Abstract
The NADPH oxidase enzymatic complex participates in the oxidative burst by producing ROS (reactive oxygen species). Altered levels of ROS production may have pathogenetic implications due to the loss of some innate immune functions such as oxidative burst and phagocytosis. Considering that HIV-1 Nef protein plays a primary role in AIDS pathogenesis, by affecting the immune system, we sought to dissect possible effects of Nef on the release of superoxide anions. We show here that the inducible expression of Nef in human phagocytic cells modulates the superoxide release in a biphasic manner. In particular, an early Nef-induced increase of the superoxide release was followed by a dramatic decrease starting from 10 h after the Nef induction. This was observed whatever the presence of cell activators such as GM-CSF (granulocyte/macrophage colony-stimulating factor) or fMLP (N-formyl-L-methionyl-L-leucyl-L-phenylalanine). Whereas the early increase in superoxide release is probably the result of the already described Nef-dependent activation of PAK-2 (p21-activated kinase 2)–Rac2, we were interested in investigating the mechanisms underlying the late inhibition of superoxide release observed originally. In this regard, we individuated at least three independent requirements for the Nef-induced blockade of superoxide release: (i) the active protein synthesis; (ii) both the membrane localization and the interaction with endocytotic machinery of Nef; and (iii) the release of soluble factor(s). Moreover, we observed that IL-10 (interleukin-10) inhibits superoxide release, whereas its depletion restored NADPH oxidase activity. We propose that the cell membrane-to-lysosome Nef transit leads to the synthesis and release of soluble factor(s) and, among them, IL-10 might significantly contribute to the inhibition of NAPDH oxidase activity.
Keywords: HIV-1 Nef, interleukin-10, NADPH oxidase, oxidative burst, phagocytic cell, superoxide anion
Abbreviations: CHX, cycloheximide; DPI, diphenyliodonium; ER, oestrogen receptor; dFCS, decomplemented fetal calf serum; ERK1/2, extracellular-signal-regulated kinases 1/2; fMLP, N-formyl-L-methionyl-L-leucyl-L-phenylalanine; GM-CSF, granulocyte/macrophage colony-stimulating factor; 4-HT, 4-hydroxytamoxifen; IL-10, interleukin-10; MAPK, mitogen-activated protein kinase; MDM, monocyte-derived macrophage; MIP-1α/β, macrophage inflammatory-1α/β; NF-κB, nuclear factor κB; PE, phycoerythrin; PI3K, phosphoinositide 3-kinase; rNef, recombinant Nef; ROS, reactive oxygen species; SOD, superoxide dismutase; STAT1/3, signal transducer and activator of transcription 1/3; wt, wild-type
INTRODUCTION
Human phagocytic cells play a crucial role in host defence against pathogens, in killing tumour cells and in removal of apoptotic and necrotic cells. These functions are triggered by the release of a large amount of superoxide anion (O2•−) by means of a process known as oxidative (or respiratory) burst. The most important O2•− producer enzyme is NADPH oxidase, a multimolecular complex that catalyses NADPH-dependent reduction of oxygen to superoxide anion [1,2]. The NADPH oxidase complex is composed of two flavocytochrome subunits located in the plasma membrane, i.e. gp91phox and p22phox constituting the catalytic core of the enzyme, and by three cytosolic subunits, namely p47phox, p67phox and p40phox [3]. Upon specific stimulation, the cytosolic factors are activated through the phosphorylation of p47phox and p67phox. These activated factors translocate to the plasma membrane and bind the flavocytochrome, thereby completing the active form of the enzyme. The functional complex is completed by translocation of GTP-activated form of the small GTPase Rac1 (or Rac2) protein [4].
Besides participating in the removal of pathogens, ROS (reactive oxygen species) have been involved in inflammation and tissue injury. Dysregulation of ROS production may contribute to the development of HIV-1 pathogenesis, playing a role in the loss of specific immune functions such as oxidative burst and phagocytosis [5–7].
Nef is a 27–34 kDa myristoylated protein produced exclusively by HIV-1/2 and simian immunodeficiency virus, playing a pivotal role in AIDS pathogenesis [8,9]. Its expression down-regulates the cell-surface levels of both CD4 and MHC-I molecules, and interferes with a number of intracellular pathways, leading to the dysregulation of cellular signalling and activation [10]. Recently, it has also been observed that Nef induces the secretion of chemotactic factors such as the CC-chemokines MIP-1α (macrophage inflammatory-1α) and MIP-1β from primary human monocyte/macrophages [11]. This is accompanied by the activation of AP-1 (activating protein-1), NF-κB (nuclear factor κB), STAT1 (signal transducer and activator of transcription 1) and STAT3 [12–16]. Of note, it has been reported that, in microglia cell lines or primary cultures, Nef activates the Vav/Rac/PAK signalling pathway (where PAK stands for p21-activated kinase), leading to the lowering of the activation threshold of NAPDH oxidase to a variety of stimuli, such as Ca2+ ionophores or endotoxins [17]. In view of the impairment of the antimicrobial functions against invading pathogens in HIV-1-infected people, as well as the primary role of Nef in AIDS pathogenesis, we were interested in studying the possible influences of Nef on the control of ROS production in human monocytes/macrophages. To this end, we used a U937 human promonocytic cell line stably transfected with a vector expressing a Nef–ER (where ER stands for oestrogen receptor) fusion protein kept in an inactive state until the treatment with 4-HT (4-hydroxytamoxifen) [18]. We found that active Nef regulates superoxide production in a biphasic manner, with an early increase of superoxide release followed by a late strong inhibition starting at 10 h from the onset of 4-HT treatment. These effects were similarly observed in cells unstimulated or stimulated by cell activators, such as GM-CSF (granulocyte/macrophage colony-stimulating factor) or fMLP (N-formyl-L-methionyl-L-leucyl-L-phenylalanine). Consistently, we determined that Nef exerted the inhibitory effect on the enzyme activity without affecting ERK1/2 (extracellular-signal-regulated kinases 1/2) phosphorylation, in spite of MAPK (mitogen-activated protein kinase) pathway involvement in GM-CSF- or fMLP-induced NADPH oxidase activation. Finally, on the basis of co-culture experiments, we concluded that the inhibition of superoxide release is probably mediated by Nef-induced soluble factor(s). Noteworthy, we proved that IL-10 (interleukin-10) is probably part of such a mechanism.
EXPERIMENTAL
Cell culture and materials
U937 cells and the stable-transfected cell lines U937-Nef and U937-NefG2A were maintained in RPMI 1640 medium supplemented with 10% (v/v) dFCS (decomplemented fetal calf serum), L-glutamine and antibiotics. dFCS was substituted by 0.2% BSA in cell-starvation procedures. All experiments were performed keeping the cells at a concentration of 1×106 cells/ml. Puromycin was obtained from Sigma–Aldrich (Milan, Italy). Co-cultures of U937 and U937-Nef cells were performed in six-well plates (Falcon, Lincoln Park, NJ, U.S.A.), and Cell Culture Insert Clyclopore® membrane (25 mm diameter, 0.45 μm pore size; BD Biosciences, Erembodegem, Belgium) was used to separate cells. Vivaspin 20 (5 kDa molecular mass cut-off; Sartorius, Göttingen, Germany) was used to concentrate U937-Nef conditioned medium. Human GM-CSF (Serotec, Kidlington, Oxford, U.K.) and fMLP (Sigma–Aldrich) were used at the concentration of 100 ng/ml and 1 μM respectively. Human IL-10 was purchased from Biosource (Camarillo, CA, U.S.A.). 4-HT (Sigma–Aldrich) was used to activate the Nef–ER fusion protein at a concentration of 50 nM and DMSO was used as vehicle. CHX (cycloheximide; Sigma–Aldrich) was added overnight at a concentration of 15 μM to inhibit protein synthesis. [35S]Methionine/cysteine (185 MBq, 10 mCi/ml) was obtained from ICN Biomedicals (Irvine, CA, U.S.A.). DPI (diphenyliodonium), PD98059 and LY294002 were from Sigma–Aldrich. PMA (Sigma–Aldrich) was used at a concentration of 10 nM to induce U937 differentiation.
Plasmids and stable transfection experiments
The pEBB wtNef-ER-IRES-puro vector (where wt stands for wild-type and IRES stands for internal ribosomal entry sequence) encoding the Nef–ER fusion protein was provided by Dr S. F. Walk (Carter Immunology Center, University of Virginia, Charlottesville, VA, U.S.A.) [18]. Briefly, the Nef–ER is a part of the bicistronic gene with an IRES followed by the coding sequence for the puromycin resistance gene (Puro). The Nef–ER expression is driven by the EF-1α (elongation factor 1α) promoter. The pEBB NefG2A-ER-IRES-puro vector was obtained by modifying the Nef sequence by PCR, using as forward primer 5′-GGTGGATCCACGCGTATGGCCGCAAAGTGG-3′ and as reverse primer 5′-CGGCGGCCGCATCGATACTAGTGCAGTTCTTGAAGTACTCCGGATG-3′. The fragment was subcloned into the pEBB vector between BamHI and NotI sites. The sequence of the mutant was verified by sequencing.
Stable transfectants of U937 cells (U937-Nef and U937-NefG2A) were obtained by electroporation of 107 cells with 20 μg of DNA at 250 Vs and 960 μF, using a Bio-Rad gene pulser transfection apparatus (Bio-Rad, Hercules, CA, U.S.A.). Cells were grown for 2 weeks in a medium containing puromycin (0.8 mg/ml). Selected populations were tested for Nef–ER expression, frozen and aliquots periodically thawed to maintain the identity of the population.
IL-10 immunodepletion
To remove IL-10, the HT-treated U937-Nef conditioned medium was incubated for 3 h at 4 °C with specific neutralizing rat anti-human IL-10 (2 μg/ml; Pharmingen, BD Biosciences) and, as control, with equal amounts of irrelevant isotype. Then, immunocomplexes were reacted with Ultralink Immobilized Protein G Plus (Pierce, Rockford, IL, U.S.A.) overnight at 4 °C. Afterwards, the immunocomplexes bound to Protein G were discarded through centrifugation and supernatants were filtered (0.22 μm pore diameter) and added to U937 culture.
Preparation of recombinant proteins
rNef (recombinant Nef) protein and the mutants thereof AXXA, DDAA and EEQQ were obtained as a His6-tagged fusion protein as described previously [16]; the nef gene from NL4-3 HIV-1 strain and Nef mutants were amplified by PCR and cloned in frame with the His6 tag into the 5′-BamHI/3′-SalI sites of pQE 30 vector (Qiagen, Chatsworth, CA, U.S.A.). rNefs were purified from IPTG (isopropyl β-D-thiogalactoside)-induced bacterial lysates in an 8 M urea buffer using Ni2+-nitrilotriacetate resin (Qiagen) according to the manufacturer's instructions. rNefs were eluted with 250 mM imidazole and the fractions were analysed by SDS/PAGE (12% polyacrylamide). The rNef-containing fractions were pooled and dialysed extensively against 1×PBS to remove the urea completely. All recombinant protein preparations were scored as negative for the presence of bacterial endotoxin by using the Lymulus amaebocyte lysate assay (BioWhittaker, Walkersville, MD, U.S.A.).
Flow cytometry analysis
Cells were suspended in Ca2+- and Mg2+-free PBS supplemented with 0.5% BSA and labelled with PE (phycoerythrin)- or allophycocyanin-conjugated anti-CD4, PE-conjugated anti-GM-CSF receptor α-chain (CDw116) and FITC-conjugated anti-CD11c monoclonal antibodies. All the antibodies were obtained from BD Biosciences. The staining was performed for 30 min on ice using antibodies at a concentration of 1 μg/106 cells. The cell fluorescence was analysed with the Epics Elite ESP Cell Sorter (Beckman Coulter, Miami, FL, U.S.A.) or with FACSAria (BD Biosciences). Cells incubated with appropriately fluorochrome-conjugated isotype controls (BD Biosciences) were used to gate non-specific fluorescence signals and dead cells were excluded by propidium iodide staining (5 μg/ml; Sigma–Aldrich).
Superoxide release measurement and SOD (superoxide dismutase)-like activity
The rate of O2•− formation was measured as SOD-inhibitable reduction of ferrocytochrome c [19]. Briefly, 1×106 cells were washed in phosphate buffer containing the metal chelator DTPA (diethylenetriaminepenta-acetic acid; 0.1 mM, pH 7.4) and reseeded in the same buffer (1 ml) containing cytochrome c (10 μM) in the presence or absence of SOD (10 μg/ml). After incubation at 37 °C for 30 min, the reaction was monitored spectrophotometrically with Lambda 14 P UV–Vis (PerkinElmer, Norwalk, CT, U.S.A.) at 550 nm (molar absorption coefficient ϵ=21 mM−1·cm−1).
The SOD-like activity of cells or medium from cells was measured as the ability to inhibit the superoxide production from a superoxide-generating system. Briefly, 1 ml of a solution containing xanthine (25 μM), xanthine oxidase (0.25 unit/ml), catalase (2750 units/ml), DTPA (25 μM) and cytochrome c (5 μM) was allowed to react in phosphate buffer (pH 7.4) at 37 °C for 30 min in the absence or presence of a fixed volume of medium from cells, or 106 cells/ml. As a control for superoxide production, SOD (30 μg/ml) was added to the solution of superoxide-generating system. The amount of superoxide produced was calculated spectrophotometrically (Δϵ550-468 nm=21.5 mM−1·cm−1). All the reagents in this section were obtained from Sigma–Aldrich.
Western blotting assay
For Western-blot analyses, cells were lysed in 1% Triton X-100 in the presence of 20 mM Tris/HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 10 mM sodium orthovanadate, 100 mM sodium fluoride and 0.5 mM PMSF for 20 min on ice. Whole cell lysates were centrifuged at 10000 g for 20 min at 4 °C and the supernatants were collected. The protein concentrations were determined by the Bio-Rad protein assay. Samples of 50 μg were separated by SDS/PAGE (10% polyacrylamide) and transferred by electroblotting on to a nitrocellulose membrane (Hybond; Amersham Biosciences, Milan, Italy). For the immunoassay, the membranes were blocked in 5% (w/v) non-fat dry milk in 1×PBS/0.1% Triton X-100 for 1 h at room temperature (15–25 °C) and then incubated overnight at 4 °C with specific primary antibodies. Antibodies used in the immunoblotting are as follows: polyclonal sheep anti-Nef antiserum (a gift from M. Harris, University of Leeds, Leeds, U.K.); polyclonal rabbit anti-phospho-p44/42 MAPK (Cell Signaling Technology, Beverly, MA, U.S.A.); polyclonal rabbit anti-ERK1/2 (Promega, Madison, WI, U.S.A.); monoclonal mouse anti-actin (Amersham Biosciences). Immunocomplexes were detected through horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse antisera (Amersham Biosciences), followed by an enhanced chemiluminescence reaction (ECL®, Amersham Biosciences).
Preparation of cell membranes
Plasma membranes from U937 cells were obtained after purification with plasma membrane protein extraction kit (MBL, Watertown, MA, U.S.A.) according to the manufacturer's instructions. Briefly, 5–10×107 cells were collected by centrifugation at 600 g for 5 min at 4 °C and washed once with 1 ml of ice-cold PBS. The cells were resuspended in 1 ml of Homogenize Buffer Mix and homogenized in an ice-cold dounce on ice for 30–50 times. The homogenate was centrifuged at 700 g for 10 min at 4 °C and the resultant supernatant was collected and centrifuged at 10000 g for 30 min at 4 °C. The total membrane protein pellet was resuspended in 200 μl of the upper phase solution and then 200 μl of the lower phase solution were added. The sample was mixed, incubated on ice for 5 min, and centrifuged in a microfuge at 1000 g for 5 min. The upper phase was transferred to a new test tube and the lower phase was extracted again by adding 100 μl of the upper phase solution and spinning for 5 min. The two upper phases were combined and extracted by adding 100 μl of lower phase solution. The upper phase obtained was diluted in 5 vol. of water, kept on ice for 5 min and then centrifuged at top speed in a microcentrifuge for 10 min at 4 °C. The recovered pellet includes the plasma-membrane proteins.
Chemokine release
Detection of MIP-1β in supernatants of PMA-differentiated U937 was performed through an ELISA kit obtained from R & D Systems (Minneapolis, MN, U.S.A.) according to the manufacturer's instructions.
Statistical analysis
Results are presented as means±S.D. Statistical analysis was performed according to paired Student's t test. P<0.05 was considered significant.
RESULTS
Nef–ER is expressed in U937 cells and efficiently activated upon 4-HT treatment
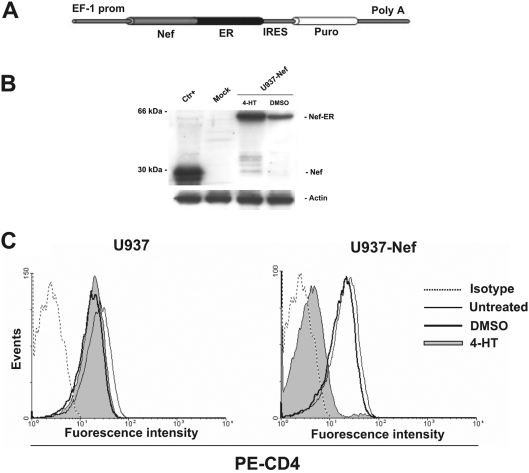
In order to gain a human monocytic cell system where the Nef expression can be tightly and efficiently regulated, U937 cells transfected with the pEBB Nef-ER-IRES-puro construct (Figure 1A) [18] were selected in puromycin for 15–20 days, and a cell population homogeneously expressing the Nef–ER fusion product was recovered. In this configuration, the ER domain keeps Nef in an inactive state by means of a steric hindrance that can be relieved by the binding of the oestrogen antagonist 4-HT to the ER domain [20–22]. In this respect, one can expect that the Nef–ER production should not be influenced by 4-HT. However, we noticed that the overnight treatment with 4-HT induced a moderate increase of the steady-state levels of the fusion product. This was already reported and interpreted as the consequence of an increased stability of Nef–ER in the presence of 4-HT (Figure 1B) [18].
Figure 1. The Nef–ER construct and its expression in stably transfected cells.
(A) Schematic representation of pEBB Nef-ER-IRES-puro vector. The NL4-3 Nef (Nef) strain is fused at its C-terminus in frame to the hormone-binding region of murine ER. The elongation EF-1α promoter (EF-1 prom) controls the expression of Nef–ER that was transcribed as a part of the bicistronic message containing puromycin resistance sequence (puro) separated by an IRES. Poly A, polyadenylation site. (B) Expression of the Nef–ER fusion protein in stably transfected U937 cells. In each lane, 50 μg of cell protein extract was loaded. Ctr+, protein extracts from pNL4-3-transfected HEK-293T cells used as positive control for the specificity of Nef antibody; mock, U937 parental cells; U937-Nef, pEBB Nef-ER-IRES-puro U937-transfected cells treated with 50 nM 4-HT or DMSO for 18 h. (C) U937-Nef (histogram on the right-hand side) and parental cells (histogram on the left-hand side) were treated with 50 nM 4-HT or DMSO for 18 h. To ensure the presence of equal amounts of protein, the membranes were reprobed with anti-actin antibodies. (C) The expression of CD4 was analysed by flow using PE-labelled anti-CD4 antibody and fluorescence intensity in 4-HT- (filled grey histogram) or DMSO (thick solid line)-treated cells was compared with untreated cells (thin solid line). Matched isotype (dotted line) was used as control of non-specific fluorescence signals and propidium iodide was used to exclude dead cells.
To verify the functionality of Nef upon the 4-HT treatment, we assessed the down-regulation of cell-surface CD4 expression, one of the best-characterized Nef cellular functions. The cytofluorimetric analyses we performed clearly proved that the 4-HT treatment for 18 h did induce a potent CD4 down-modulation (Figure 1C). Significantly, a similar result, even though in reduced extent, has been obtained by treating cells for as short a time as 4 h (results not shown and see below). Thus we formally validate the U937-Nef cells as a reliable monocytic cell system expressing Nef in a tightly regulated way.
Nef modulates the release of superoxide anions
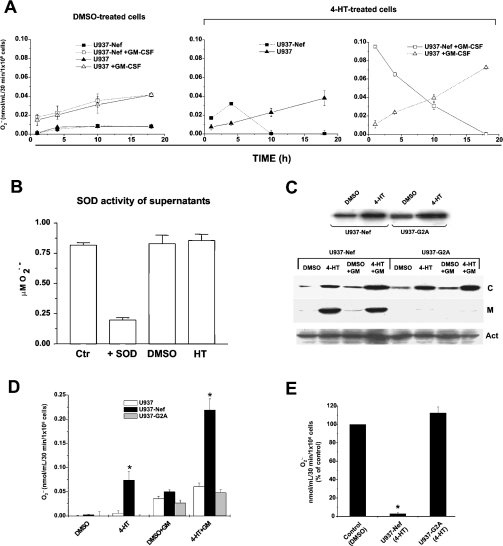
We were first interested in assessing whether Nef has some influence on the superoxide release. For this purpose, U937-Nef and parental U937 cells were treated with 4-HT or, as control, with the 4-HT vehicle (i.e. DMSO) and, at different time points of treatment, were analysed for their capability to release superoxide in the supernatant. As shown in Figure 2(A), Nef appeared to increase the release of superoxide at the early time points analysed (i.e. 1 and 4 h), whereas at later time points (i.e. 10 and 18 h) of treatment with 4-HT, the levels of superoxide decreased below the sensitivity threshold of the assay. On the contrary, a slight increase with time was observed in control cells.
Figure 2. Nef modulation of superoxide anion release in GM-CSF-stimulated cells and G2A Nef mutant analysis.
(A) U937-Nef and parental cells were cultured for different time periods (1, 4, 10 and 18 h) in the presence of DMSO or 4-HT. The cells were centrifuged and resuspended in phosphate buffer for 30 min and superoxide release was determined in unstimulated or GM-CSF-stimulated (100 ng/ml) cells. The NADPH oxidase activity, determined as extracellular superoxide release, was measured by the SOD-inhibitable reduction of ferricytochrome c, as described in the Experimental section. (B) Extracellular SOD-like activity was measured in HT- and DMSO-treated U937-Nef cells. Cells were cultured for 18 h in the presence of DMSO or 4-HT and, afterwards, were centrifuged and resuspended in phosphate buffer for 30 min in the presence of GM-CSF. Thereafter, the cells were centrifuged and the supernatants were analysed for the SOD-like activity. Ctr, superoxide production from a superoxide-generating system; +SOD, superoxide production from a superoxide-generating system in the presence of SOD; DMSO, extracellular SOD-like activity measured on the supernatant of DMSO-treated U937-Nef cells; HT, extracellular SOD-like activity measured on the supernatant of HT-treated U937-Nef cells. (C, top) Western-blot analysis of Nef–ER and G2A–ER fusion protein expression in stably transfected U937 cells. Cell extracts were prepared from U937-Nef and U937-G2A cells cultured overnight in the presence of DMSO or 4-HT. Protein extracts were separated by SDS/PAGE and immunoblotted with polyclonal sheep anti-Nef antiserum. In each lane, 50 μg/sample was loaded. (C, bottom) Western-blot analysis of Nef–ER and G2A–ER fusion protein expression in cytosolic or plasma-membrane fractions. The plasma membrane and the cytosolic fractions were separated with a protein extraction kit (MBL). Cell extracts were prepared from U937-Nef and U937-G2A cells cultured overnight in the presence of DMSO or 4-HT and half of single cultures were stimulated with 100 ng/ml GM-CSF 30 min before cell extraction. In each lane, 50 μg of proteins extracted from the cytosolic fraction (C) was loaded, whereas all protein extracts of plasma membrane (M) from 107 cells were loaded in each lane. Protein extracts were separated by SDS/PAGE and immunoblotted with polyclonal sheep anti-Nef antiserum. To ensure the presence of equal amounts of proteins, the membranes were reprobed with anti-actin antibodies (Act). (D) Superoxide release was measured in U937 parental cells, U937-Nef and U937-G2A transfected cells. The cells were cultured overnight in RPMI 1640 medium supplemented with 0.2% BSA; afterwards, 4-HT or DMSO was added to the cultures for 1 h and superoxide release was determined in unstimulated and GM-CSF-stimulated cells. In order to improve the response to GM-CSF, cells were cultured overnight in RPMI 1640 medium supplemented with 0.2% BSA before stimulation with GM-CSF (100 ng/ml). (E) Superoxide release was determined in U937 parental cells, U937-Nef and U937-G2A transfected cells cultured for 18 h in RPMI 1640 medium supplemented with 0.2% BSA in the presence of 4-HT or DMSO in GM-CSF-stimulated cells. The superoxide anion release was reported as a percentage of the control, represented by cell culture in the presence of DMSO. These results (means±S.D.) are representative of at least three independent experiments performed in duplicate. *P<0.05.
In order to validate our results, we repeated the experiment in the presence of GM-CSF, a growth factor capable of eliciting a strong superoxide production by NADPH oxidase induction during phagocytic oxidative burst [23]. Both U937 and U937-Nef cells, starved overnight in 0.2% BSA-supplemented medium and stimulated with GM-CSF for 30 min, significantly increased the superoxide anion release. We established the optimal stimulation at the concentration of 100 ng/ml GM-CSF (results not shown), which was used in all subsequent experiments. The time-course analysis of 4-HT-treated cells stimulated with GM-CSF gave results that reproduced, but to a much greater extent, the results already obtained in the absence of GM-CSF (Figure 2A, right panel). Of note, similar results were obtained also upon stimulation with fMLP (results not shown). Taken together, these results support the idea that Nef influences the release of superoxide by means of a bimodal, time-dependent mechanism, independent of the presence of GM-CSF or fMLP.
To monitor the presence of extracellular SOD-like activity in the supernatants of HT-treated U937-Nef cells potentially interfering with the above-described results, we measured the SOD-like activity of supernatants from both HT- and DMSO-treated U937-Nef cells. The superoxide was generated by xanthine/xanthine oxidase. As shown in Figure 2(B), the supernatants did not appear to contain SOD-like activity. Moreover, as expected, U937-Nef showed a slight SOD-like activity (0.3 unit/106 cells), but no difference was detected between HT- and DMSO-treated U937-Nef (P=0.44; results not shown). Thus we concluded that the possible presence of SOD-like activity associated with either the supernatants or the cell membrane did not significantly contribute to the results described in Figure 2(A).
We next performed experiments including the analysis of the G2A Nef negative mutant. This is a mutant lacking the myristoylation site, thus becoming incapable of reaching the cell membrane [10]. The use of Nef G2A as a negative control was justified considering that it lacks most of the functions attributed to Nef. For the first time, we demonstrated that U937-NefG2A cells underwent Nef induction as observed in cells expressing wtNef, and, as expected, that NefG2A did not localize on the cell membrane (Figure 2B). Next, we repeated the superoxide measurements in the supernatants of cells stably transfected with a vector expressing a 4-HT-inducible Nef G2A mutant. Superoxide measurements performed on the supernatants, recovered at both early (Figure 2C) and late times (Figure 2D) after 4-HT treatment and in the presence or absence of GM-CSF co-stimulation, demonstrated that Nef G2A was ineffective, thus enforcing the relevance of the results we obtained with the wt counterpart.
4-HT-activated Nef inhibits NADPH oxidase without affecting GM-CSF-induced MAPK phosphorylation
Although the stimulation-dependent induction of NADPH oxidase activity by Nef has already been described in human microglia [17], in the present study, we report for the first time that delayed effects of Nef can lead to inhibition of the respiratory burst. We were therefore interested in inspecting the mechanism underlying such a new biological function of Nef.
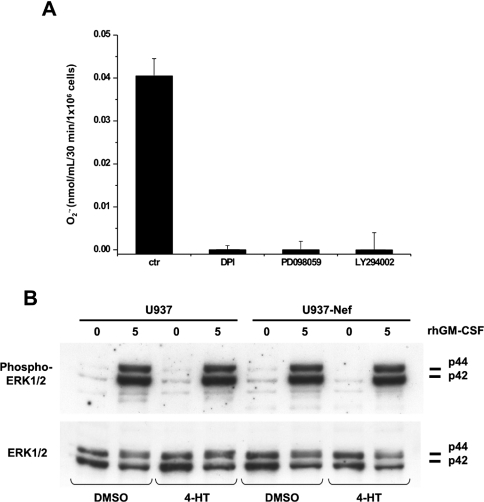
The GM-CSF is able to induce NADPH oxidase activity through the activation of the MAPK pathway [24,25]. In this regard, we observed that the addition of the MEK (MAPK/ERK kinase)-specific inhibitor PD98059 and the PI3K (phosphoinositide 3-kinase)-specific inhibitor LY294002 produced a complete inhibition of superoxide release with a magnitude comparable with the flavoproteins inhibitor DPI (Figure 3A). In order to determine whether active Nef could inhibit GM-CSF-stimulated superoxide release interfering with the MAPK pathway, we studied the effect of 4-HT on GM-CSF-induced phosphorylation of ERK1/2 proteins. Both parental U937 and U937-Nef, treated for 18 h with DMSO or 4-HT, were stimulated with GM-CSF for 5 min, showing a rapid phosphorylation of ERK1/2 (Figure 3B). Moreover, GM-CSF-stimulated ERK1/2 phosphorylation levels remained approximately constant at least for 180 min in either DMSO- or 4-HT-treated U937-Nef (results not shown). These results, while confirming the already published observations concerning the role of MAPK in the stimulation of the NADPH oxidase upon GM-CSF treatment [24], also demonstrate that active Nef did not impair the MAPK pathway, probably inhibiting the NADPH oxidase activity independent of or downstream of this pathway.
Figure 3. Nef does not affect GM-CSF-induced ERK1/2 phosphorylation even though the NADPH oxidase activity is mediated by the MAPK pathway.
(A) Superoxide release was determined in U937 cells cultured for 18 h in RPMI 1640 medium supplemented with 0.2% BSA. The inhibitors DPI (10 μM), PD098059 (5 μM) and LY294002 (5 μM) were added to the cultures 30 min before GM-CSF stimulation. (B) U937-Nef and parental cells were cultured in RPMI 1640 medium supplemented with 0.2% BSA for 18 h with 4-HT or DMSO and stimulated with GM-CSF (100 ng/ml) for 5 min. Phosphorylated MAPKs (Phospho-ERK1/2) were detected by Western blotting by incubating the membranes with anti-phospho-p44/42 MAPK. To ensure the presence of equal amounts of protein, the membranes were reprobed with anti-ERK1/2.
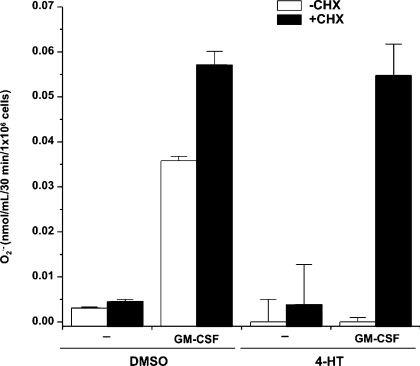
CHX suppresses the Nef-mediated inhibition of GM-CSF-induced superoxide release but not the CD4 down-regulation
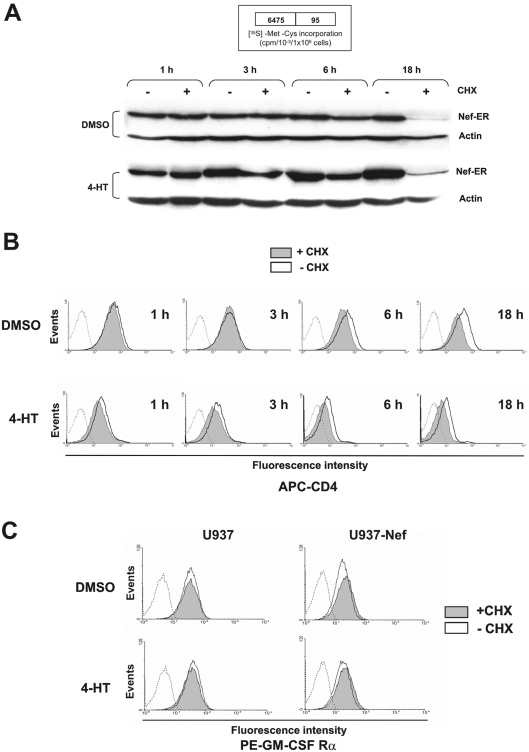
The time-course analysis we performed demonstrates that Nef induced the NADPH oxidase activity in 1 h from the onset of 4-HT treatment, while the inhibition of superoxide release was observed at a late time of treatment. To establish whether Nef could play a direct or an indirect role in such an inhibition, we assayed the effects of the blockade of protein synthesis during the treatment of U937-Nef cells with 4-HT for 18 h. The results show that the treatment with CHX neutralized the inhibitory effect of Nef, restoring also the responsiveness to GM-CSF of NADPH oxidase activity (Figure 4). Although the treatment for 18 h with CHX induced a dramatic decrease, but not the disappearance, in Nef–ER protein (Figure 5A), 4-HT-activated Nef was still able to induce CD4 down-regulation (Figure 5B), thus supporting the idea that the blockade of protein synthesis did not completely hinder the Nef activity. Western-blot analysis also demonstrates that the levels of Nef–ER protein were only slightly decreased after 6 h of CHX treatment, while the increased 4-HT stability of Nef–ER partially compensates the effect of CHX (Figure 5A). Parallel analysis of CD4 expression at the same time points showed that Nef was able to reduce the levels of CD4 membrane localization already after 1 h from its activation by 4-HT. 4-HT treatment for 6 h appears sufficient to determine a nearly complete reduction of CD4 expression (Figure 5B). However, the treatment with CHX did not significantly modify the effect of active Nef on CD4 expression levels. Moreover, as predicted by the full responsiveness to GM-CSF stimulation in the presence of CHX (Figure 4), no change in the expression levels of GM-CSF receptor α-chain was observed (Figure 5C).
Figure 4. CHX suppresses the Nef-mediated inhibition of GM-CSF-induced superoxide release.
U937-Nef cells were cultured in RPMI 1640 medium supplemented with 0.2% BSA for 18 h with 4-HT or DMSO in the presence or absence of CHX (15 μM). Superoxide release was determined in unstimulated (–) and GM-CSF-stimulated cells (GM-CSF). These results (means±S.D.) are representative of at least three independent experiments performed in duplicate.
Figure 5. Time-course analysis of the Nef–ER, CD4 and GM-CSF receptor α-chain expression in cells treated with CHX.
U937-Nef cells were cultured in RPMI 1640 medium supplemented with 0.2% BSA with 4-HT or DMSO in the presence or absence of CHX for 1, 3, 6 and 18 h. (A) Western-blot analysis of Nef–ER expression in U937-Nef cells after 1, 3, 6 and 18 h of treatment. As a control for equal amount of protein loading, the membranes were reprobed with anti-actin monoclonal antibody. As a control for CHX-mediated inhibition of protein synthesis, the [35S]methionine/cysteine incorporation was determined at 18 h (inset). Briefly, cells were starved in methionine/cysteine-free medium supplemented with 5% (v/v) dialysed fetal bovine serum for 2 h followed by incubation for 16 h with [35S]methionine/cysteine (1.85 MBq/ml), in the presence or absence of CHX. [35S]Methionine/cysteine incorporation was evaluated by protein extract precipitation in 20% (w/v) trichloroacetic acid. (B) Flow cytometry analysis of CD4 in U937-Nef cells cultured in RPMI 1640 medium supplemented with 0.2% BSA and treated with 4-HT or DMSO in the presence or absence of CHX for 1, 3, 6 and 18 h. Cells were stained with allophycocyanin-conjugated anti-CD4 as described under the Experimental section. The fluorescence intensities in CHX-treated (filled grey histogram) or untreated (solid line) cells were compared with matched isotype-stained cells (dotted line). (C) Flow cytometry analysis of GM-CSF receptor α-chain in U937-Nef and parental cells cultured in RPMI 1640 medium supplemented with 0.2% BSA and treated for 18 h with 4-HT or DMSO in the presence or absence of CHX. Cells were stained with PE-conjugated anti-GM-CSF receptor α-chain as described in the Experimental section. The fluorescence intensities in CHX-treated (filled grey histogram) or untreated (solid line) cells were compared with matched isotype stained cells (dotted line). Propidium iodide was used to exclude dead cells.
Taken together, these results strongly suggest that the Nef-mediated inhibitory effect on superoxide release is dependent on the synthesis of protein(s) intermediates, although we cannot definitely exclude the possibility that Nef could be required for more than 6 h and/or at a higher concentration to inhibit superoxide release.
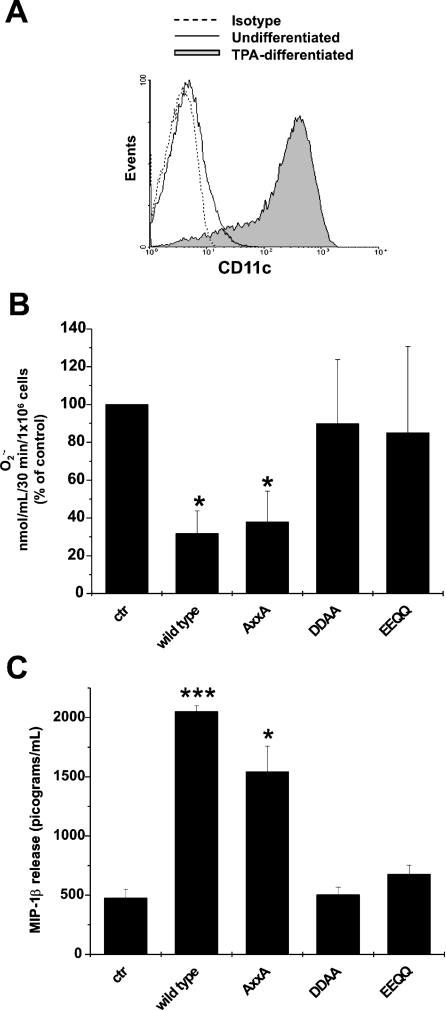
The Nef-induced inhibition of superoxide release couples with the chemokine release
Attempting to deepen our investigations on the mechanisms underlying the Nef-induced inhibition of superoxide release, we extended our observations by studying the effects of a number of Nef mutants. In particular, we carried out a series of experiments by treating cells with soluble rNefs, whose consequences, as we have already demonstrated, largely reproduce the effects induced by endogenous Nef [16,26]. Due to the increase in internalization activity we observed (results not shown), our studies were focused on U937 cells induced to differentiation upon PMA treatment. In particular, 2 days treatment of U937 cells with PMA induces a differentiated phenotype, characterized by growth arrest, adhesion and expression of macrophage-specific surface proteins such as CD11c (Figure 6A). In addition, these cells can be more responsive to different stimuli, such as GM-CSF or fMLP [27]. We evaluated the effects of three mutants of rNef upon GM-CSF stimulation, in particular a mutant defective for the interaction with the SH3-binding domain (AXXA) [28], and two mutants defective for the interaction with the endocytotic machinery (DDAA and EEQQ) [29,30]. The results show that, as observed in wtNef-expressing cells, the treatment with wt-rNef inhibits the release of superoxide anions. Although the same result was obtained by treating cells with the AXXA mutant, conversely both the DDAA and EEQQ mutants were ineffective (Figure 6B). Of a major importance, we already demonstrated that all rNef mutants we used underwent cell internalization as efficiently as the wt counterpart did [16].
Figure 6. Recombinant Nef protein inhibits superoxide release and induces secretion of MIP-1β in PMA-differentiated U937 cells.
(A) U937 cells were treated with PMA (TPA; 10 nM) for 2 days in RPMI 1640 medium supplemented with 10% dFCS and CD11c expression was analysed by FACS. PMA-differentiated U937: filled grey histogram; U937: solid line; matched isotype control: dotted line. (B) U937 cells were treated for 2 days with PMA to induce differentiation. PMA was then removed and cells were cultured for 1 additional day in RPMI 1640 medium supplemented with 10% dFCS. After 24 h, 100 ng/ml of either wt-rNef or AXXA, DDAA and EEQQ mutants were added to cells that were cultivated for an additional 18 h in RPMI 1640 medium supplemented with 0.2% BSA. Superoxide release was determined in GM-CSF-stimulated cells and the results were reported as a percentage of control, represented by the cells cultured without rNef proteins. (C) MIP-1β release was measured in supernatants collected from the cultures of U937 cells treated for 18 h with 100 ng/ml wt-rNef, AXXA, DDAA and EEQQ mutants. The culture conditions were identical with those described for (B). The results were reported as pg·ml−1·(106 cells)−1. These results (means±S.D.) are representative of at least three independent experiments performed in duplicate. *P<0.05, ***P<0.005.
It was reproducibly described that Nef induces the release of inflammatory factors from monocytes/macrophages, including CCL2/MIP-1α and CCL3/MIP-1β [13,16]. We were interested in verifying whether the treatment with wt-rNef and mutants thereof were able to induce release of MIP-1β from PMA-differentiated U937 cells. Analysis of the supernatants of cultures contemporarily tested for superoxide release demonstrated that the treatment with either wt or AXXA rNefs induced the secretion of MIP-1β, whereas the mutants DDAA and EEQQ lacked such a function (Figure 6C). These results indicate that the Nef domains important for lysosome trafficking are involved in the inhibition of both superoxide release and soluble factor(s) production, thus suggesting a link between these two functions.
Superoxide release was inhibited by Nef-induced soluble factor(s)
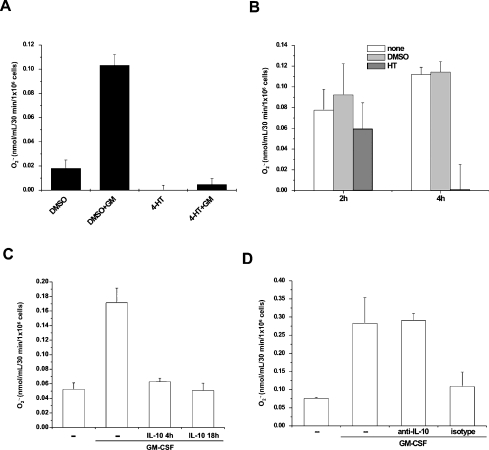
The data presented above imply that Nef-mediated inhibition of the NADPH oxidase activity depends on de novo protein synthesis. Moreover, the correlation we found by means of the Nef mutant analysis between the Nef-induced inhibition of the superoxide release and the Nef-dependent soluble factor release prompted us to investigate the possibility that soluble factor(s) induced by Nef could mediate the inhibitory effect on NADPH oxidase activity. This hypothesis was tested by co-cultivating U937-Nef with U937 parental cells. The co-cultivation was carried out for 18 h in the presence of 4-HT or vehicle by keeping the cells separated by a 0.45 μm pore size membrane. After the incubation, the U937-Nef and U937 parental cells were separately collected in phosphate buffer and the respective superoxide releases were measured in unstimulated or GM-CSF-stimulated conditions. The results clearly demonstrate that the co-cultivation with U937-Nef cells in the presence of HT, but not in the presence of vehicle, led to a strong inhibition of the superoxide release from U937 cells (Figure 7A). We conclude that the release of newly synthesized soluble factor(s) upon Nef stimulation is involved at least in part in the Nef-induced inhibition of the superoxide release. Additional experimental evidence supporting the involvement of macromolecular soluble factor(s) was achieved by incubating U937 parental cells with the medium from U937-Nef cultivated for 18 h in the presence of 4-HT. The medium was concentrated by using a Vivaspin concentrator with 5 kDa molecular mass cut-off, thus allowing the removal of small molecules and catabolism products, the latter possibly involved in the basal level of superoxide release from U937 parental cells (results not shown). As shown in Figure 7(B), the treatment of U937 cells for 4 h with the concentrated medium fairly reproduced the strong inhibition of the superoxide release we previously observed, thus strongly suggesting that this phenomenon is mediated by macromolecules.
Figure 7. Nef inhibits superoxide release in co-cultured U937 parental cells through the release of soluble factor(s).
(A) U937-Nef and U937 parental cells were co-cultured in trans-well 6-well plates separated by a cell-culture membrane (0.45 μm pore size) in RPMI 1640 medium supplemented with 0.2% BSA in the presence of 4-HT or DMSO for 18 h. After incubation, the two cell lines were separately collected, centrifuged and resuspended in phosphate buffer for the extracellular superoxide release assay. The results (means±S.D.) are representative of at least three independent experiments performed in duplicate. (B) U937-Nef cells were cultured in RPMI 1640 medium supplemented with 0.2% BSA in the presence of 4-HT or DMSO for 18 h. The respective media were 10-fold concentrated with Vivaspin 20 concentrator with 5 kDa molecular mass cut-off. U937 parental cells were then cultured in RPMI 1640 medium supplemented with 0.2% BSA for 18 h and 100 μl of concentrated medium was added to 1 ml of cell culture for the last 2 or 4 h. After incubation, untreated cells (none), cells treated with concentrated medium from DMSO-treated U937-Nef cells (DMSO) and HT-treated U937-Nef cells (HT) were centrifuged and resuspended in phosphate buffer for the GM-CSF-stimulated extracellular superoxide release assay. The results (means±S.D.) are representative of at least three independent experiments performed in duplicate. (C) U937 parental cells were cultured in RPMI 1640 medium supplemented with 0.2% BSA for 18 h. IL-10 (10 ng/ml) was added during either the whole (18 h) or the last 4 h of the starvation time. After incubation, cells were resuspended in phosphate buffer for the GM-CSF-stimulated extracellular superoxide release assay. (D) U937 parental cells were cultured in RPMI 1640 medium supplemented with 0.2% BSA for 18 h. During the last 4 h of incubation, the concentrated medium from IL-10-depleted HT-treated U937-Nef cells (anti-IL-10) or treated with isotype as negative control (isotype) were added to the cultures. Then the cells were resuspended in phosphate buffer for the GM-CSF-stimulated extracellular superoxide release assay. The results (means±S.D.) are representative of at least three independent experiments performed in duplicate.
IL-10 inhibits superoxide release
Several studies reported that HIV-1 Nef induced IL-10 expression in either peripheral blood mononuclear cells or in cell lines, U937 included [31,32]. On the other hand, IL-10 has recently emerged as an anti-inflammatory cytokine capable of inhibiting superoxide release in human monocytic leukaemia cell lines and in human neutrophils [33,34]. Consistently, we found that the addition of IL-10 to U937 parental cells inhibited GM-CSF-stimulated superoxide release (Figure 7C). To define better the role of IL-10 in the Nef-mediated inhibitory effect on superoxide release, we treated U937 cells with conditioned medium from HT-treated U937-Nef cells upon IL-10 immunodepletion. The results we obtained clearly demonstrate that the depletion of IL-10 restored the GM-CSF-stimulated superoxide release, while the conditioned medium incubated with an isotype control still inhibited the superoxide release (Figure 7D). These results strongly suggest that the IL-10 release could be a part of the mechanism of Nef action, of course not excluding the involvement of additional soluble factors.
DISCUSSION
The NADPH oxidase represents a key enzyme involved in the generation of ROS during the respiratory burst in phagocytic cells, such as monocytes and macrophages. Both preserved [35,36] and impaired [5–7,37,38] oxidative burst response in monocytes/macrophages from HIV-1-infected patients have been reported. Studies on human alveolar macrophages from HIV-1-infected individuals demonstrated an impaired phagocytosis of Pneumocystis carinii pneumonia [39] that is also associated with a reduced oxidative burst response to in vitro challenge with P. carinii [6].
In the present study, we have investigated the role of HIV-1 Nef in the regulation of superoxide anion production in the U937 monoblastic cell line. The results presented here are strongly suggestive of a biphasic effect of Nef in the control of the enzymatic activity of NAPDH oxidase, i.e. a rapid activation followed, within 10 h, by a dramatic inhibition. This latter depended on de novo protein synthesis and was apparently mediated by soluble factor(s). Whereas the early apparent activation of NAPDH oxidase can be ascribed to the already reported ability of Nef to induce phosphorylation and cell-membrane translocation of p47phox and p67phox by means of the activation of PAK-2, a typical substrate for the Nef activity [40,41], the late blockade of superoxide anion release appeared as an original finding, thus deserving detailed investigation.
Concerning the early Nef-dependent increase of superoxide anion release, similar results have been described by Vilhardt et al. [17] in Ra2 microglia mouse astrocytic cell line expressing Nef by means of a lentiviral construct. They reported a sort of priming effect of Nef that induced a strong NADPH oxidase activity after stimulation with the Ca2+ ionophore, fMLP or endotoxin. They also reported that Nef was unable to activate NADPH oxidase by itself and the priming effect was mediated by increasing the levels of the activated Rac1 fraction. Differently, we observed increased levels of superoxide anion release also in the absence of specific stimuli, and this difference could be explained by the more stringent control of Nef activity in our cellular system. Indeed, the U937 cell line, which constitutively expresses Nef–ER in an inactivated but rapidly inducible form by the addition of 4-HT to the culture, could be considered a synchronized cell culture concerning the Nef activities. These conditions are likely to allow a more sensible evaluation of superoxide anion release, permitting the detection of low activity of NADPH oxidase in the presence of activated Nef only. Furthermore, by prolonging the observation time points, we originally describe an Nef-dependent blockade of the superoxide anion release after the stimulation effect.
U937 are cells of myeloid lineage derivation already committed towards a monocytic differentiation pathway. The macrophage-like phenotype induced by several factors, including PMA, is characterized by quantitative rather than qualitative modifications of specific biochemical and cellular functions, and correlates with the developing properties of host-defence specialized functions [42]. A recent study [43], based on a combination of proteomics, transcriptomics and principal component analysis, indicates the U937 cells as a suitable model system to study the macrophage differentiation process.
In our experiments, the superoxide release produced by the NADPH oxidase was measured in U937 cells also stimulated by GM-CSF or fMLP. During the inflammatory response, GM-CSF, as well as other pro-inflammatory cytokines such as tumour necrosis factor and IL-8, is considered a phagocytic priming factor capable of inducing a weak oxidative response [44]. This process is crucial to induce a more responsive state in phagocytic cells necessary to produce high levels of ROS following a secondary stimulus such as bacterial fMLP [24,45]. In the present study, we report that both the PI3K-specific inhibitor LY294002 and the MEK-specific inhibitor PD98059 significantly reduced superoxide release in GM-CSF-stimulated U937 cells. Furthermore, LY294002 inhibited the GM-CSF-induced ERK1/2 phosphorylation, placing PI3K probably upstream of MAPKs in GM-CSF-initiated signalling pathways. Although the PI3K/MAPK pathway appears essential for the NADPH oxidase activation in cells stimulated by GM-CSF ([24,25,46,47] and results of the present study), the Nef protein inhibited superoxide release without interfering with the GM-CSF-induced ERK1/2 phosphorylation. These observations suggest that Nef should act through an independent way or downstream of the PI3K/MAPK signalling pathway.
In order to reveal the mechanism of Nef-mediated inhibition of the NADPH oxidase activity, we analysed the effects of three different exogenous Nef mutants on the release of superoxide in PMA-differentiated U937 cells. The internalization of rNef in human MDMs (monocyte-derived macrophages) has already been reported [16,26] and, more recently, it has been described that rNef may activate NF-κB in U937 cells [48]. Our results demonstrate that exogenous wt-rNef inhibited superoxide release as well as the endogenously expressed Nef. Furthermore, analysis of the rNef mutants indicates that the integrity of domains involved in the endosome-to-lysosome trafficking is required for the Nef inhibitory activity, whereas the proline-rich domain seems to be dispensable. Considering the crucial role of the PXXP domain in the activation of Rac1 and Pak [49], this result suggests that the two activities, i.e. activation and inhibition of superoxide release, could be uncoupled. A further analysis of the effects of Nef mutants on PMA-differentiated U937 cultures demonstrated that wt or AXXA rNefs were able to induce the MIP-1β released, while the mutants DDAA and EEQQ lacked such a function. These results are in agreement with already described observations concerning the Nef-induced release of inflammatory factors from MDM [11,16].
A possible correlation between the Nef activities involved in the inhibition of superoxide release and those underlying the secretion of inflammatory factors such as MIP-1β has been suggested by co-culture experiments in which we demonstrated that the culture medium of U937-Nef treated with 4-HT included soluble factors(s) capable of inhibiting superoxide release in co-cultured parental U937.
We present here experimental evidence supporting the idea that IL-10 participates in the Nef-induced inhibition of superoxide release. Of note, this appears to be rather consistent with the previously reported data highlighting IL-10 as an anti-inflammatory cytokine with marked antioxidant properties [50]. However, although the role of soluble factor(s) in the superoxide release inhibition is strongly suggested by these experiments and is consistent with the requirement of de novo protein synthesis for the Nef effect, we cannot formally exclude that Nef could inhibit the NADPH oxidase enzymatic activity also by activating additional ‘inside-in’ mechanisms.
Our studies on the mechanisms underlying the Nef-induced inhibition of superoxide anion release bring us to the conclusion that this is the result of the autocrine/paracrine action of soluble factor(s) produced in response to Nef and, in particular, to Nef domains involved in the endosome-to-lysosome trafficking. This mechanism readily reminds us of the results we have already described for the Nef-dependent STAT1 and STAT3 activations [14,16]. In this regard, we previously claimed that in primary human MDM, the Nef-dependent activation of the p50/p50 NF-κB homodimer correlated with the activation of a number of genes, including those coding for inflammatory chemokines/cytokines, whose release leads, upon the engagement with their specific receptors, to a loop of cell activation. Considering also that both the Nef-dependent NF-κB activation and the Nef-dependent inhibition of superoxide anion release rely on the presence of intact Nef domains involved in its endosome-to-lysosome cell trafficking (i.e. EEQQ and DDAA), one may hypothesize that the NF-κB activation is the upstream event ultimately leading to the NAPDH inhibition. In this perspective, the blockade of the NAPDH oxidase may be considered as a part of the pathological consequences induced by the presence of Nef in monocytes/macrophages. The inhibition of the NADPH oxidase by Nef could thus represent a new function directed to the impairment of the oxidative burst response of the phagocytic cells, more generally contributing to the pathogenesis of HIV-1 infection.
Acknowledgments
This work was supported by institutional funds from ISS (Istituto Superiore di Sanità, Rome, Italy) and by the ISS–NIH collaborative project ‘Peripheral blood determinants of redox changes in human respiratory disease: biochemical and pathophysiological evaluations’, Rif. 0F14 (2004–2005). We thank our students in Biotechnology (A. Mallano, V. Ruggieri and E. Ruggiero) for excellent technical assistance.
References
- 1.Chanock S. J., el Benna J., Smith R. M., Babior B. M. The respiratory burst oxidase. J. Biol. Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- 2.Roos D., van Bruggen R., Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–1315. doi: 10.1016/j.micinf.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Babior B. M. NADPH oxidase. Curr. Opin. Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Vignais P. V. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell. Mol. Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitrak D. L., Mullane K. M., Bilek M. L., Stevens P., Allen R. C. Impaired phagocyte oxidative capacity in patients with human immunodeficiency virus infection. J. Lab. Clin. Med. 1998;132:284–293. doi: 10.1016/s0022-2143(98)90041-5. [DOI] [PubMed] [Google Scholar]
- 6.Koziel H., Li X., Armstrong M. Y., Richards F. F., Rose R. M. Alveolar macrophages from human immunodeficiency virus-infected persons demonstrate impaired oxidative burst response to Pneumocystis carinii in vitro. Am. J. Respir. Cell Mol. Biol. 2000;23:452–459. doi: 10.1165/ajrcmb.23.4.4084. [DOI] [PubMed] [Google Scholar]
- 7.Elbim C., Pillet S., Prevost M. H., Preira A., Girard P. M., Rogine N., Hakim J., Israel N., Gougerot-Pocidalo M. A. The role of phagocytes in HIV-related oxidative stress. J. Clin. Virol. 2001;20:99–109. doi: 10.1016/s1386-6532(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 8.Kestler H. W., III, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell (Cambridge, Mass.) 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 9.Hanna Z., Kay D. G., Rebai N., Guimond A., Jothy S., Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell (Cambridge, Mass.) 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 10.Geyer M., Fackler O. T., Peterlin B. M. Structure–function relationships in HIV-1 Nef. EMBO Rep. 2001;2:580–585. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swingler S., Mann A., Jacque J., Brichacek B., Sasseville V. G., Williams K., Lackner A. A., Janoff E. N., Wang R., Fisher D., et al. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat. Med. 1999;5:997–103. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biggs T. E., Cooke S. J., Barton C. H., Harris M. P., Saksela K., Mann D. A. Induction of activator protein 1 (AP-1) in macrophages by human immunodeficiency virus type-1 NEF is a cell-type-specific response that requires both hck and MAPK signaling events. J. Mol. Biol. 1999;290:21–35. doi: 10.1006/jmbi.1999.2849. [DOI] [PubMed] [Google Scholar]
- 13.Olivetta E., Percario Z., Fiorucci G., Mattia G., Schiavoni I., Dennis C., Jager J., Harris M., Romeo G., Affabris E., et al. HIV-1 Nef induces the release of inflammatory factors from human monocyte/macrophages: involvement of Nef endocytotic signals and NF-kappa B activation. J. Immunol. 2003;170:1716–1727. doi: 10.4049/jimmunol.170.4.1716. [DOI] [PubMed] [Google Scholar]
- 14.Federico M., Percario Z., Olivetta E., Fiorucci G., Muratori C., Micheli A., Romeo G., Affabris E. HIV-1 Nef activates STAT1 in human monocytes/macrophages through the release of soluble factors. Blood. 2001;98:2752–2761. doi: 10.1182/blood.v98.9.2752. [DOI] [PubMed] [Google Scholar]
- 15.Briggs S. D., Scholtz B., Jacque J. M., Swingler S., Stevenson M., Smithgall T. E. HIV-1 Nef promotes survival of myeloid cells by a Stat3-dependent pathway. J. Biol. Chem. 2001;276:25605–25611. doi: 10.1074/jbc.M103244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percario Z., Olivetta E., Fiorucci G., Mangino G., Peretti S., Romeo G., Affabris E., Federico M. Human immunodeficiency virus type 1 (HIV-1) Nef activates STAT3 in primary human monocyte/macrophages through the release of soluble factors: involvement of Nef domains interacting with the cell endocytotic machinery. J. Leukocyte Biol. 2003;74:821–832. doi: 10.1189/jlb.0403161. [DOI] [PubMed] [Google Scholar]
- 17.Vilhardt F., Plastre O., Sawada M., Suzuki K., Wiznerowicz M., Kiyokawa E., Trono D., Krause K. H. The HIV-1 Nef protein and phagocyte NADPH oxidase activation. J. Biol. Chem. 2002;277:42136–42143. doi: 10.1074/jbc.M200862200. [DOI] [PubMed] [Google Scholar]
- 18.Walk S. F., Alexander M., Maier B., Hammarskjold M. L., Rekosh D. M., Ravichandran K. S. Design and use of an inducibly activated human immunodeficiency virus type 1 Nef to study immune modulation. J. Virol. 2001;75:834–843. doi: 10.1128/JVI.75.2.834-843.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarpey M. M., Fridovich I. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ. Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- 20.Klippel A., Escobedo M. A., Wachowicz M. S., Apell G., Brown T. W., Giedlin M. A., Kavanaugh W. M., Williams L. T. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol. Cell. Biol. 1998;18:5699–5711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohn A. D., Barthel A., Kovacina K. S., Boge A., Wallach B., Summers S. A., Birnbaum M. J., Scott P. H., Lawrence J. C., Jr, Roth R. A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J. Biol. Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 22.Pritchard C. A., Samuels M. L., Bosch E., McMahon M. Conditionally oncogenic forms of the A-Raf and B-Raf protein kinases display different biological and biochemical properties in NIH 3T3 cells. Mol. Cell. Biol. 1995;15:6430–6442. doi: 10.1128/mcb.15.11.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang P. M., Dewas C., Gaudry M., Fay M., Pedruzzi E., Gougerot-Pocidalo M. A., El Benna J. Priming of human neutrophil respiratory burst by granulocyte/macrophage colony-stimulating factor (GM-CSF) involves partial phosphorylation of p47(phox) J. Biol. Chem. 1999;274:20704–20708. doi: 10.1074/jbc.274.29.20704. [DOI] [PubMed] [Google Scholar]
- 24.Mollapour E., Linch D. C., Roberts P. J. Activation and priming of neutrophil nicotinamide adenine dinucleotide phosphate oxidase and phospholipase A(2) are dissociated by inhibitors of the kinases p42(ERK2) and p38(SAPK) and by methyl arachidonyl fluorophosphonate, the dual inhibitor of cytosolic and calcium-independent phospholipase A(2) Blood. 2001;97:2469–2477. doi: 10.1182/blood.v97.8.2469. [DOI] [PubMed] [Google Scholar]
- 25.Coffer P. J., Koenderman L. Granulocyte signal transduction and priming: cause without effect? Immunol. Lett. 1997;57:27–31. doi: 10.1016/s0165-2478(97)00067-9. [DOI] [PubMed] [Google Scholar]
- 26.Alessandrini L., Santarcangelo A. C., Olivetta E., Ferrantelli F., d'Aloja P., Pugliese K., Pelosi E., Chelucci C., Mattia G., Peschle C., et al. T-tropic human immunodeficiency virus (HIV) type 1 Nef protein enters human monocyte-macrophages and induces resistance to HIV replication: a possible mechanism of HIV T-tropic emergence in AIDS. J. Gen. Virol. 2000;81:2905–2917. doi: 10.1099/0022-1317-81-12-2905. [DOI] [PubMed] [Google Scholar]
- 27.Joyce D. A., Steer J. H. Differentiation of the U-937 promonocytic cell line induced by phorbol myristate acetate or retinoic acid: effect of aurothiomalate. Agents Actions. 1992;37:305–310. doi: 10.1007/BF02028124. [DOI] [PubMed] [Google Scholar]
- 28.Saksela K., Cheng G., Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piguet V., Gu F., Foti M., Demaurex N., Gruenberg J., Carpentier J. L., Trono D. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell (Cambridge, Mass.) 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 30.Lu X., Yu H., Liu S. H., Brodsky F. M., Peterlin B. M. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 31.Brigino E., Haraguchi S., Koutsonikolis A., Cianciolo G. J., Owens U., Good R. A., Day N. K. Interleukin 10 is induced by recombinant HIV-1 Nef protein involving the calcium/calmodulin-dependent phosphodiesterase signal transduction pathway. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3178–3182. doi: 10.1073/pnas.94.7.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tangsinmankong N., Day N. K., Good R. A., Haraguchi S. Monocytes are target cells for IL-10 induction by HIV-1 Nef protein. Cytokine. 2000;12:1506–1511. doi: 10.1006/cyto.2000.0741. [DOI] [PubMed] [Google Scholar]
- 33.Kuga S., Otsuka T., Niiro H., Nunoi H., Nemoto Y., Nakano T., Ogo T., Umei T., Niho Y. Suppression of superoxide anion production by interleukin-10 is accompanied by a downregulation of the genes for subunit proteins of NADPH oxidase. Exp. Hematol. 1996;24:151–157. [PubMed] [Google Scholar]
- 34.Capsoni F., Minonzio F., Ongari A. M., Carbonelli V., Galli A., Zanussi C. Interleukin-10 down-regulates oxidative metabolism and antibody-dependent cellular cytotoxicity of human neutrophils. Scand. J. Immunol. 1997;45:269–275. doi: 10.1046/j.1365-3083.1997.d01-393.x. [DOI] [PubMed] [Google Scholar]
- 35.Kimura T., Kameoka M., Ikuta K. Amplification of superoxide anion generation in phagocytic cells by HIV-1 infection. FEBS Lett. 1993;326:232–236. doi: 10.1016/0014-5793(93)81797-4. [DOI] [PubMed] [Google Scholar]
- 36.Dukes C. S., Matthews T. J., Weinberg J. B. Human immunodeficiency virus type 1 infection of human monocytes and macrophages does not alter their ability to generate an oxidative burst. J. Infect. Dis. 1993;168:459–462. doi: 10.1093/infdis/168.2.459. [DOI] [PubMed] [Google Scholar]
- 37.Muller F., Rollag H., Froland S. S. Reduced oxidative burst responses in monocytes and monocyte-derived macrophages from HIV-infected subjects. Clin. Exp. Immunol. 1990;82:10–15. doi: 10.1111/j.1365-2249.1990.tb05396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torre D., Gennero L., Baccino F. M., Speranza F., Biondi G., Pugliese A. Impaired macrophage phagocytosis of apoptotic neutrophils in patients with human immunodeficiency virus type 1 infection. Clin. Diagn. Lab. Immunol. 2002;9:983–986. doi: 10.1128/CDLI.9.5.983-986.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koziel H., Phelps D. S., Fishman J. A., Armstrong M. Y., Richards F. F., Rose R. M. Surfactant protein-A reduces binding and phagocytosis of Pneumocystis carinii by human alveolar macrophages in vitro. Am. J. Respir. Cell Mol. Biol. 1998;18:834–843. doi: 10.1165/ajrcmb.18.6.3059. [DOI] [PubMed] [Google Scholar]
- 40.Knaus U. G., Morris S., Dong H. J., Chernoff J., Bokoch G. M. Regulation of human leukocyte p21-activated kinases through G protein-coupled receptors. Science. 1995;269:221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed S., Prigmore E., Govind S., Veryard C., Kozma R., Wientjes F. B., Segal A. W., Lim L. Cryptic Rac-binding and p21(Cdc42Hs/Rac)-activated kinase phosphorylation sites of NADPH oxidase component p67(phox) J. Biol. Chem. 1998;273:15693–15701. doi: 10.1074/jbc.273.25.15693. [DOI] [PubMed] [Google Scholar]
- 42.Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J. Leukoc. Biol. 1985;37:407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 43.Verhoeckx K. C., Bijlsma S., de Groene E. M., Witkamp R. F., van der Greef J., Rodenburg R. J. A combination of proteomics, principal component analysis and transcriptomics is a powerful tool for the identification of biomarkers for macrophage maturation in the U937 cell line. Proteomics. 2004;4:1014–1028. doi: 10.1002/pmic.200300669. [DOI] [PubMed] [Google Scholar]
- 44.Hallett M. B., Lloyds D. Neutrophil priming: the cellular signals that say ‘amber’ but not ‘green’. Immunol. Today. 1995;16:264–268. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- 45.Elbim C., Bailly S., Chollet-Martin S., Hakim J., Gougerot-Pocidalo M. A. Differential priming effects of proinflammatory cytokines on human neutrophil oxidative burst in response to bacterial N-formyl peptides. Infect. Immunol. 1994;62:2195–2201. doi: 10.1128/iai.62.6.2195-2201.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki K., Hino M., Hato F., Tatsumi N., Kitagawa S. Cytokine-specific activation of distinct mitogen-activated protein kinase subtype cascades in human neutrophils stimulated by granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor-alpha. Blood. 1999;93:341–349. [PubMed] [Google Scholar]
- 47.Yamamori T., Inanami O., Nagahata H., Kuwabara M. Phosphoinositide 3-kinase regulates the phosphorylation of NADPH oxidase component p47(phox) by controlling cPKC/PKCdelta but not Akt. Biochem. Biophys. Res. Commun. 2004;316:720–730. doi: 10.1016/j.bbrc.2004.02.108. [DOI] [PubMed] [Google Scholar]
- 48.Varin A., Manna S. K., Quivy V., Decrion A. Z., Van Lint C., Herbein G., Aggarwal B. B. Exogenous Nef protein activates NF-kappa B, AP-1, and c-Jun N-terminal kinase and stimulates HIV transcription in promonocytic cells. Role in AIDS pathogenesis. J. Biol. Chem. 2003;278:2219–2227. doi: 10.1074/jbc.M209622200. [DOI] [PubMed] [Google Scholar]
- 49.Fackler O. T., Luo W., Geyer M., Alberts A. S., Peterlin B. M. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell. 1999;3:729–739. doi: 10.1016/s1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- 50.Haddad J. J., Fahlman C. S. Redox- and oxidant-mediated regulation of interleukin-10: an anti-inflammatory, antioxidant cytokine? Biochem. Biophys. Res. Commun. 2002;297:163–176. doi: 10.1016/s0006-291x(02)02094-6. [DOI] [PubMed] [Google Scholar]