Abstract
GTN (nitroglycerin; glycerol trinitrate) causes dilation of blood vessels via activation of nitric oxide (NO)-sensitive sGC (soluble guanylate cyclase), a heterodimeric haem protein that catalyses the conversion of GTP into cGMP. Activation of sGC by GTN requires enzymatic or non-enzymatic bioactivation of the nitrate. Based on insufficient NO release and lack of spectroscopic evidence for formation of NO–sGC, the cysteine (Cys)-dependent activation of sGC by GTN was proposed to occur in an NO-independent manner. This extraordinary claim is questioned by the present findings. First, the effect of GTN/Cys was blocked by the NO scavenger oxyhaemoglobin, the superoxide-generating compound flavin mononucleotide and the haem-site sGC inhibitor ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one). Secondly, at equi-effective concentrations, GTN/Cys and the NO donor 2,2-diethyl-1-nitroso-oxyhydrazine released identical amounts of NO. Finally, at sufficiently high rates of NO release, activation of sGC by GTN/Cys was accompanied by a shift of the Soret band from 431 to 399 nm, indicating formation of NO–sGC. In the absence of Cys, GTN caused haem oxidation, apparent as a shift of the Soret band to 392 nm, which was accompanied by inactivation of the NO-stimulated enzyme. These results suggest that the effect of GTN/Cys is the result of an activation/inactivation equilibrium that is controlled by the rate of NO release and haem oxidation.
Keywords: haem oxidation, nitric oxide (NO), nitroglycerin, soluble guanylate cyclase
Abbreviations: DEA/NO, 2,2-diethyl-1-nitroso-oxyhydrazine; GTN, glycerol trinitrate (nitroglycerin); ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; sGC, soluble guanylate cyclase; TEA, triethanolamine; YC-1, 1-benzyl-3-(5-hydroxymethylfur-2-yl)indazole
INTRODUCTION
sGC (soluble guanylate cyclase; E.C.4.6.1.2), which catalyses conversion of GTP into the cyclic nucleotide cGMP, is the major effector enzyme of nitric oxide (NO) in mammalian cells (for recent reviews, see [1–3]). Binding of NO to a regulatory haem group at the β-subunit of the protein results in a several hundredfold stimulation of cGMP formation. The NO/cGMP signalling pathway is involved in a number of biological processes, including vasodilation, haemostasis and cell-to-cell communication in the brain. Several cardiovascular diseases, including coronary artery disease, atherosclerosis and hypertension, are associated with impaired release of NO from the endothelium, resulting in vasoconstriction, increased platelet adhesion and reduction in blood flow due to diminished formation of vascular cGMP [4]. The NO deficiency can be overcome by NO-releasing drugs, the so-called nitrovasodilators, with GTN (nitroglycerin; glycerol trinitrate) being the most prominent representative that has been used for the treatment of coronary artery disease since the second half of the 19th century [5].
Despite this long medical history and extensive clinical use of GTN, the precise mechanism of its action is still unclear. Since the pioneering work of Dr Murad and co-workers in the late 1970s [6], there is general agreement that the drug does not activate sGC directly, but requires bioactivation in blood vessels to release NO. Besides several enzymatic mechanisms of bioactivation that have been proposed in the last few decades (for recent reviews, see [7,8]), there is a puzzling chemical reaction that takes place between GTN and cysteine (Cys). Like virtually all thiol compounds, including the physiologically most abundant low-molecular-mass thiol GSH (reduced glutathione), Cys reacts with GTN to yield 1,2- and 1,3-dinitroglycerin, together with nitrite, as stable end-products [9]. We have reinvestigated this issue, and observed no significant differences between six different thiols, either in reaction rates or in product distribution (A. Hofer, A. Kollau, K. Schmidt and B. Mayer, unpublished work). However, in contrast with non-enzymatic GTN metabolism, bioactivation of the drug, i.e. formation of an intermediate that activates sGC, specifically requires Cys or related thiols, such as N-acetylcysteine [9].
The mechanism of Cys-triggered GTN bioactivation is unclear. Most intriguingly, two recent studies have suggested that stimulation of sGC by GTN/Cys is not mediated by NO [10,11]. This conclusion was based on two pieces of evidence: first, the GTN/Cys system was found to release insufficient quantities of NO to explain sGC activation [10], and secondly, neither light absorbance nor EPR spectra of GTN/Cys-treated sGC indicated formation of a haem–NO sGC species [11]. Obviously, if correct, these observations would suggest a new, previously unrecognized mechanism of sGC activation, which could contribute to cGMP formation in vivo and certainly deserves further investigation. Another interesting observation has been made with the GTN/Cys system and YC-1 [1-benzyl-3-(5-hydroxymethylfur-2-yl)-indazole], a drug shown previously to sensitize sGC for activation by NO and CO [12]. It is generally accepted that YC-1 increases the apparent affinity of purified sGC for NO without affecting maximal enzyme activity [1]. However, when tested in the presence of GTN/Cys, YC-1 showed a pronounced, 3–4-fold effect on maximal cGMP formation, resulting in virtually identical enzyme activities with GTN/Cys and DEA/NO (2,2-diethyl-1-nitroso-oxyhydrazine) [10]. In that paper, both the ‘partial agonism’ of the GTN/Cys system, as well as the effect of YC-1, have remained unexplained.
The present study was designed to clarify whether or not sGC activation by GTN/Cys is mediated by NO, and to explain the apparent ‘partial agonism’ of this system. Using NO chemiluminescence for determination of NO release, and by simultaneous spectroscopic and functional analysis of purified sGC, we obtained unambiguous evidence that enzyme activation by GTN/Cys is mediated by NO. In addition, the ‘partial agonism’ of GTN/Cys is explained by an activation/inactivation equilibrium that is controlled by NO release and GTN-triggered haem oxidation. Since this model inevitably implies that a compound that increases the apparent NO affinity of sGC will shift the equilibrium towards enzyme activation, it also provides a simple explanation for the effect of YC-1 on maximal GTN/Cys-stimulated sGC activity.
MATERIALS AND METHODS
Materials
Bovine lung sGC was purified as described previously [13]. [α-32P]GTP (400 Ci/mmol) was obtained from Amersham Biosciences (Vienna, Austria). Approx. 5 mg of protein was obtained from 5 kg of fresh lung. Purity of sGC was >95%, as judged by SDS/PAGE. The protein (≈10 mg/ml) was stored at −70 °C. Nitropohl® ampoules (G. Pohl-Boskamp GmbH and Co., Hohenlockstedt, Germany), containing 4.4 mM GTN in 250 mM glucose, were obtained from a local pharmacy. The experiments shown in Figure 5 were performed with GTN (Aquo-trinitrosan) from Merck (Darmstadt, Germany). Dilutions were made in buffer containing 250 mM glucose. DEA/NO, ODQ (1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one) and YC-1 (Alexis Corporation, Lausen, Switzerland) were purchased through Eubio (Vienna, Austria). DEA/NO was dissolved and diluted in 10 mM NaOH. Stock solutions of YC-1 (100 mM) and ODQ (10 mM) were prepared in DMSO and diluted with 25% DMSO in H2O (v/v). Oxyhaemoglobin was prepared by reduction of bovine haemoglobin (Sigma, Vienna, Austria) with sodium dithionite, as described previously [14]. All other chemicals were from Sigma.
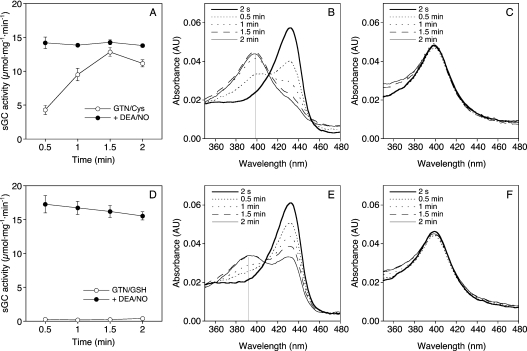
Figure 5. Simultaneous determination of sGC activity and light absorbance in the presence of DEA/NO and GTN/Cys.
GTN (1 mM) was pre-incubated for 10 min at 37 °C in 5 mM [α-32P]GTP, 15 mM MgCl2, 1 mM cGMP, 50 mM TEA/HCl, pH 7.4, in the presence of 10 mM Cys (A–C) or GSH (D–F) in a final volume of 450 μl, followed by addition of 30 μg of GC in 50 μl of 50 TEA/HCl, pH 7.4, containing 10 mM Cys (A–C) or GSH (D, E), at the zero time point. Light absorbance spectra were recorded every 30 s using the 8453 diode array device from Agilent Technologies. For determination of sGC activity, 0.1 ml aliquots were removed 30, 60, 90 and 120 s after addition of the enzyme and quenched in 450 μl of zinc acetate (120 μM), followed by isolation of [32P]cGMP. When indicated, DEA/NO (0.1 mM final) was added 1 min before addition of sGC. Results are means±S.E.M. for four experiments performed in duplicate. (A) sGC activity in the presence of 1 mM GTN and 10 mM Cys alone (○) or in combination with 0.1 mM DEA/NO (●). (B) Light absorbance spectra of GTN/Cys-treated sGC recorded at the indicated time points after addition of the enzyme. (C) Light absorbance spectra of sGC in the additional presence of DEA/NO. (D) sGC activity in the presence of 1 mM GTN and 10 mM GSH alone (○) or in combination with 0.1 mM DEA/NO (●). (E) Light absorbance spectra of GTN/GSH-treated sGC recorded at the indicated time points after addition of the enzyme. (F) Light absorbance spectra of sGC in the additional presence of DEA/NO.
Determination of sGC activity
Unless otherwise indicated, purified bovine lung sGC (50 ng) was incubated at 37 °C for 10 min in a final volume of 0.1 ml with the indicated concentrations of GTN or DEA/NO. Assay mixtures contained 50 mM TEA (triethanolamine)/HCl, pH 7.4, 0.5 mM [α-32P]GTP (approx. 150000 c.p.m.), 3 mM MgCl2, 1 mM cGMP and 2 mM GSH or Cys. Reactions were terminated by addition of 450 μl of zinc acetate (120 mM) and 450 μl of sodium bicarbonate (120 mM), followed by isolation of [32P]cGMP, as described previously [15]. Blank values were determined in the absence of sGC.
To study GTN-induced sGC inactivation, 0.5 μg of the enzyme was pre-incubated with 0.3 mM GTN or vehicle in 0.1 ml of 50 mM TEA/HCl, pH 7.4, for 10 min at 37 °C. Subsequently, samples were put on ice for 5 min, and 10 μl aliquots were incubated for 10 min at 37 °C in 0.1 ml of 50 mM TEA/HCl, pH 7.4, in the presence of 0.5 mM [α-32P]GTP, 3 mM MgCl2, 1 mM cGMP, 2 mM GSH and the indicated concentrations of DEA/NO (0–100 μM). For the time course of sGC inactivation (see Figure 7B), 1 μg of the enzyme was pre-incubated with 0.3 mM GTN or vehicle in 100 μl of TEA/HCl, pH 7.4, at 37 °C. At the indicated time points, 10 μl aliquots were removed and incubated for 1 min at 37 °C in 0.1 ml of 50 mM TEA/HCl, pH 7.4, in the presence of 0.5 mM [α-32P]GTP, 3 mM MgCl2, 1 mM cGMP, 2 mM GSH and 1 μM DEA/NO.
Figure 7. Inactivation of DEA/NO-stimulated sGC by GTN.
(A) Purified sGC (0.5 μg) was pre-incubated with 0.3 mM GTN or vehicle in 0.1 ml of TEA/HCl, pH 7.4, for 10 min at 37 °C. Subsequently, samples were put on ice for 5 min, and 10 μl aliquots were incubated for 10 min at 37 °C in the presence of [α-32P]GTP (0.5 mM, ≈150000 c.p.m.), 3 mM MgCl2, 1 mM cGMP, 2 mM GSH and the indicated concentrations of DEA/NO. (B) Purified sGC (0.5 μg) was pre-incubated with 0.3 mM GTN or vehicle in 0.1 ml of TEA/HCl, pH 7.4, at 37 °C for 0–10 min. At the indicated time points, 10 μl aliquots were removed and incubated for 1 min at 37 °C in the presence of [α-32P]GTP (0.5 mM, ≈150000 c.p.m.), 3 mM MgCl2, 1 mM cGMP, 2 mM GSH and 1 μM DEA/NO. Results are expressed as percentages of the activity of sGC pre-incubated with vehicle, and represent means±S.E.M. for three experiments.
Light absorbance spectroscopy (standard conditions)
Light absorbance spectra were recorded with a Hewlett–Packard 8452A Diode Array spectrophotometer in 1 cm light-path Ultra-Micro Suprasil quartz cuvettes (Hellma, Müllheim/Baden, Germany) that permit measurements in volumes of 10 or 50 μl (type 105.210-QS and 105.202-QS respectively). Spectra were obtained upon addition of DEA/NO, GTN, GTN/Cys or ferricyanide (for final concentrations, see the legend to Figure 2) to 2.5 μM sGC in 50 mM TEA/HCl, pH 7.4, 75 mM NaCl, 2 mM GSH, 1 mM EDTA and 20% glycerol at 18 °C. When GTN was present, the final concentrations of these agents were slightly lower: 37 mM TEA/HCl, pH 7.4, 56 mM NaCl, 1.5 mM GSH, 0.7 mM EDTA, 15% (v/v) glycerol and 36–50 mM glucose. Raw spectra were processed as described previously [16] with small modifications. Average absorbances between 720 and 820 nm, where sGC absorbance is negligible, were subtracted from each spectrum. The resulting spectra were corrected for volume changes (maximally 25%). Signal-to-noise ratios were improved by the application of a 5-point moving average smoothing procedure. Absorbances at specific wavelengths that tended to yield erratic results due to the proximity of strong narrow emission bands of the measuring light source (486 nm, 604–606 nm and 656–658 nm) were sometimes discarded. To enable a better comparison of the spectra obtained in the presence of ferricyanide, DEA/NO and GTN/Cys, differences in the actual protein concentrations for these experiments, inherent to the use of very small volumes, were accounted for by normalization of the Soret maxima of the starting ferrous haem spectra to a value of 0.21.
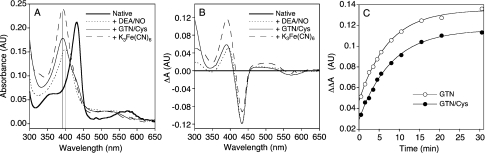
Figure 2. Effects of GTN/Cys, DEA/NO and ferricyanide on light absorbance of sGC.
Spectroscopy was performed with 2.5 μM sGC in 50 mM TEA/HCl, pH 7.4, 75 mM NaCl, 2 mM GSH, 1 mM EDTA and 20% glycerol at 18 °C. When GTN was present, the final concentrations of these agents were slightly lower: 37 mM TEA/HCl, pH 7.4, 56 mM NaCl, 1.5 mM GSH, 0.7 mM EDTA, 15% glycerol and 36–50 mM glucose. The spectra are representative of three (DEA/NO), five (ferricyanide), eight (GTN/Cys), and 19 (no additions) experiments, performed with two different enzyme preparations. (A) Light absorption spectra of sGC (2.5 μM) measured in the absence (thick continuous line) or presence of 125 μM DEA/NO (dotted line), 0.88 mM GTN/2 mM Cys (thin continuous line) or 25 μM ferricyanide (broken line). The spectra obtained with DEA/NO and GTN/Cys were recorded after 30 min, the spectrum obtained with ferricyanide after ≈1 min. Raw spectra were processed as described in the Materials and methods section. (B) Corresponding absorbance difference spectra illustrating the effects of addition of DEA/NO (dotted line), GTN/Cys (thin continuous line), and ferricyanide (broken line) to native (ferrous) sGC (thick continuous line at ΔΔA=0). (C) The kinetics of the reaction between sGC (2.5 μM) and 0. 7 mM GTN alone (○) and 0.67 mM GTN/1.5 mM Cys (●) is presented as the change in the absorbance difference (ΔΔA) between 472 and 432 nm over time. For reasons of clarity, the absorbance difference at the zero time point was arbitrarily set to 0.02 and 0 for the results obtained with GTN alone and GTN/Cys respectively. The lines drawn through the data points are best fits to single exponentials, with first-order rate constants of 0.138±0.007 and 0.126±0.007 min−1 for GTN alone and GTN/Cys respectively. Note that the fits intersect the y-axis well above the expected points for both reactions, indicating the presence of a fast phase that contributes approx. 25% to the total absorbance change.
Simultaneous determination of light absorbance and sGC activity
GTN (1 mM) was pre-incubated in a disposable cuvette for 10 min at 37 °C in 450 μl of 50 mM TEA/HCl, pH 7.4, containing 5 mM [α-32P]GTP (≈10 kBq), 15 mM MgCl2 and 1 mM cGMP in the presence of 10 mM Cys or GSH, followed by addition of 30 μg of GC (0.4 μM final concentration) in 50 μl of 50 mM TEA/HCl, pH 7.4, containing 10 mM Cys or GSH, at the zero time point. Light absorbance spectra were recorded immediately after mixing (≈2 s), and then every 30 s using the 8453 diode array device from Agilent Technologies. For determination of sGC activity, 0.1 ml aliquots were removed 30, 60, 90 and 120 s after addition of the enzyme and quenched in 450 μl of zinc acetate (120 mM), followed by isolation of [32P]cGMP as described previously [15]. When indicated, DEA/NO (0.1 mM final concntration) was added 1 min before addition of the enzyme.
Determination of NO
NO was measured with a chemiluminescence detector (Sievers Instruments, Boulder, CO, U.S.A.; model NOA 280), which was calibrated by injection of aqueous nitrite standards (10–200 pmol in 0.1–0.5 ml) into the purge vessel filled with a mixture of 50 mg NaI, dissolved in 1 ml of H2O and 5 ml of acetic acid. To determine the release of NO from DEA/NO or GTN, 1.8 ml glass vials were filled with 1 ml of buffer (50 mM Tris/HCl, pH 7.4) and sealed with a rubber septum. Reactions were started by injection of the drugs (3–10 μl) and vortex-mixing the vials for 5 s. After incubation for 10 min at 37 °C, aliquots of 0.1–0.8 ml were removed with a gas-tight syringe (previously flushed with argon) and injected into the water-filled purge vessel of the analyser. Data are expressed as the NO concentration of the injected samples.
RESULTS
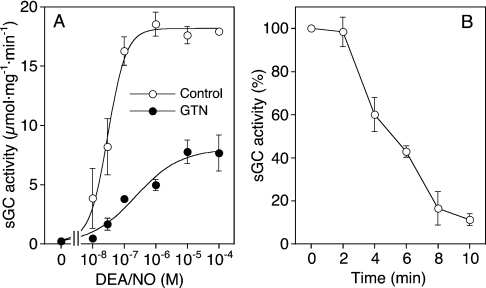
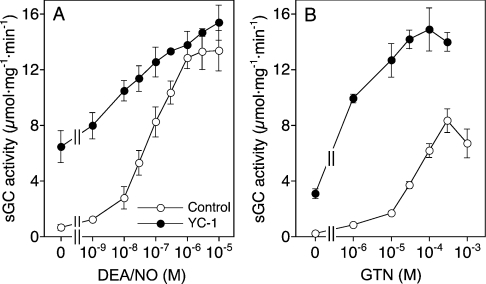
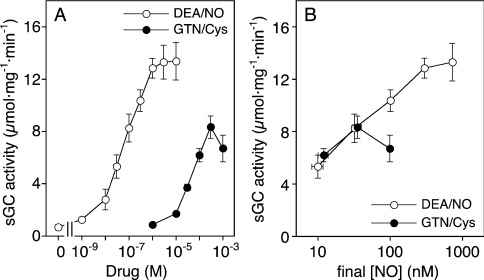
The activation of sGC by DEA/NO and GTN/Cys was measured in the absence and presence of the NO sensitizer YC-1. As shown in Figure 1(A), YC-1 led to an approx. 10-fold increase in the basal sGC activity, and caused a 2-fold increase in the potency of DEA/NO (EC50 values ≈100 and ≈40 nM respectively), while maximal enzyme activity was not significantly affected. Stimulation of sGC by GTN was strictly Cys-dependent (results not shown). In the presence of 2 mM Cys, basal sGC activity was approx. 3-fold lower than with GSH. GTN/Cys led to ≈30-fold enzyme stimulation, with an EC50 of 35.4±5.9 μM. Maximal enzyme activity was approx. 40% of that measured with DEA/NO. YC-1 caused a pronounced, ≈40-fold increase in the potency of GTN/Cys (EC50 1.40±0.6 μM). Under these conditions, the efficacy of the GTN/Cys system was virtually identical with that of DEA/NO (Figure 1B).
Figure 1. Effects of (A) DEA/NO and (B) GTN/Cys on sGC activity in the absence and presence of YC-1.
Purified sGC (50 ng) was incubated at 37 °C for 10 min in a final volume of 0.1 ml with the indicated concentrations of DEA/NO (A) and GTN (B) in the absence (○) or presence (●) of 0.2 mM YC-1. Assay mixtures contained 50 mM TEA/HCl, pH 7.4, [α-32P]GTP (0.5 mM, ≈150000 c.p.m.), 3 mM MgCl2, 1 mM cGMP and 2 mM GSH or Cys. Samples were analysed for [32P]cGMP as described in the Materials and methods section. Results are shown as the means±S.E.M. for three to five experiments.
Figure 2(A) shows light absorbance spectra of sGC treated with either DEA/NO or GTN in the presence of 2 mM Cys at 18 °C under standard conditions. As isolated, the enzyme was in the ferrous oxidation state with a Soret band at 431 nm. DEA/NO triggered transition of the Soret band to 399 nm, indicating the expected formation of NO–sGC. Oxidation of the haem with 25 μM ferricyanide resulted in a transition from 431 to 392 nm. Upon treatment with GTN/Cys, the enzyme showed a λmax of 392 nm without appearance of the 399 nm band, showing that haem oxidation occurs without formation of NO–sGC under these conditions. The corresponding difference spectra are shown in Figure 2(B). The kinetics of haem oxidation by GTN and GTN/Cys, measured as time-dependence of the 431→392 nm transition, is shown in Figure 2(C). The reaction followed first-order kinetics, with half-times of 5.5 and 5.0 min in the absence and presence of Cys respectively.
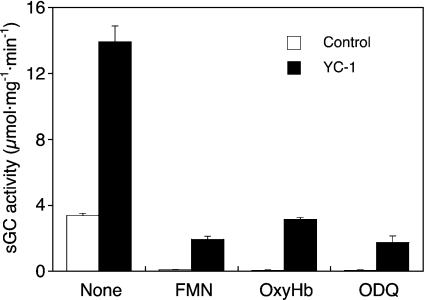
So far, our results confirmed the observations reported by Artz et al. [11], suggesting that the activation of sGC by GTN/Cys is not mediated by NO binding to the haem. To substantiate further this intriguing conclusion, we tested several pharmacological tools known to interfere with NO stimulation of sGC. As shown in Figure 3, the effect of GTN/Cys was fully inhibited by the superoxide-generating compound FMN, the NO scavenger oxyhaemoglobin and the haem-site inhibitor of sGC, ODQ, both in the absence and presence of YC-1. Thus, in contrast with the spectroscopic results, the functional data strongly suggest that the active intermediate is NO. To resolve this conflict, we measured the concentration of NO released from GTN in the presence of Cys under sGC assay conditions (pH 7.4, 37 °C and 10 min). The detection limit of the chemiluminescence method was ≈10 nM NO, precluding reliable measurements at GTN concentrations <0.1 mM. The NO concentrations measured after 10 min of incubation of 0.1, 0.3 and 1 mM GTN with 2 mM Cys were 11±1.9, 38±4.4 and 99±3.6 nM (means±S.E.M; n=4–6). These values were compared with NO release from DEA/NO and correlated with sGC activity. Figure 4(A) shows a re-plot of the DEA/NO and GTN/Cys control curves from Figures 1(A) and 1(B) respectively; the correlation with NO release is shown in Figure 4(B). As illustrated in the Figure, NO release from 0.1 and 0.3 mM GTN was identical with that from equi-effective concentrations of DEA/NO (30 and 100 nM), suggesting that NO release from GTN/Cys fully accounts for sGC activation. However, a pronounced mismatch between NO release and sGC activity was observed at 1 mM GTN. At that concentration, GTN released 3-fold more NO than at 0.3 mM, but caused a decrease in the rate of cGMP formation, indicating that sGC activation was impaired by a secondary effect of GTN.
Figure 3. Effects of FMN, oxyhaemoglobin and ODQ on GTN/Cys-activated sGC in the absence and presence of YC-1.
Purified sGC (50 ng) was incubated at 37 °C for 10 min in a final volume of 0.1 ml with 0.1 mM GTN and 2 mM Cys in the absence (○) or presence (●) of 0.2 mM YC-1 and 0.1 mM FMN, 10 μM oxyhaemoglobin (OxyHb) or 30 μM ODQ. Results are the means±S.E.M. for three experiments.
Figure 4. Correlation of sGC activity and NO release from DEA/NO and GTN/Cys.
(A) Re-plot of the control curves from Figures 1(A) and 1(B). (B) DEA/NO (0.03, 0.1, 0.3, 1 and 3 μM) or GTN (0.1, 0.3 and 1 mM) were incubated at 37 °C for 10 min in the presence of 2 mM Cys, followed by determination of NO by chemiluminescence, as described in the Materials and methods section. NO concentrations were plotted against the corresponding sGC activities shown in (A). Results are the means±S.E.M. for four to six measurements.
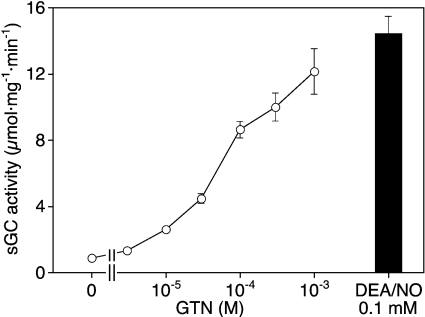
In addition to the excellent correlation between NO release and sGC activity, the NO measurements revealed that the sGC concentration used for light absorbance spectroscopy (2.5 μM) was 50–100-fold higher than the NO release expected from GTN/Cys (50–100 nM), indicating that the lack of NO–sGC formation was probably due to substoichiometric NO availability. To settle this issue, we sought to correlate sGC activity with the spectral transitions of enzyme-bound haem measured under identical conditions, and accordingly changed the experimental setup. The enzyme concentration was reduced to 0.4 μM, incubation temperature was raised to 37 °C, and GTN (1 mM) was pre-incubated with 10 mM Cys for 10 min before addition of sGC. Under these conditions, the NO concentration measured after 10 min was 0.51±0.05 μM, pointing to an at least approximately matched protein-to-ligand ratio. In this experimental setup, maximal sGC activity was identical in the presence of GTN and DEA/NO, although the onset of enzyme activation by GTN was slower, presumably due to the lower NO steady-state concentration, and showed a tendency to decrease after 2 min of incubation (Figure 5A). As shown in Figure 5(B), the increase in enzyme activity triggered by GTN/Cys was accompanied by a transition of the Soret band from 430 to 399 nm, indicating formation of the ferrous haem–NO complex. In the presence of DEA/NO, the 430→399 nm transition was complete within 2 s (Figure 5C). Prolonged incubation revealed a time-dependent 399→392 nm transition that was virtually complete after 20 min (results not shown). Figures 5(D)–5(F) show control experiments in which Cys was replaced by GSH. Whereas cGMP formation and spectral transitions triggered by DEA/NO were identical with both thiols, GTN was inactive and caused transition of the Soret band to 392 nm without detectable formation of the haem–NO complex in the presence of GSH.
These results suggested that the apparent ‘partial agonism’ of GTN/Cys is a consequence of comparably slow haem oxidation occurring during the 10 min incubation routinely used for activity measurements. Indeed, the activity of GTN/Cys-activated sGC approached that of the NO-stimulated enzyme (86% of the DEA/NO control at 1 mM) when the incubation time was reduced to 2 min (Figure 6). Instead of the characteristic decrease in cGMP formation at 1 mM GTN observed under standard conditions, the enzyme activity was increased further without reaching a maximum in the short-term assays, confirming that the mismatch between NO release and sGC activity apparent in Figure 3 reflects relatively slow haem oxidation that impairs sGC activation upon prolonged exposure of the enzyme to GTN.
Figure 6. Effect of GTN/Cys on sGC activity measured after 2 min of incubation.
GTN (1 μM to 1 mM) was pre-incubated with 10 mM Cys for 10 min at 37 °C in the sGC assay mixture described in the legend to Figure 1, followed by 2 min of incubation with 0.1 μg of sGC in a final volume of 0.1 ml and determination of [32P]cGMP, as described in the Materials and methods section. Where indicated, DEA/NO (0.1 mM final) was added 1 min before addition of sGC. Results are the means±S.E.M. for three experiments.
The effect of haem oxidation was studied in more detail by measuring NO-stimulated sGC activity upon pre-incubation of the enzyme with GTN. As shown in Figure 7(A), pre-incubation with 0.3 mM GTN markedly impaired sGC activation by DEA/NO both in terms of potency and efficacy of the donor. The EC50 was increased approx. 5-fold (from ≈40 to ≈200 nM), and maximal activity was reduced from ≈18 to ≈8 μmol·min−1·mg−1. The effect of pre-incubation time on enzyme stimulation by DEA/NO is shown in Figure 7(B). The loss of activity triggered by GTN was time-dependent, with a half-time of 4–5 min. This value agrees reasonably well with the kinetics of GTN-induced haem oxidation measured by light absorbance spectroscopy (see Figure 2C).
DISCUSSION
The present data indicate that stimulation of sGC by GTN/Cys is mediated by release of free NO. This conclusion is supported by three lines of evidence. First, cGMP formation was highly sensitive to agents known to interfere with NO stimulation of sGC. Oxyhaemoglobin, which reacts at nearly diffusion-controlled rates with NO to yield methaemoglobin and nitrate, is established as the gold standard to test for the involvement of NO in a given biological process [17]. In our experiments, oxyhaemoglobin reduced the activity of GTN/Cys-stimulated sGC to basal levels both in the absence and in the presence of YC-1. The sensitivity of NO to superoxide is also well established. Stabilization of endothelium-derived relaxing factor by superoxide dismutase was one of the key observations that led to the identification of this factor as NO [18–20], and many of the toxic effects of NO are now recognized as being mediated by the product of the NO/superoxide reaction, peroxynitrite [21]. FMN is a well-established non-enzymatic source of superoxide in the presence of light (e.g. see [22]). Based on detailed previous studies on the use of FMN for superoxide generation [23,24], we used this compound to test for superoxide sensitivity of the active intermediate and observed complete inhibition of GTN/Cys-induced sGC activation.
Finally, ODQ is a well-established sGC inhibitor that acts via oxidation of the haem [25–27]. Full inhibition of the GTN/Cys effect by ODQ indicates the essential involvement of ferrous haem in enzyme stimulation.
The second piece of evidence in favour of NO as an active intermediate was provided by the excellent correlation between NO release from two equi-effective concentrations of GTN/Cys and DEA/NO measured under identical conditions. Our data indicate that NO stimulates sGC activity with an apparent EC50 of 10–30 nM (see Figure 4B). Garthwaite and colleagues [28,29] have carefully measured the NO binding affinity of sGC at clamped NO concentrations, and arrived at an EC50 of approx. 1 nM. Our less sophisticated approach is expected to give only an approximate estimate of the upper limit of the EC50, since we related the NO concentration measured at a single time point to the cumulative enzyme activity during 10 min of incubation. Thus our data agree with the more precise measurements from the Garthwaite laboratory, but strikingly disagree with the approx.-100-fold-higher value (EC50 1.6 μM) reported by Artz et al. [10]. We think that this enormous underestimation of NO potency is due to a methodological problem. NO release from established donor compounds was calculated from first-order rate constants determined with a Clark-type electrode, and is expressed as total NO released during incubations, without considering autoxidation or other pathways of NO decay. Obviously, such an approach must yield markedly higher values than the determination of actual NO concentrations at a given time point. Even more troubling is the fact that these results were compared with the amount of NO released from GTN/Cys determined with a basically different method, i.e. chemiluminescence measurements of the actual NO concentrations after 10 min of incubation. We think that this unjustified comparison of calculated and measured NO concentrations explains the discrepancy, but cannot exclude the contribution of additional factors, e.g. the use of crude as opposed to pure sGC preparations.
Finally, our spectroscopic analysis of GTN/Cys-treated sGC under conditions of sufficient NO availability (≈0.5 μM NO compared with 0.4 μM protein) unambiguously showed formation of the ferrous haem–NO complex. The time-dependent activation of sGC by GTN/Cys was accompanied by transition of the Soret band from 430 to 399 nm, as expected for formation of NO–sGC [30]. The contrasting report published previously [11] is explained by substoichiometric NO availability. The authors recorded their spectra with 0.3 μM (light absorbance experiments) or 10 μM (EPR experiments) sGC in the presence of 1 mM GTN and 2 mM Cys at 10 °C, with the release of 51±6.8 nM NO (n=3) after 10 min under anaerobic conditions (K. Schmidt and B. Mayer, unpublished work). Thus the protein was present at an approx. 6-fold excess over NO in the light absorbance experiments. Such a minor 399 nm band would be hardly detectable alongside the much more pronounced Soret band at 392 nm. The situation is even worse in the EPR experiments, which were obtained with a 200-fold excess of sGC.
It is well established that oxidation of sGC-bound haem impairs NO stimulation of the enzyme due to low NO binding affinity of the ferric oxidation state [17]. Haem oxidation appears to be the mechanism of action of ODQ and related haem-site sGC inhibitors [26,27,31], and it may be the key reaction triggering sGC inactivation by GTN. In accordance with previous studies [32,33], we found that pre-treatment of cultured porcine aortic endothelial cells with 10–300 μM GTN resulted in time- and concentration-dependent loss of NO-stimulated sGC activity, whereas the sensitivity of the enzyme to protoporphyrin IX remained unaffected (K. Schmidt and B. Mayer, unpublished work). The half-time of haem oxidation measured as transition of the Soret band from 431 to 392 nm was approx. 5 min, a value that agrees well with the kinetics of GTN-triggered inactivation of purified sGC (Figure 7B), as well as the time-dependent decrease in sGC activity observed in GTN-treated crude supernatants of bovine coronary arteries [34]. These results confirm previous proposals suggesting that sGC desensitization by GTN is due to oxidation of enzyme-bound haem [32,33]. The decreased NO-sensitivity of sGC exposed to GTN either in vitro or in vivo has led to the speculation that this effect might contribute to nitrate tolerance [34], but this issue is still controversial, and reduced GTN bioactivation may be more relevant for tolerance development [7]. Further studies are needed to clarify whether impaired vascular cGMP accumulation upon prolonged exposure of sGC to GTN contributes to nitrate tolerance in vivo.
The proposed model of GTN/Cys action, involving an activation/inactivation equilibrium controlled by NO release and haem oxidation respectively, necessarily implies that increasing the apparent NO-binding affinity of sGC by YC-1 or related NO sensitizers will result in increased maximal enzyme activity due to decreased rates of haem oxidation at the lower GTN concentrations needed for sGC activation. Thus our model provides a simple explanation for the effect of YC-1 on maximal activity of GTN/Cys-stimulated sGC.
In the presence of YC-1 and Cys, GTN exhibited a fairly high potency (EC50≈1 μM), reviving Ignarro and Gruetter's early proposal that a non-enzymatic reaction with thiols may contribute to vascular bioactivation of GTN [35]. This proposal has received less attention in recent years, because there seems to be a striking discrepancy between the high (nanomolar) potency of GTN to relax blood vessels and the 10–100 μM concentrations needed to activate sGC in vitro. However, recent data from our laboratory [36] showed that the EC50 value needed of GTN to dilate rat aortic rings is at least ≈100-fold lower than the concentration required for cGMP accumulation measured in the same tissue samples (EC50 values of 0.1 and >10 μM respectively), suggesting that maximal relaxation requires only a relatively small increment in cGMP. With respect to the potentiating effect of YC-1, one might speculate about the occurrence of an endogenous analogue. Pronounced accumulation of tissue cGMP in response to CO, which does not considerably activate purified sGC in the absence of YC-1 [12,37,38], suggests that sGC may be sensitized to haem ligands by an endogenous factor with YC-1-like properties. If so, the non-enzymatic reaction could play a significant role in GTN bioactivation in vivo.
Another issue questioning the in vivo relevance of non-enzymatic GTN bioactivation is the specificity of the reaction for Cys, a thiol that, unlike GSH, is not abundantly available in a free form in cells. Certainly, there is no evidence for a cellular compartment containing millimolar concentrations of free Cys as required to boost significant NO release from GTN. However, it cannot be excluded that another, as-yet-unidentified SH compound present in cells reacts with GTN to yield NO as an intermediate. Interestingly, enzymatic GTN bioactivation catalysed by mitochondrial aldehyde dehydrogenase raises a similar problem. The reaction requires the presence of a thiol for regeneration of the oxidized enzyme, but neither GSH nor Cys can perform this role [39]. In both cases, identification of the putative cofactor appears to be an interesting task for future studies.
In summary, our functional, analytical and spectroscopic data unambiguously show that stimulation of sGC by GTN/Cys occurs via formation of free NO as an active intermediate. In addition, our results indicate that sGC activation by GTN is counteracted by Cys-independent haem oxidation. This activation/inactivation equilibrium explains both the only partial activation of the enzyme by GTN/Cys upon prolonged incubation and the increase in maximal activity caused by NO-sensitizing drugs such as YC-1. Further studies are necessary to clarify the mechanism of the GTN/Cys reaction and to explore the contribution of non-enzymatic NO release to GTN bioactivation.
Acknowledgments
The excellent technical assistance of B. Heiling is gratefully acknowledged. This work was supported by the Fonds zur Förderung der Wissenschaftlichen Forschung in Austria (to B. M. and K. S.) and the Deutsche Forschungsgemeinschaft (to D. K.).
References
- 1.Friebe A., Koesling D. Regulation of nitric oxide-sensitive soluble guanylyl cyclase. Circ. Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- 2.Krumenacker J. S., Hanafy K. A., Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain. Res. Bull. 2004;15:505–515. doi: 10.1016/S0361-9230(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 3.Denninger J. W., Marletta M. A. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim. Biophys. Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 4.Gewaltig M., Kojda G. Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc. Res. 2002;55:250–260. doi: 10.1016/s0008-6363(02)00327-9. [DOI] [PubMed] [Google Scholar]
- 5.Marsh N., Marsh A. A short history of nitroglycerin and nitric oxide in pharmacology and physiology. Clin. Exp. Pharmacol. Physiol. 2000;27:313–319. doi: 10.1046/j.1440-1681.2000.03240.x. [DOI] [PubMed] [Google Scholar]
- 6.Katsuki S., Arnold W. P., Mittal C. K., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J. Cyclic Nucleotide Res. 1977;3:23–35. [PubMed] [Google Scholar]
- 7.Mayer B. Bioactivation of nitroglycerin – a new piece in the puzzle. Angew. Chem. Int. Edit. 2003;42:388–391. doi: 10.1002/anie.200390124. [DOI] [PubMed] [Google Scholar]
- 8.Fung H. L. Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Annu. Rev. Pharmacol. Toxicol. 2004;44:67–85. doi: 10.1146/annurev.pharmtox.44.101802.121646. [DOI] [PubMed] [Google Scholar]
- 9.Noack E., Feelisch M. Molecular mechanisms of nitrovasodilator bioactivation. Basic Res. Cardiol. 1991;86(suppl. 2):37–50. doi: 10.1007/978-3-642-72461-9_5. [DOI] [PubMed] [Google Scholar]
- 10.Artz J. D., Toader V., Zavorin S. I., Bennett B. M., Thatcher G. R. J. In vitro activation of soluble guanylyl cyclase and nitric oxide release: a comparison of NO donors and NO mimetics. Biochemistry. 2001;40:9256–9264. doi: 10.1021/bi002885x. [DOI] [PubMed] [Google Scholar]
- 11.Artz J. D., Schmidt B., McCracken J. L., Marletta M. A. Effects of nitroglycerin on soluble guanylate cyclase – implications for nitrate tolerance. J. Biol. Chem. 2002;277:18253–18256. doi: 10.1074/jbc.C200170200. [DOI] [PubMed] [Google Scholar]
- 12.Friebe A., Schultz G., Koesling D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- 13.Humbert P., Niroomand F., Fischer G., Mayer B., Koesling D., Hinsch K., Gausepohl H., Frank R., Schultz G., Böhme E. Purification of soluble guanylyl cyclase from bovine lung by a new immunoaffinity chromatographic method. Eur. J. Biochem. 1990;190:273–278. doi: 10.1111/j.1432-1033.1990.tb15572.x. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt K., Klatt P., Mayer B. Reaction of peroxynitrite with oxyhaemoglobin: interference with photometrical determination of nitric oxide. Biochem. J. 1994;301:645–647. doi: 10.1042/bj3010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz G., Böhme E. Guanylate cyclase. GTP pyrophosphate-lyase (cyclizing), E.C.4.6.1.2. In: Bergmeyer H. U., Bergmeyer J., Graßl M., editors. Methods of Enzymatic Analysis. Germany: Verlag Chemie, Weinheim; 1984. pp. 379–389. [Google Scholar]
- 16.Gorren A. C. F., Schmidt K., Mayer B. Binding of L-arginine and imidazole suggests heterogeneity of rat brain neuronal nitric oxide synthase. Biochemistry. 2002;41:7819–7829. doi: 10.1021/bi025675o. [DOI] [PubMed] [Google Scholar]
- 17.Cooper C. E. Nitric oxide and iron proteins. Biochim. Biophys. Acta. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 18.Gryglewski R. J., Palmer R. M. J., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature (London) 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 19.Palmer R. M. J., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature (London) 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 20.Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckman J. S., Koppenol W. H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am. J. Physiol. 1996;40:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 22.Roubaud V., Sankarapandi S., Kuppusamy P., Tordo P., Zweier J. L. Quantitative measurement of superoxide generation using the spin trap 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide. Anal. Biochem. 1997;247:404–411. doi: 10.1006/abio.1997.2067. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer S., Schmidt K., Mayer B. Dityrosine formation outcompetes tyrosine nitration at low steady-state concentrations of peroxynitrite – implications for tyrosine modification by nitric oxide/superoxide in vivo. J. Biol. Chem. 2000;275:6346–6352. doi: 10.1074/jbc.275.9.6346. [DOI] [PubMed] [Google Scholar]
- 24.Schrammel A., Gorren A. C. F., Schmidt K., Pfeiffer S., Mayer B. S-nitrosation of glutathione by nitric oxide, peroxynitrite, and •NO/O2•−. Free Radical Biol. Med. 2003;34:1078–1088. doi: 10.1016/s0891-5849(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 25.Garthwaite J., Southam E., Boulton C. L., Nielsen E. B., Schmidt K., Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 26.Schrammel A., Behrends S., Schmidt K., Koesling D., Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- 27.Zhao Y. D., Brandish P. E., DiValentin M., Schelvis J. P. M., Babcock G. T., Marletta M. A. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry. 2000;39:10848–10854. doi: 10.1021/bi9929296. [DOI] [PubMed] [Google Scholar]
- 28.Bellamy T. C., Griffiths C., Garthwaite J. Differential sensitivity of guanylyl cyclase and mitochondrial respiration to nitric oxide measured using clamped concentrations. J. Biol. Chem. 2002;277:31801–31807. doi: 10.1074/jbc.M205936200. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths C., Wykes V., Bellamy T. C., Garthwaite J. A new and simple method for delivering clamped nitric oxide concentrations in the physiological range: application to activation of guanylyl cyclase-coupled nitric oxide receptors. Mol. Pharmacol. 2003;64:1349–1356. doi: 10.1124/mol.64.6.1349. [DOI] [PubMed] [Google Scholar]
- 30.Gerzer R., Böhme E., Hofmann F., Schultz G. Soluble guanylate cyclase purified from bovine lung contains heme and copper. FEBS Lett. 1981;132:71–74. doi: 10.1016/0014-5793(81)80429-2. [DOI] [PubMed] [Google Scholar]
- 31.Olesen S. P., Drejer J., Axelsson O., Moldt P., Bang L., Nielsen-Kudsk J. E., Busse R., Mülsch A. Characterization of NS 2028 as a specific inhibitor of soluble guanylyl cyclase. Br. J. Pharmacol. 1998;123:299–309. doi: 10.1038/sj.bjp.0701603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schröder H., Leitman D. C., Bennett B. M., Waldman S. A., Murad F. Glyceryl trinitrate-induced desensitization of guanylate cyclase in cultured rat lung fibroblasts. J. Pharmacol. Exp. Ther. 1988;245:413–418. [PubMed] [Google Scholar]
- 33.Waldman S. A., Rapoport R. M., Ginsburg R., Murad F. Desensitization to nitroglycerin in vascular smooth muscle from rat and human. Biochem. Pharmacol. 1986;35:3525–3531. doi: 10.1016/0006-2952(86)90622-2. [DOI] [PubMed] [Google Scholar]
- 34.Romanin C., Kukovetz W. R. Tolerance to nitroglycerin is caused by reduced guanylate cyclase activation. J. Mol. Cell. Cardiol. 1989;21:41–48. doi: 10.1016/0022-2828(89)91491-0. [DOI] [PubMed] [Google Scholar]
- 35.Ignarro L. J., Gruetter C. A. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite: possible involvement of S-nitrosothiols. Biochim. Biophys. Acta. 1980;631:221–231. doi: 10.1016/0304-4165(80)90297-4. [DOI] [PubMed] [Google Scholar]
- 36.Kollau A., Hofer A., Russwurm M., Koesling D., Keung W. M., Schmidt K., Brunner F., Mayer B. Contribution of aldehyde dehydrogenase to mitochondrial bioactivation of nitroglycerin. Evidence for activation of purified soluble guanylyl cyclase via direct formation of nitric oxide. Biochem. J. 2005;385:769–777. doi: 10.1042/BJ20041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burstyn J. N., Yu A. E., Dierks E. A., Hawkins B. K., Dawson J. H. Studies of the heme coordination and ligand binding properties of soluble guanylyl cyclase (sGC): Characterization of Fe(II)sGC and Fe(II)sGC(CO) by electronic absorption and magnetic circular dichroism spectroscopies and failure of CO to activate the enzyme. Biochemistry. 1995;34:5896–5903. doi: 10.1021/bi00017a019. [DOI] [PubMed] [Google Scholar]
- 38.Stone J. R., Marletta M. A. The ferrous heme of soluble guanylate cyclase: formation of hexacoordinate complexes with carbon monoxide and nitrosomethane. Biochemistry. 1995;34:16397–16403. doi: 10.1021/bi00050a021. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z. Q., Zhang J., Stamler J. S. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]