locations where lung tissue showed hyperplastic cells in these animals, AP-IR was intense and in all areas lacking hyperplastic cells, AP-IR intensity was minimal and similar to that of controls. Thus, increased AP-IR intensity and distribution occurred only in regions with hyperplastic cells and was consistently present in such cells.
FIG. 1.
Individual bronchiolar epithelial cells exhibit AP-IR in lungs 1 day p.i. with saline, while many cells lack AP-IR. Most cells lack or have only minimal amounts of AP-IR in the cytoplasm. The airways lack epithelial cells undergoing hyperplasia. Magnification, ×20.
TABLE 1.
Distribution and intensity of AP-IR in lung lobes contralateral (noninoculated) to inoculated lobesa
| No. of days p.i. (no. of sheep) | AP-IR distribution score | AP-IR intensity score |
|---|---|---|
| 1 (3) | 1 | 1 |
| 15 (3) | 1 | 1 |
| 45 (3) | 1 | 1 |
Distribution and intensity of AP-IR were determined as outlined in Materials and Methods.
TABLE 2.
Distribution and intensity of AP-IR in lung lobes of sheep inoculated with pyrogen-free salinea
| No. of days p.i. (no. of sheep) | AP-IR distribution score (range) | AP-IR intensity score (range) |
|---|---|---|
| 1 (2) | 1.167 (1–1.5) | 1.333 (1–2) |
| 15 (3) | 1.333 (1–2) | 1.333 (1–2) |
| 45 (3) | 1 | 1 |
Distribution and intensity of AP-IR were determined as outlined in Materials and Methods.
FIG. 2.
There is increased AP-IR in subacute and chronic lesions of pneumonia, and the AP-IR is especially prominent in the cytoplasm of hyperplastic cells. (A) Hyperplastic bronchiolar epithelium 15 days p.i. with bacteria. Intense cytoplasmic AP-IR is present in cuboidal epithelial cells. Magnification, ×40. (B) Bronchioles 15 days p.i. with bacteria. In the cytoplasm of hyperplastic epithelial cells in this focus of bronchiolitis fibrosa obliterans, there is intense AP-IR. Magnification, ×40. (C) Bronchioles 45 days p.i. with bacteria are lined by cuboidal, hyperplastic epithelial cells, and many have intense cytoplasmic AP-IR. Magnification, ×20.
TABLE 3.
Distribution and intensity of AP-IR in lungs of sheep with acute, subacute, and chronic M. (Pasteurella) haemolytica infections and in hyperplastic epithelial cells of sheep with subacute and chronic pneumoniaa
| No. of days p.i. (no. of sheep) | AP-IR distribution score (range) | AP-IR intensity score (range) |
|---|---|---|
| 1 (2) | 2.5 (2–3) | 2 |
| 15 (3) | 3 | 3 |
| 45 (3) | 3 (2–4) | 3 (2–4) |
| 15 and 45 (4)b | 4* | 4* |
Distribution and intensity of AP-IR were determined as outlined in Materials and Methods.
Only areas of bronchioles and alveoli with hyperplastic epithelial cells were assessed. These cells were present in three lambs 15 days p.i. and one lamb 45 days PI. *, significant difference in results for epithelial cells of bronchioles and alveoli in contralateral (noninoculated) and control (pyrogen-free saline) lobes.
The time points for euthanasia were chosen as representative of the acute, subacute, and chronic stages of the pneumonic lesions (1, 12, 17). In the present study, all of the changes characteristic of natural infection were present in the lungs of animals from all groups. The lesions of mild hyperplasia of peribronchiolar lymphoid tissue present in lambs of the control group were likely secondary to Mycoplasma ovipneumoniae infection (17); however, these changes were minimally present in both the control and bacterium-inoculated lungs and were not associated with AP-IR.
DISCUSSION
The mild bronchial and bronchiolar AP-IR in the lungs of the control animals, as well as in contralateral lung sections from noninoculated animals, and the lack of increased AP-IR in the lung tissues from animals with acute pneumonia were similar to the results for humans, sheep, and cattle in previous studies (6, 8).
In contrast, the expression of AP in the lung tissues of lambs with subacute and chronic pneumonia due to infection with M. (Pasteurella) haemolytica was strikingly increased in the lungs of animals with subacute-to-chronic bacterial pneumonia. The increased AP-IR was located in the cytoplasm of hyperplastic bronchiolar epithelial cells and alveolar type II epithelial cells of all areas of the lungs of the animals with these cells. This finding suggests that, while AP expression is not up-regulated in response to acute inflammation, there is a relative increase in AP accumulation within the proliferative (hyperplastic) cells involved in repair. This increase may be due to either increased basal AP expression, increased cytoplasmic storage, or a lack of release or secretion of AP by these cells. An enhanced constitutive production of AP (or its parent zymogen compound) (5, 7) may be an essential part of the biological activity of hyperplastic regenerative epithelial cells during lesion resolution. The mechanism(s) and relevance of the apparent maturity-dependent expression of AP by epithelial cells are unknown, but AP may serve to protect immature lung epithelial cells from microbial infection during repair and allow them time for additional differentation to the point where they can express the inducible families of antimicrobial peptides.
Although most epithelial cells in normal lungs exhibit AP expression in the cytoplasm and nucleus, the increased AP in hyperplastic cells is due to cytoplasmic rather than nuclear production. AP expression in the cytoplasm is at a location closer to the site of initial microbial adherence and colonization. In addition, the cytoplasm also has organelles (e.g., vesicles and vacuoles) for immediate release or secretion that are clearly lacking in the nucleus. Therefore, preferential AP expression in the cytoplasm rather than the nucleus is another feature that differs from AP expression by nonhyperplastic cells and is consistent with AP’s antimicrobial activity. The identification and characterization of the parent zymogen compound are necessary to understand this and other aspects of AP. Unidentified 31- and 64-kDa proteins show positive AP-IR during Western analysis of sheep lung extract.
This study also shows that in this model of M. haemolytica pneumonia, lesions are located primarily at the site of inoculum deposition, with no compromise of the contralateral, noninoculated lung. Bacteria-inoculated animals develop focal lesions of pneumonic pasteurellosis that are similar to those seen in natural infections but do not alter the remaining lobes. The noninflamed areas had AP-IR identical to that of the corresponding areas of the control animals, which suggests that AP expression in noninfected areas of lungs is not altered by localized infections in an animal. The effect of localized lesions in various stages of pneumonia on the expression of inducible antimicrobial peptides, such as certain β-defensins, has not been determined.
In summary, this study demonstrates that the distribution of AP is not altered during the progressive stages of pneumonia in most bronchi and bronchioles; however, in bronchioles and alveoli with hyperplastic epithelial cells, there is a marked increase in the cytoplasmic expression of AP. This expression may be a general characteristic of hyperplastic respiratory epithelial cells and may have a role in protection against additional microbial infections during the resolution and repair of lesions resultant of bronchopneumonia.
Acknowledgments
We thank E. Huffman and K. DeJong for outstanding technical assistance, Jim Fosse of the ISU Biomedical Communications Department for excellent assistance with photo preparation, and Tammy Benson for help in statistical analysis.
REFERENCES
- 1.Ackermann, M. R., and K. A. Brogden. 2000. Response of the ruminant respiratory tract to Mannheimia (Pasteurella) haemolytica infection. Microbes Infect. 2:1079–1088. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann, M. R., K. A. Brogden, A. F. Florance, and M. E. Kehrli, Jr. 1999. Induction of CD18-mediated passage of neutrophils into pulmonary bronchi and bronchioles. Infect. Immun. 67:659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brogden, K. A. 1992. Ovine pulmonary surfactant induces killing of Pasteurella haemolytica, Escherichia coli, and Klebsiella pneumoniae by normal serum. Infect. Immun. 60:5182–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brogden, K. A., M. R. Ackermann, and B. M. DeBey. 1995. Pasteurella haemolytica lipopolysaccharide-associated protein induces pulmonary inflammation after bronchoscopic deposition in calves and sheep. Infect. Immun. 63:3595–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogden, K. A., A. J. De Lucca, J. Bland, and S. Elliot. 1996. Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc. Natl. Acad. Sci. USA 93:412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brogden, K. A., M. Ackermann, and K. M. Huttner. 1998. Detection of anionic antimicrobial peptides in ovine bronchoalveolar lavage fluid and respiratory epithelium. Infect. Immun. 66:5948–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brogden, K. A., M. Ackermann, and K. M. Huttner. 1997. Small, anionic, and charge-neutralizing propeptide fragments of zymogens are antimicrobial. Antimicrob. Agents Chemother. 41:1615–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brogden, K. A., M. R. Ackermann, P. B. McCray, Jr., and K. M. Huttner. 1999. Differences in the concentrations of small, anionic, antimicrobial peptides in bronchoalveolar lavage fluid and in respiratory epithelia of patients with and without cystic fibrosis. Infect. Immun. 67:4256–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond, G., D. Legarda, and L. K. Ryan. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27–38. [DOI] [PubMed] [Google Scholar]
- 10.Diamond, G., J. P. Russell, and C. L. Bevins. 1996. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc. Natl. Acad. Sci. USA 93:5156–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia, H. P., B. C. Schutte, A. Schudy, R. Linzmeier, J. M. Guthmiller, G. K. Johnson, B. F. Tack, J. P. Mitros, A. Rosenthal, T. Ganz, and P. B. McCray, Jr. 2001. Discovery of new human beta-defensins using a genomics-based approach. Gene 263:211–218. [DOI] [PubMed] [Google Scholar]
- 12.López, A. 1995. Respiratory system, p.142–151. In W. W. Carlton and M. D. McGavin (ed.), Thomson’s special veterinary pathology, 2nd ed. Mosby, St. Louis, Mo.
- 13.Martin, E., T. Ganz, and R. I. Lehrer. 1995. Defensins and other endogenous peptide antibiotics of vertebrates. J. Leukoc. Biol. 58:128–136. [DOI] [PubMed] [Google Scholar]
- 14.Morrison, G. M., D. J. Davidson, and J. R. Dorin. 1999. A novel mouse β defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett. 442:112–116. [DOI] [PubMed] [Google Scholar]
- 15.Ramírez-Romero, R., and K. A. Brogden. 2000. The potential role of the Arthus and Shwartzman reactions in the pathogenesis of pneumonic pasteurellosis. Inflamm. Res. 49:98–101. [DOI] [PubMed] [Google Scholar]
- 16.Ramírez-Romero, R., K. A. Brogden, J. M. Gallup, R. A. F. Dixon, and M. R. Ackermann. 2000. Reduction of pulmonary mast cells in areas of acute inflammation in calves with Mannheimia (Pasteurella) haemolytica pneumonia. J. Comp. Pathol. 123:29–35. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Romero, R., K. A. Brogden, J. M. Gallup, I. M. Sonea, and M. R. Ackermann. 2001. Mast cell density and substance P-like immunoreactivity during the initiation and progression of lung lesions in ovine Mannheimia (Pasteurella) haemolytica pneumonia. Microb. Pathog. 30:325–335. [DOI] [PubMed] [Google Scholar]
- 18.Russell, J. P., G. Diamond, A. P. Tarver, T. F. Scanlin, and C. L. Bevins. 1996. Coordinate induction of two antibiotic genes in tracheal epithelial cells exposed to the inflammatory mediators lipopolysaccharide and tumor necrosis factor alpha. Infect. Immun. 64:1565–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schonwetter, B., E. D. Stolzenberg, and M. A. Zasloff. 1995. Epithelial antibiotics induced at sites of inflammation. Science 267:1645–1648. [DOI] [PubMed] [Google Scholar]
- 20.Schröder, J.-M., and J. Harder. 1999. Molecules in focus: human β-defensin-2. Int. J. Biochem. Cell Biol. 31:645–651. [DOI] [PubMed] [Google Scholar]
- 21.Stolzenberg, E. D., G. M. Anderson, M. R. Ackermann, R. H. Whitlock, and M. Zasloff. 1997. Epithelial antibiotic induced in states of disease. Proc. Natl. Acad. Sci. USA 94:8686–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, O. M. Howard, and J. J. Oppenheim. 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525–528. [DOI] [PubMed] [Google Scholar]