Abstract
Recent isolation of Tropheryma whipplei (formerly Trophyrema whippelii), the agent of Whipple’s disease, from the cardiac valve of a patient with Whipple’s disease endocarditis now allows the detection of reactive epitopes that could be used in a serological assay. In order to propose an enzyme-linked immunosorbent assay (ELISA) that uses recombinant T. whipplei antigen, we first determined by Western blotting of human, mouse, and rabbit antisera that the common immunodominant epitope is an 84-kDa protein. We then produced 13 monoclonal antibodies (MAbs) against T. whipplei, 12 of which recognize this immunodominant epitope. These MAbs did not react with phylogenetically closely related bacteria or bacteria previously shown to be cross-reactive with T. whipplei, but they did react with two other strains of T. whipplei isolated, one from an ocular sample and the other from a duodenal biopsy specimen. By confocal microscopy, the MAbs allowed detection of T. whipplei within infected fibroblasts. The identification of the 84-kDa antigen with our MAbs will make it possible to develop a diagnostic antigen for use in a diagnostic ELISA for Whipple’s disease.
Whipple’s disease is a systemic bacterial infection characterized by fever, weight loss, diarrhea, lymphadenopathy, and polyarthritis. Occasionally, it is also associated with cardiac manifestations such as myocarditis, pericarditis, and endocarditis or central nervous system involvement (13, 18, 21). Diagnosis of infection is usually based on histopathological examination of a duodenal biopsy specimen showing infiltration by large macrophages that contain periodic acid-Schiff-positive, non-acid-fast bacteria (1). The determination of the nucleotide sequence of the 16S rRNA gene of Tropheryma whipplei (formerly Trophyrema whippelii), the agent of Whipple’s disease (10, 19, 23), provided the basis for the development of species-specific diagnostic PCR systems (15, 22). These PCR-based diagnostic methods have become standards for the diagnosis of Whipple’s disease. Recent studies have demonstrated that T. whipplei is probably a ubiquitous or commensal microorganism, thereby questioning the diagnostic validity of a positive PCR result (2, 3, 12, 20). Using a shell vial cell culture system, we recently isolated the Whipple’s disease bacterium from the cardiac valve of a patient with Whipple’s disease-related endocarditis and successfully established a stable culture (16). In the same work, we developed a specific microimmunofluorescence (MIF) assay with Labteck slide-grown bacteria (16). This technique presents several major drawbacks, including the difficulty of antigen production. An enzyme-linked immunosorbent assay (ELISA) with a recombinant antigen that is an immunodominant epitope could thus be an alternative approach to the serological diagnosis of Whipple’s disease. In the present study, we first identified immunodominant epitopes by Western immunoblotting and then produced highly specific monoclonal antibodies (MAbs) directed against an immunopredominant antigen of T. whipplei. These MAbs will later be used to screen an expression bank of the T. whipplei genome cloned in Escherichia coli for a suitable recombinant antigen for use in an ELISA for Whipple’s disease.
MATERIALS AND METHODS
Antigen preparation.
The type strain of T. whipplei, Twist-Marseille (CNCM I-2202), was routinely subcultured on HEL cell monolayers in 150-cm2 cell culture flasks grown in 30 ml of minimal essential medium as described previously (16). HEL cells infected with bacteria were harvested from 40 150-cm2 flasks into 40 ml of phosphate-buffered saline (PBS). Trypsin (Gibco) was added to a final concentration of 5 mg ml−1, and the suspension was incubated at 30°C for 45 min. The suspension was then subjected to sonication (three times for 1 min each time on ice), after which the unlysed cells were removed by centrifugation at 100 × g for 15 min. The supernatant was layered onto a 25% (wt/vol) sucrose solution in PBS. After centrifugation at 9,000 × g for 30 min at 4°C, the pellet containing the bacteria was resuspended in 2 ml of PBS and carefully layered onto a 25 to 45% (wt/vol) Renografin step gradient (in PBS). This gradient was subjected to centrifugation at 130,000 × g for 1 h at 5°C. The bacteria were then harvested from the interface of the 25 to 45% Renografin gradient and washed twice in PBS. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the bacteria were resuspended in sterile distilled water at a final concentration of 1 mg ml−1.
The MAbs produced were tested against antigens from 2 other T. whipplei strains isolated in our laboratory (strains Slow2-Marseille and Eye) and 22 diverse bacterial strains isolated in our laboratory from clinical samples including Actinomyces meyeri, Actinomyces viscosus, Actinomyces pyogenes, Nocardia asteroides, Propionibacterium acnes, Mycobacterium marinum, Mycobacterium avium, Bacillus cereus, Listeria monocytogenes, Corynebacterium ANF group, Corynebacterium striatum, Streptococcus bovis, Streptococcus agalactiae (group B Streptococcus), Clostridium perfringens, Clostridium bifermentans, Fusobacterium necrophorum, E. coli, Yersinia enterocolitica, Shigella sonnei, Shigella flexneri, Salmonella enterica, and Campylobacter jejuni.
Human, mouse, and rabbit sera.
Six- to 8-week-old immunocompetent BALB/c mice were inoculated subcutaneously with a total of 0.1 mg of purified strain Twist-MarseilleT and Freund’s complete adjuvant. Mice were inoculated on days 0, 10, 20, and 30. On day 40, the mice were killed and their sera were frozen at −80°C. A rabbit was immunized by intradermal inoculation of a total of 1 mg of purified strain Twist-MarseilleT and Freund’s complete adjuvant. The rabbit was given a booster immunization on day 20, and serum sampled 10 days later was frozen at −80°C. Before use, mouse and rabbit polyclonal antisera were adsorbed with HEL cells in order to remove possible nonspecific anti-HEL cell antibodies. The sera were mixed with a pellet of washed HEL cells. The cells were suspended in the serum, and the mixture was shaken overnight at room temperature. After centrifugation at 10,000 × g for 10 min the supernatant was removed and frozen at −80°C. Human sera were obtained from a patient with Whipple’s disease endocarditis (16) and from a patient with typical intestinal Whipple’s disease. Control sera were obtained from patients who underwent duodenal biopsy for diseases other than Whipple’s disease and for whom the biopsy specimen was negative for T. whipplei by PCR and immunohistochemistry (17).
Production of MAbs.
MAbs were produced as described previously (5, 11). Six-week-old female BALB/c mice were inoculated three times intraperitoneally with 0.1 mg of purified strain Twist-MarseilleT, suspended in 0.5 ml of PBS, at 7-day intervals. One week after the final intraperitoneal inoculation, the mice were injected intravenously with 0.1 mg of bacteria suspended in 0.1 ml of PBS. Three days later, spleen cells from the mice were fused with SP2/0-Ag14 myeloma cells (10:1) by using 50% polyethylene glycol (molecular weight, 1,300 to 1,600; Sigma Chemical Co., St. Louis, Mo.). The fusion cells were grown in hybridoma medium (Seromed, Berlin, Germany) with 17% fetal bovine serum (Gibco BRL) and hypoxanthine-aminopterin-thymidine selective medium (Sigma Chemical Co.) at 37°C in an humidified atmosphere supplemented with 5% CO2. The supernatants were screened for antibodies to T. whipplei by MIF (14). Sera from the immunized mice were used as positive controls, and sera from healthy mice were used as negative controls. Positive hybridomas were subcloned twice by limiting dilution. The isotypes of the MAbs were determined with an ImmunoType Mouse Monoclonal Antibody (MAb) Isotyping kit with antisera to mouse immunoglobulin M (IgM), IgA, IgG1, IgG2a, IgG2b, and IgG3 (Sigma Chemical Co.). The specificities of the MAbs were tested by Western immunoblotting.
SDS-PAGE and Western blot study.
SDS-PAGE and Western blotting were performed by the method described by Laemmli (9). Antigens were used in the native state or were treated with proteinase K or boiled. Proteinase K digestion was performed by incubating antigen in a solution with 2 mg of proteinase K (Boehringer Mannheim, Mannheim, Germany) per liter at 37°C for 2 h. Heat denaturation was performed by boiling the antigens at 100°C for 10 min. For Western blotting, the strips were incubated with undiluted supernatants of MAbs and polyclonal mouse, rabbit, and human T. whipplei antisera diluted 1:100 in PBS at room temperature for 1 h and washed as described above.
Immunofluorescence detection of intracellular T. whipplei using confocal microscopy.
Immunofluorescence detection of T. whipplei-infected HEL cells within shell vials has been detailed elsewhere (10). In the present work, 100 μl of hybridoma supernatant with 3% (wt/vol) nonfat dry milk was used as the primary antibody. The coverslips were examined with a laser confocal fluorescence microscope (Leica, Lyon, France) equipped with a ×100 oil immersion lens.
RESULTS
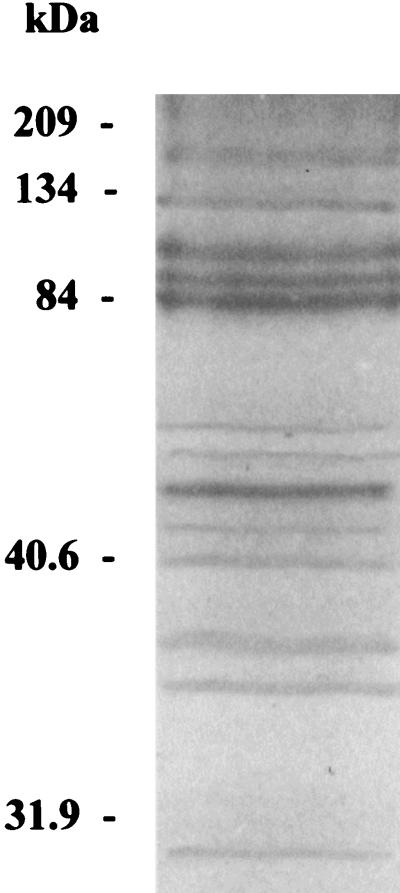
Study of SDS-PAGE revealed the presence of 21 protein bands, 13 of which were well visualized (Fig. 1). Of these bands, three were more prominent between 84 and 100 kDa and one was more predominant at 53 kDa. On Western blotting with human sera, reactivity was observed with antigens spanning the region between 84 and 105 kDa, with specific prominent reactivity against an 84-kDa antigen and against an antigen at 134 kDa being noted. For one human serum specimen, two reactive bands at 60 and 68 kDa were also observed. No reactivity was observed with control sera. The reactivity obtained with animal sera was comparable to that obtained with human sera for the antigens recognized, but reactivity with other antigens was included. Rabbit antiserum also reacted with antigens of 105, 100, 90, 60, 53, 40, 33, 32, and 23 kDa. Mouse serum did not react with high-molecular-mass antigens but reacted with antigens of 84, 60, 53, and 40 kDa. Thus, for all sera, reactivity against the 84-kDa antigen was the most prominent, but only animal sera reacted with the 53-kDa antigen.
FIG. 1.
Protein profile of T. whipplei obtained by SDS-PAGE with Coomassie brilliant blue staining of an 8% polyacrylamide gel.
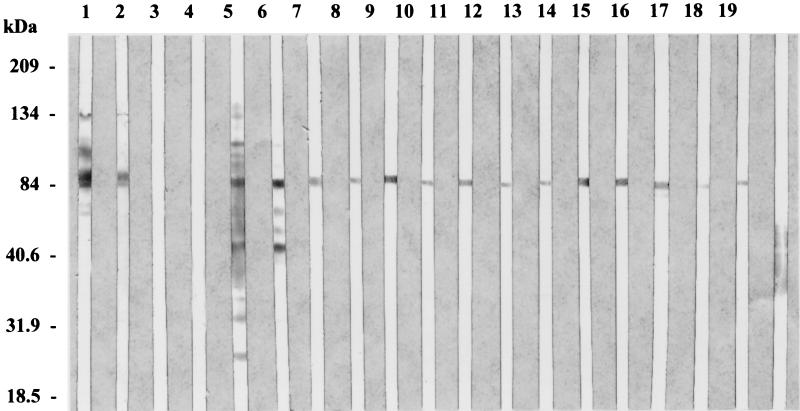
We obtained 13 MAbs: MAbs Tw17G2, Tw17B10, Tw18D5, Tw15F2, Tw24B9, Tw24E11, Tw25A6, Tw25A10, Tw25C5, Tw25D7, Tw25E5, Tw25H9, and Tw26A4. The isotypes of the MAbs were determined to be IgG1 for MAbs Tw25A10, Tw25C5, Tw25D7, Tw25E5, and Tw25H9 and IgM for the remainder of the MAbs. The titers of the MAbs in the supernatants were 1:160 (MAb Tw25E5), 1:320 (MAb Tw25H9), 1:640 (MAbs Tw17B10, Tw15F2, Tw24E11, Tw25A6, and Tw26A4), 1:1,280 (MAb Tw17G2, Tw24B9, and Tw25D7), and 1:2,560 (MAbs Tw18D5, Tw25A10, and Tw25C5). All these MAbs reacted with T. whipplei but not with HEL cells. Both intracellular and extracellular bacteria were recognized. Reactivity was indistinguishable from that observed with mouse and rabbit polyclonal antibodies. None of the MAbs obtained reacted with any of the other 22 diverse bacterial strains tested, but the MAbs did react with the 2 other T. whipplei strains tested. Western immunoblotting indicated that 12 of these MAbs reacted with the 84-kDa antigen that was recognized by SDS-PAGE analysis as one of the most prominent bands (Fig. 2). MAb Tw26A4 recognized a large antigenic region that extended from 43 to 57 kDa but in which there was no clear distinguishable reactive band. The antigenic reactivities of the MAbs were not modified by boiling of the antigens, whereas the reactivities disappeared after treatment with proteinase K, thereby demonstrating that the reacting antigen is protein in nature.
FIG. 2.
Immunoblots of antigens of T. whipplei with mono- and polyclonal antibodies. Lanes 1 and 2, sera of Whipple’s disease patients; lanes 3 and 4, sera of control patients; lane 5, polyclonal rabbit antiserum; lane 6, polyclonal mouse antiserum; lane 7, MAb Tw17G2; lane 8, MAb Tw17B10; lane 9, MAb Tw18D5; lane 10, MAb Tw15F2; lane 11, MAb Tw24B9; lane 12, Tw24E11; lane 13, MAb Tw25A6; lane 14, MAb Tw25A10; lane 15, MAb Tw25C5; lane 16, MAb Tw25D7; lane 17, MAb Tw25E5; lane 18, MAb Tw25H9; lane 19, Tw26A4.
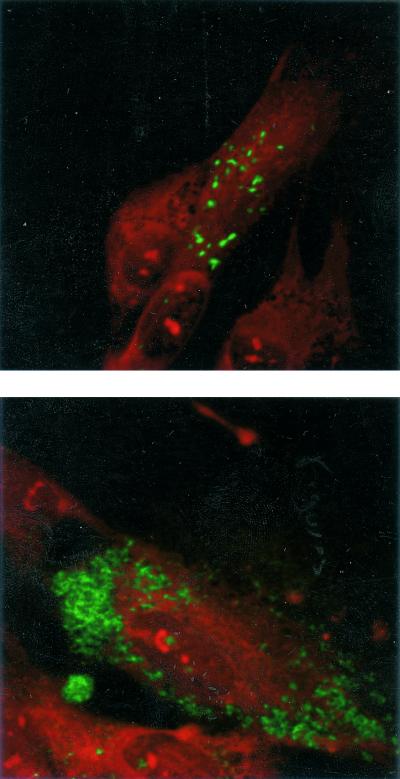
Confocal microscopy allowed detection of numerous fluorescent bacteria mostly aggregated within intracellular vacuoles (Fig. 3). The appearance was comparable to that of strains Twist-MarseilleT and Slow-Marseille detected with mouse polyclonal antisera (10, 17).
FIG. 3.
T. whipplei isolate Twist-MarseilleT resolved by immunofluorescence with the supernatant of the Tw18D5 hybridoma with a laser confocal fluorescence microscope. The isolate was grown in human HEL fibroblasts. Top, slight infection; below, heavy infection. Magnifications, ×1,000.
DISCUSSION
In a previous work, we used an immunofluorescence technique for antibody determination in patients with Whipple’s disease (16). Using this technique, we examined sera from 9 patients with Whipple’s disease and 40 control subjects (16). When a cutoff value of 1:100 was selected, IgG antibodies against the bacillus were detected in the serum samples of all nine patients with Whipple’s disease, as well as almost 75% of the samples from the control subjects. Thus, antibodies of the IgG subclass are produced at high levels in people without Whipple’s disease and cannot be used for diagnosis. In the same study, detection of IgM class antibodies was demonstrated to be more specific for Whipple’s disease, as, using a cutoff value of 1:50, we found that the results were positive for 7 of 9 patients with Whipple’s disease, whereas they were positive for 3 of 40 control subjects. Higher titers of IgM antibodies (≥1:400) were present in three of seven patients with classic Whipple’s disease and in both patients with Whipple’s disease endocarditis but in none of the control subjects (16). The high frequency of IgG antibodies against the Whipple’s disease isolate suggests that this pathogen is ubiquitous, causing illness only occasionally, perhaps because of differences in virulence among the strains or in host factors or as a result of the patient’s exposure to other immunologically cross-reacting microorganisms. Although the results obtained by this serological technique are encouraging, this technique presents several drawbacks that render its routine use difficult. First, the excessively slow multiplication of the bacterium, with a doubling time calculated to be about 18 days, limits the amount of available antigens (10). Second, the procedure used for MIF requires the use of Lab-Tek slides, which is very time-consuming (16). Finally, we have observed that as the number of passages in subculture increases, the specificity observed with IgM antibodies has a tendency to decrease (unpublished data). Our goal was thus to first identify immunodominant epitopes of T. whipplei and then to construct hybridomas that produce MAbs against this immunodominant epitope in order to produce it in a large scale for use in an ELISA.
In the present work, we generated 13 MAbs that were as efficient as mouse and rabbit polyclonal antibodies in recognizing T. whipplei strain Twist-MarseilleT by the MIF assay. These MAbs were demonstrated to be specific since they did not react with 22 other pathogenic, phylogenetically closely related, gram-positive bacteria with high G+C contents, nor did they react with common gastrointestinal pathogenic bacteria. Furthermore, bacterial species that have been shown to be cross-reactive with the Whipple’s diseases bacillus, such as S. agalactiae and S. flexneri, were not recognized by the MAbs (4, 8). These MAbs also reacted with two other T. whipplei strains, one of genotype 1A (strain Slow 2) and the other of genotype 2A (6, 7). On Western blotting the reactivity appears to be different according to the animal serum tested. Human sera recognized few antigens, and all antigens recognized by human sera were ≥60 kDa. Mouse antiserum also recognized few antigens, but not the 134-kDa antigen recognized by human sera, and mouse sera reacted with lower-molecular-mass antigens. The most reactive serum was that of the rabbit, which recognized the same antigens recognized by human and mouse sera as well as three additional low-molecular-mass antigens. Recognition of the 53-kDa antigen was specific to animal sera, and an 84-kDa antigen appeared to be the immunodominant antigen, with all sera tested having strong reactivity to this antigen. Among the 13 MAbs obtained, 12 reacted with the unique 84-kDa antigen. This antigen is a heat-resistant protein since its reactivity was not modified by boiling but disappeared after treatment with proteinase K. The reactivity of MAb Tw26A4 was different, as it recognized on Western blotting a protein antigen that was not a distinct band on SDS-PAGE and that extended from 43 to 57 kDa.
The recent isolation and large-scale production of T. whipplei in cell cultures have opened the way for the development of new diagnostic procedures (16). Using mouse polyclonal antibodies, we have demonstrated the efficiency of immunochemistry in detecting T. whipplei in a cardiac valve specimen (16) and a duodenal biopsy specimen (17). The MAbs produced in the present study were used for immunochemistry with the same tissue samples. Labeling of T. whipplei was weak and not convenient for diagnostic purposes. We think that this poor efficacy could be due to degradation of the antigen recognized by formalin because, using these MAbs in an MIF assay with methanol-fixed cell monolayers, we achieved good visualization of the bacteria by confocal microscopy. The evidence that the 84-kDa antigen recognized by our MAbs is an immunodominant epitope recognized by sera from patients with Whipple’s disease will allow the development of an ELISA. After cloning of the T. whipplei genome to obtain an expression bank, these MAbs could be used for the screening of products of clones in order to obtain a protein antigen that could be used in an ELISA for the detection of antibodies to T. whipplei.
Acknowledgments
We are indebted to Sarah Wyllie for reviewing the manuscript.
REFERENCES
- 1.Black-Schaffer, B. 1949. The tinctoral demonstration of a glycoprotein in Whipple’s disease. Proc. Soc. Exp. Biol. Med. 72:225–227. [DOI] [PubMed] [Google Scholar]
- 2.Dutly, F. 2000. Tropheryma whippelii DNA in saliva of patients without Whipple’s disease. Infection 28:219–222. [DOI] [PubMed] [Google Scholar]
- 3.Ehrbar, H. U., P. Bauerfeind, F. Dutly, H. R. Koelz, and M. Altwegg. 1999. PCR-positive tests for Tropheryma whippelii in patients without Whipple’s disease. Lancet 353:2214. [DOI] [PubMed] [Google Scholar]
- 4.Evans, D., and M. H. Ali. 1985. Immunohistochemistry in the diagnosis of Whipple’s disease. J. Clin. Pathol. 38:372–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harlow, E., and D. Lane. 1988. Monoclonal antibodies & growing hybridomas, p.139–282. In E. Harlow and D. Lane (ed.), Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 6.Hinrikson, H. P., F. Dutly, and M. Altwegg. 2000. Analysis of the actinobacterial insertion in domain III of the 23S rRNA gene of uncultured variants of the bacterium associated with Whipple’s disease using broad-range and “Tropheryma whipplei”-specific PCR. Int. J. Syst. Evol. Microbiol. 50:1007–1011. [DOI] [PubMed] [Google Scholar]
- 7.Hinrikson, H. P., F. Dutly, S. Nair, and M. Altwegg. 1999. Detection of three different types of Tropheryma whipplei directly from clinical specimens by sequencing, single-strand conformation polymorphism (SSCP) analysis, and type-specific PCR of their 16S–23S ribosomal intergenic spacer region. Int. J. Syst. Bacteriol. 49:1701–1706. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick, P. M., Jr., S. P. Kent, A. Milhas, and P. Pritchett. 1978. Whipple’s disease: case report with immunological studies. Gastroenterology 75:297–301. [PubMed] [Google Scholar]
- 9.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680–685. [DOI] [PubMed] [Google Scholar]
- 10.La Scola, B., F. Fenollar, P. E. Fournier, M. Altwegg, M. N. Mallet, and D. Raoult. 2001. Description of Tropheryma whipplei gen. nov. sp. nov., the Whipple’ s disease bacillus. Int. J. Syst. Evol. Microbiol. 51:1471–1479. [DOI] [PubMed] [Google Scholar]
- 11.Liang, Z., and D. Raoult. 2000. Species-specific monoclonal antibodies for rapid identification of Bartonella quintana. Clin. Diagn. Lab. Immunol. 7:21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiwald, M., F. Schuhmacher, H. J. Ditton, and A. Von Herbay. 1999. Environmental occurrence of the Whipple’s disease bacterium (Tropheryma whippelii). Appl. Environ. Microbiol. 64:760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maizel, H., J. M. Ruffin, and W. O. Dobbins. 1970. Whipple’s disease: a review of 19 patients from one hospital and a review of the literature since 1950. Medicine 49:175–205. [PubMed] [Google Scholar]
- 14.Philip, R. N., E. A. Casper, W. Burgdorfer, R. K. Gerloff, L. B. Hughes, and E. J. Bell. 1978. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J. Immunol. 121:1961–1968. [PubMed] [Google Scholar]
- 15.Ramzan, N. N., E. Loftus, Jr., L. J. Burgart, M. Rooney, K. P. Batts, R. H. Wiesner, D. N. Fredricks, D. A. Relman, and H. H. Persing. 1997. Diagnosis and monitoring of Whipple disease by polymerase chain reaction. Ann. Intern. Med. 126:520–527. [DOI] [PubMed] [Google Scholar]
- 16.Raoult, D., M. L. Birg, B. La Scola, P. E. Fournier, M. Enea, H. Lepidi, V. Roux, J. C. Piette, F. Vandenesch, D. Vital-Durand, and T. J. Marrie. 2000. Cultivation of the bacillus of Whipple’s disease. N. Engl. J. Med. 342:620–625. [DOI] [PubMed] [Google Scholar]
- 17.Raoult, D., B. La Scola, P. Lecocq, H. Lepidi, and P. E. Fournier. 2001. Culture and immunological detection of Tropheryma whipplei from the duodenum of a patient with Whipple’s disease. JAMA 285:1039–1043. [DOI] [PubMed] [Google Scholar]
- 18.Ratliff, N. B., J. T. McMahon, T. J. Naab, and D. M. Cosgrove. 1984. Whipple’s disease in the porcine leaflets of a Carpentier-Edwards prosthetic mitral valve. N. Engl. J. Med. 311:902–903. [DOI] [PubMed] [Google Scholar]
- 19.Relman, D. A., T. M. Schmidt, R. P. MacDermott, and S. Falkow. 1992. Identification of the uncultured bacillus of Whipple’s disease. N. Engl. J. Med. 327:293–301. [DOI] [PubMed] [Google Scholar]
- 20.Street, S., H. D. Donoghue, and G. H. Neild. 1999. Tropheryma whipplei DNA in saliva of healthy people. Lancet 354:1178–1179. [DOI] [PubMed] [Google Scholar]
- 21.Vital-Durand, D., C. Lecomte, P. Cathebras, H. Rousset, and P. Godeau. 1997. Whipple disease. Clinical review of 52 cases. Medicine 76:170–184. [DOI] [PubMed] [Google Scholar]
- 22.Von Herbay, A., H. J. Ditton, and M. Maiwald. 1996. Diagnostic application of a polymerase chain reaction assay for the Whipple’s disease bacterium to intestinal biopsies. Gastroenterology 110:1735–1743. [DOI] [PubMed] [Google Scholar]
- 23.Wilson, K. H., R. Blitchington, R. Frothingham, and J. A. P. Wilson. 1991. Phylogeny of the Whipple’s-disease-associated bacterium. Lancet 338:474–475. [DOI] [PubMed] [Google Scholar]