Abstract
Evidence linking Chlamydia pneumoniae infection to atherosclerosis and to atherothrombotic events has recently emerged. A primary candidate implicated in these pathogenetic events is the 60-kDa chlamydial heat shock protein (HSP60). Another putative candidate to activate a potential proinflammatory mechanism is the chlamydial outer membrane protein 2 (OMP2). We have generated both HSP60 and OMP2 recombinant antigens in a nondenatured form and shown that (i) the two antigens were highly immunogenic in mice and (ii) murine antisera thus generated recognized the native C. pneumoniae proteins. We measured by enzyme linked immunosorbent assay (ELISA) and immunoblot assay antibody titers to the recombinant antigens in samples from 219 patients with coronary heart disease (CHD), 179 patients with unstable angina (UA), 40 patients with acute myocardial infarction (AMI), and 100 age-, sex-, and risk factor-matched healthy controls. We also examined whether anti-HSP60 and/or anti-OMP2 antibodies correlated with anti-C. pneumoniae antibodies assessed by a commercial microimmunofluorescence (MIF) assay. Immunoglobulin G (IgG), but neither IgA nor IgM, antibodies against the two recombinant proteins were detected by ELISA. In particular, anti-HSP60 antibodies were detected in >99% of CHD patients versus 0% of the controls, whereas the proportions of anti-OMP2 positive subjects were >70 and 27%, respectively. Nonetheless, among CHD patients, similar frequencies of positive subjects and titers of anti-HSP60 or anti-OMP2 antibodies were present in UA and AMI subjects. The anti-OMP2, but not the anti-HSP60, antibodies showed high specificity. Consistently, high serological correlation was observed between IgG MIF titers and IgG ELISA reactivity to OMP2 but not to HSP60. Overall, the results of this study demonstrate a strong correlation between CHD and anti-HSP60 IgG levels, as measured by our in-house ELISA. They also suggest that recombinant OMP2 ELISA, because of its high specificity and strong correlation with MIF assay, could be a candidate diagnostic marker for C. pneumoniae infection, which would be of potential usefulness for its specificity and nonsubjective nature.
Coronary heart disease (CHD) and atherosclerotic and atherothrombotic cardiac, cerebrovascular, and peripheral vascular disease, is a major cause of death and disability in industrialized countries. Atherosclerosis is generally asymptomatic in the early stages, but progressive plaque development leads to arterial stenosis, atherosclerosis, and ischemic clinical manifestation. A systemically detected inflammatory component commonly appears in unstable angina (UA) and is correlated with development of acute myocardial infarction (AMI) and cardiac death (31).
Recently, there has been renewed interest in the possibility that one or more CHD pathologies might be associated with chronic infection by Chlamydia pneumoniae, a common respiratory pathogen. The evidence for a link between C. pneumoniae and CHD is much stronger than for any other infectious organism, but the specific C. pneumoniae traits implicated in this association are still poorly defined. Inflammation in response to antigenic or nonantigenic stimulation of local or systemic reactivity by the host is commonly thought of as a putative mechanism of disease (11).
In this context, several findings indicate that the chlamydial heat shock proteins (HSPs), in particular the 60-kDa HSP (HSP60), may represent a particular antigenic stimulus capable of eliciting strong humoral and cell-mediated immune responses with immunopathological sequelae of chronic chlamydial infections (19). In chronic Chlamydia trachomatis infection, expression of the chlamydial HSP60 was observed, suggesting the involvement of this stress response bacterial protein in the induction and exacerbation of the immunopathological disorder following persistent antigenic stimulation (3). Reaction to C. trachomatis HSP60 has therefore been suggested as a predictive serological marker for the development of chronic sequelae leading to tubal occlusion and secondary infertility (12).
An additional antigen candidate to activate a potential proinflammatory mechanism is outer membrane protein 2 (OMP2). This is a developmentally regulated, cysteine-rich protein and a major component of the chlamydial cell wall. This structural protein is expressed late in the growth cycle, being prevalent in the extracellular infectious elementary body. Although OMP2 is poorly surface accessible to antibody binding in intact cells (41), pronounced antibody responses to OMP2 following both C. trachomatis and C. pneumoniae infections have been reported (30, 39). Genus- and species-specific B- and T-cell epitopes have been identified in OMP2 (1, 41). A peptide conserved between murine and human α-myosin heavy chains that has sequence homology to OMP2 was shown to induce autoimmune inflammatory heart disease in mice. Injection of the homologous chlamydial peptide into mice also induced perivascular inflammation, fibrotic changes, and blood vessel occlusion in the heart, as well as triggering T- and B-cell reactivity to the homologous heart muscle-specific peptide (2). Recent studies demonstrated that T-cell lines from atheromatous plaques, as well as peripheral blood mononuclear cells of patients with angina, were able to respond to OMP2 (8, 13).
Because of the potential role of the two antigens mentioned above as targets of humoral and/or cellular anti-Chlamydia immune responses by the infected host, we used purified recombinant C. pneumoniae HSP60 (R-HSP60) and R-OMP2 proteins for the assessment of antibody titers to these antigens in CHD patients and in healthy individuals. In particular, the contribution of anti-R-HSP60 and anti-R-OMP2 serum antibodies to the humoral response to C. pneumoniae in patients with UA or with AMI was studied, in order to evaluate a possible association between the seroreactivity to these antigens and the severity of the cardiac events. A second aim of the study was a preliminary assessment of the potential of individual recombinant antigens (R-antigens) to perform as a valid supplement to the standard microimmunofluorescence (MIF) test for the detection of antibodies directed to C. pneumoniae.
MATERIALS AND METHODS
Propagation of C. pneumoniae.
C. pneumoniae strain Parola (kindly provided by P. Saikku, KTL, Oulu, Finland) was propagated in Hep-2 cells (American Type Culture Collection, Manassas, Va.). Hep-2 cells were routinely grown in 25-cm2 flasks with Eagle’s minimal essential medium (MEM) (Gibco Life Technologies, Grand Island, N.Y.) supplemented with 1.25% fetal calf serum and 1 μg of cycloheximide per ml. Confluent cell monolayers were infected with C. pneumoniae elementary bodies suspended in inoculation medium (MEM plus 4 mg of glucose per ml [pH 7.5]) at a multiplicity of infection of 102 infective units per cell. Infection was achieved by centrifugation at 800 × g for 1 h at 37°C. After an additional 30-min incubation at 37°C, fresh growth medium (inoculation medium plus 1 μg of cycloheximide per ml) was added, and infected cultures were incubated for 72 h at 37°C under 5% CO2. C. pneumoniae was harvested by disruption of monolayers with sterile glass beads and low-speed centrifugation at 200 × g. Aliquots of C. pneumoniae were titrated and stored at −70°C in inoculation medium until used.
Gene cloning.
The groEL and omp2 coding sequences were amplified by PCR using as a template total DNA extracted from Hep-2 cells infected with C. pneumoniae strain Parola. To make possible directional cloning of the amplicon, the sense and the antisense oligonucleotide primers were designed with suitable restriction sites at the level of the ATG start codon (SmaI and BamHI) and downstream of the stop codon (PstI and SphI), as shown in Table 1. PCR products were initially cloned into pGEM-T Easy vector (Promega, Madison, Wis.) and checked for their correct reading frame by sequencing. The cloned inserts were then excised by digestion with suitable enzymes (SmaI-PstI for groEL and BamHI-SphI for omp2) and ligated to the compatible sites of the expression vector pQE30 (Qiagen Inc., Chatsworth, Calif.) in frame with a His6 affinity tag-coding sequence at the 5′ terminus. The resulting plasmids were introduced into Escherichia coli M15 carrying the repressor plasmid pREP4 (Qiagen Inc.) (35). E. coli M15 transformants were selected and routinely grown in the presence of 100 μg of ampicillin per ml and 25 μg of kanamycin per ml.
TABLE 1.
Oligonucleotide primers for PCR amplificationa
| Gene | Sense primer | Antisense primer |
|---|---|---|
| groEL | 5′-CAACCCCGGGAGCGAAAAATATTAAATATA-3′ | 5′-GCTTGACGTCTAGTAGTCCATTCCTCGGC-3′ |
| 5′-CAACGATGGCAGCGAAAAATATTAAATATA-3′ | 5′-GCTTAAGAGACTAGTAGTCCATTCCTCGGC-3′ | |
| omp2 | 5′-GATCGGATCCTCCAAACTCATGAGACGAGT-3′ | 5′-CCTGCATGCTTAATACACGTGGGTATTTTC-3′ |
| 5′-GATCCCTATGTCCAAACTCATGAGACGAGT-3′ | 5′-CCTTAGAATTTAATACACGTGGGTATTTTC-3′ |
For each gene, the nucleotides in boldface are restriction sites within the degenerate primers (upper sequences) used for directional cloning in the pQE30 vector: groEL sense, SmaI; groEL antisense, PstI; omp2 sense, BamHI; omp2 antisense, SphI. The underlined nucleotides represent translational start codons (sense primers) and stop codons (antisense primers) in the wild-type gene (lower sequences).
Production of R-proteins.
For the expression of recombinant proteins (R-proteins), bacteria were grown exponentially in Luria broth at 37°C and induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 2.5 h. R-proteins carrying the His6 tag at their N terminis were purified under native conditions by nickel-nitrilotriacetic acid-agarose (Ni-NTA) affinity chromatography according to a standard protocol (QIAexpressionist manual; Qiagen Inc.). Briefly, cells from 500-ml cultures were harvested by centrifugation (6,000 × g, 4°C), resuspended in 10 ml of ice-cold lysis buffer (50 mM Tris-HCl [pH 8], 200 mM NaCl, 5 mM imidazole), and sonicated on ice. After centrifugation, the supernatant was supplemented with 2 ml of Ni-NTA resin and gently mixed at 4°C for 1 h. The lysate-Ni-NTA mixture was loaded into a 15-ml column and washed with 50 mM Tris-HCl [pH 8]-200 mM NaCl-25 mM imidazole. The fusion proteins were eluted from the column by applying a stepwise imidazole gradient (50 to 200 mM). Aliquots of the eluted fractions were diluted in gel loading buffer (250 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% 2-mercaptoethanol, 20% glycerol), heated at 65°C for 5 min, and analyzed by 0.1% SDS-12% polyacrylamide gel electrophoresis (SDS-PAGE). Electrophoresis was carried out at 10 V/cm in Tris-glycine buffer (25 mM Tris-HCl [pH 8.3], 192 mM glycine, 1% SDS). Homogeneous fractions containing >99% pure R-proteins were pooled, extensively dialyzed at 4°C in 10 mM phosphate-buffered saline (PBS) (pH 7.0), filtered through a 0.45-μm-pore-size membrane, and stored at −70°C until used. The protein concentration was determined by the method of Bradford (7).
Expression analysis of R-proteins and Western blot assays.
For R-protein expression analysis, bacterial lysates were obtained by heating cells in gel loading buffer for 5 min at 100°C. After SDS-PAGE, the gels were electroblotted onto a nitrocellulose membrane (Hybond-C extra; Amersham), using a semidry apparatus (Hoefer Scientific Instruments). The membrane was saturated with 1% bovine serum albumin in TBS-T buffer (125 mM Tris base, 300 mM NaCl, 0.1% Tween 20 [pH 8.0]) overnight at room temperature. Blots were probed with a commercial antibody recognizing the His6 tag epitope (Qiagen Inc.).
Human sera and MIF assay.
The characteristics of the study population are given in Table 2. A total of 219 sera from CHD patients were obtained from the Institute of Cardiology, Catholic University of Rome. Patients were divided into two groups according to the disease status: 179 presented with UA, and 40 presented with AMI. Serum specimens were collected within 6 and 24 h from the acute attacks of AMI and UA, respectively. One hundred control sera from sex- and age-matched patients without CHD were also included in the study. Conventional risk factors for atherosclerosis, namely, hyperlipidemia, hypertension, C-reactive protein, diabetes, or smoking, were recorded (Table 2). Patient and control sera were examined for anti-C. pneumoniae antibodies with a commercial MIF assay (Labsystems Oy, Helsinki, Finland), according to the manufacturer’s instructions. Immunoglobulin M (IgM) titers of >16 and IgG or IgA titers of ≥32 were regarded as positive. All sera were tested twice by two independent experienced investigators, who were blinded for the sample source (control, UA, or AMI), with high (>90%) concordance.
TABLE 2.
Clinical characteristics of the study population
| Characteristic | No. (%) of individuals with characteristic
|
|
|---|---|---|
| CHD patients | Healthy controls | |
| Sex | ||
| Male | 158 (72) | 76 (76) |
| Female | 61 (28) | 24 (24) |
| Age (yr) | ||
| 33–45 | 18 (8) | 15 (15) |
| 46–60 | 68 (31) | 39 (38) |
| 61–75 | 108 (50) | 47 (47) |
| >75 | 25 (11) | 0 (0) |
| UA | 179 (82) | 0 (0) |
| AMI | 40 (18) | 0 (0) |
| Family history of ischemic heart disease | 93 (43) | NDa |
| Hypertension | 105 (48) | 28 (28) |
| Diabetes | 35 (16) | 0 (0) |
| Smoker | 120 (55) | 59 (59) |
| Dislipidemia | 85 (39) | ND |
| C-reactive protein level of >3 mg/liter | 130 (60) | ND |
ND, not determined.
ELISA.
Indirect enzyme-linked immunosorbent assays (ELISAs) for the detection of IgM, IgG, and IgA antibodies were performed in 96-well polystyrene plates (Greiner Labortechnik) coated with a predetermined optimal quantity (100 ng/well) of the R-proteins. Before the ELISA was performed, a mouse monoclonal antibody recognizing the His6 tag of the antigens and pooled mouse hyperimmune sera raised against each R-protein were used to determine the optimal coating concentrations. Coating was performed overnight at 4°C in 50 mM NaHCO3 buffer, pH 9.6. Plates were washed three times with W1 buffer (PBS-0.1% Tween 20) and blocked with 3% ovalbumin (grade V) in W2 buffer (PBS-0.15% Tween 20) for 90 min at 37°C. Plates were washed three times with W1 buffer and incubated with a 1:50 dilution of the patient sera in W3 buffer (PBS-0.05% Tween 20-0.1% ovalbumin) for 1 h a 37°C. Plates were washed three times with W1 buffer and incubated with alkaline phosphatase-conjugated goat anti-human IgM, IgG, or IgA antibodies (Sigma Immuno Chemical, Sigma Chemical Co., St. Louis, Mo.) for 1 h at 37°C. Secondary antibodies were diluted 1:10,000 in W3 buffer. After three additional washings with W1 buffer, the substrate p-nitrophenylphosphate (SigmaFAST tablets; Sigma Chemical Co.) was added, and color was left to develop for 30 min at 37°C prior to addition of NaOH at a final concentration of 1 N. The absorbance at 405 nm (A405) of each sample was read. Each sample was assayed twice in R-protein-coated wells and in uncoated wells as the control. Cutoff values for seropositivity in ELISAs with R-proteins were defined according to previously reported criteria (4, 36). A positive sample was defined as one yielding an A405 value that was at least 2 standard deviations (SDs) above the mean value obtained with a panel of 30 randomly selected MIF-negative sera from CHD patients, that is, an A405 of >0.4 for R-HSP60 and an A405 of >0.28 for R-OMP2.
Reproducibility was assessed with 30 randomly selected samples which were tested twice at a 1-week interval by two independent staff members using the same laboratory equipment and reagents. The reproducibility values were calculated using the coefficient of variation (CV) according to the formula CV = SD/(21/2x), where the SD is that between the two determinations and x is the mean value obtained from all samples.
Immunoblot analysis with human sera.
To confirm the ELISA specificity, Western blot analyses were performed with randomly selected MIF-positive and -negative human sera using the R-proteins as antigens. To separate R-HSP60 from R-OMP (both proteins are approximately 60 kDa) in the same SDS-PAGE run, individual R-proteins (1 μg each) were sequentially loaded into the same well at 15-min intervals. Proteins were electroblotted onto nitrocellulose filters which were then cut into 5-mm strips. The nitrocellulose strips were presaturated with bovine serum albumin and incubated for 2 h with human sera diluted 1:100 in TBS-T buffer. Bound antibodies were visualized with alkaline phosphatase-conjugated anti-human IgG (Sigma Immuno Chemical, Sigma Chemical Co.) and p-nitrophenylphosphate as the substrate.
Mouse immunization and immunogenicity assay.
Purified R-proteins were mixed with complete Freund’s adjuvant (Sigma Chemical Co.) and used to immunize CD1 mice (10 animals, 10 μg of protein each). At weekly intervals, three additional boosts of purified R-proteins (10 μg) in incomplete Freund’s adjuvant (Sigma Chemical Co.) were given to each animal. After an additional 2 weeks the animals were bled, and approximately 0.7 ml of polyclonal antiserum was obtained from each animal and stored at −20°C. For immunodetection of R-proteins, blots were probed with the mouse polyclonal antisera at 1:20,000 in TBS-T buffer. An anti-mouse IgG preparation conjugated to alkaline phosphatase (Sigma Chemical Co.) was used as a secondary antibody at a concentration of 1:7,500. Antibody titers were measured by ELISA. Polystyrene microtiter plates were coated overnight at 4°C with 100 ng of R-proteins as described above. Sera from immunized animals were serially twofold diluted in W3 buffer, and 100 μl of each dilution was incubated with the immobilized R-proteins at 37°C for 1 h. Twofold-diluted sera from mice before immunization were used as negative controls. After three washes with W1 buffer, alkaline phosphatase-conjugated rabbit anti-mouse IgG (1:20,000 in PBS; Sigma Chemical Co.) was added, and the plates were incubated for 1 h at 37°C. The reaction was developed with p-nitrophenylphosphate as the substrate. Titers were defined as the highest dilution of the mouse serum yielding an A405 reading of at least twice the reading of the negative control, i.e., >0.26 (the sera of the nonimmunized animals gave A405 readings of 0.12 to 0.13).
Statistical analysis.
Statistically significant differences between groups of data were calculated by the two-tailed χ2 test. Statistical significance was set at a P value of <0.05.
RESULTS
Cloning, purification, and immunogenicity of R-HSP60 and R-OMP2.
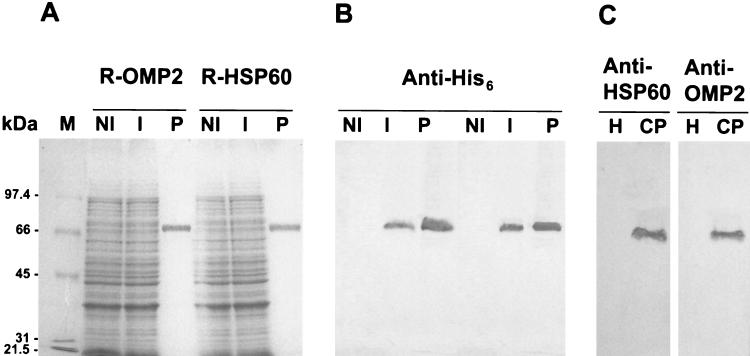
The groEL and omp2 coding sequences were amplified by PCR and cloned in frame with a His6-coding tag at the 5′ terminus under the control of the IPTG-inducible PT5-lacO promoter-operator element of the expression vector pQE30. The resulting plasmids were introduced into E. coli M15(pREP4) and used for expression of the R-proteins following IPTG induction, as outlined in Materials and Methods. The expression level was low for both R-proteins, as no difference was observed between the SDS-PAGE profiles of uninduced and induced bacterial lysates (compare lanes NI with lanes I in Fig. 1A). However, both R-OMP2 and R-HSP60 were detected as a single immunoreactive species in the soluble fraction of induced lysates by Western blot analysis with a commercial antibody recognizing the His6 tag epitope (Fig. 1B, lanes NI and I). Since there was no evidence of precipitation of R-proteins within inclusion bodies, purification was performed by nickel affinity chromatography under native (nondenaturing) conditions, using a stepwise imidazole gradient. The peak eluting at 150 mM imidazole contained >99% pure R-proteins, as revealed by SDS-PAGE and immunoblot analysis of pooled fractions of the eluate (Fig. 1A and B, lanes P).
FIG. 1.
Expression, purification, and Western blot analysis of C. pneumoniae HSP60 and OMP2 proteins. (A) SDS-PAGE analysis of whole-cell lysates of E. coli M15(pREP4) expressing the His6-tagged R-HSP60 and R-OMP2 proteins cloned in pQE30 and of the purified protein products. Bacterial cells were grown at 37°C in Luria broth and induced (lanes I) or not (lanes NI) with IPTG. R-HSP60 and R-OMP2 were purified under native conditions from the soluble cell fraction by Ni-NTA affinity chromatography (lanes P). (B) Western blot analysis, with an anti-His6 mouse monoclonal IgG antibody, of the purified R-HSP60 and R-OMP2 protein products, corresponding to lanes P of panel A. (C) Immunoblot analysis of total lysates of Hep-2 cells infected (lanes CP) or not (lanes H) with C. pneumoniae. The lysates were subjected to SDS-PAGE and immunoblot analysis with mouse polyclonal antisera raised against R-HSP60 and R-OMP2, as indicated. Molecular mass protein standards (lane M) are shown on the left of the Coomassie blue-stained gel.
The R-proteins were dialyzed to equilibrium in PBS at pH 7.0 and used to immunize CD1 mice. Western blot analysis with the anti-R-OMP2 and anti-R-HSP60 mouse antisera (1:20,000) made it possible to detect IPTG-inducible proteins of approximately 60 kDa in cell extracts of E. coli M15(pREP4) carrying the cloned omp2 and groEL genes, consistent with the predicted molecular masses of the corresponding protein products. The immunodetected proteins had electrophoretic mobilities apparently identical to those of proteins recognized by the anti-His6 antibody (data not shown), providing evidence of the authenticity of the recombinant omp2 and groEL gene products. Mice immunized with either R-HSP60 or R-OMP2 produced elevated IgG levels, with the expected specificity. The anti-R-OMP2 IgG titer of the pooled sera from 10 animals was 1:320,000 (A405 = 0.28), while the anti-R-HSP60 antibody titer was 1:180,000 (A405 = 0.27). Minor variations in the IgG response were observed between animals. Neither IgM nor IgA antibodies against R-OMP2 and R-HSP60 were found in the mouse sera at the end of the immunization protocol. The mouse hyperimmune sera raised to R-OMP2 and R-HSP60 recognized with good specificity the 60-kDa antigens from supernatants of C. pneumoniae-infected Hep-2 cells (Fig. 1C), thereby confirming that immunization with R-OMP2 and R-HSP60 induces a specific IgG response to the wild-type C. pneumoniae proteins.
MIF assay for C. pneumoniae antibody titers in CHD patients and in healthy controls.
Antibodies directed to whole C. pneumoniae antigen were assessed by MIF in serum specimens of all subjects with CHD and in control sera from 100 healthy individuals (without CHD) matched for age, sex, and some risk factors. Sera from CHD patients were divided into two subgroups according to the disease status (UA and AMI). For each antibody class, the MIF titer endpoint used as a cutoff value for seropositivity was that recommended by the manufacturer. Specific IgM antibodies were not detected. As shown in Table 3 for IgG antibodies, MIF titers were more elevated in patients with CHD than in controls without CHD. Notably, about half of the CHD patient sera (48% of UA and 45% of AMI) and none of the control sera showed IgG MIF titers of ≥128. Furthermore, IgA antibodies were detected by MIF in 47 and 37% of UA and AMI subjects, respectively, and in none of the controls (data not shown). However, there was no statistically significant difference in the level of serum IgG or IgA antibodies to C. pneumoniae between UA and AMI patients (Table 3 and data not shown).
TABLE 3.
Results of MIF assay for detection of anti-C. pneumoniae IgG in serum specimens of CHD patients and healthy controls
| MIF IgG titer | No. (%) of subjects
|
|||
|---|---|---|---|---|
| Control (n = 100) | CHD (n = 219)a | UA (n = 179) | AMI (n = 40) | |
| ≤32 | 70 (70) | 70 (32)* | 56 (31) | 14 (35) |
| 32 | 23 (23) | 24 (11)* | 20 (11) | 4 (10) |
| 64 | 7 (7) | 22 (10) | 18 (10) | 4 (10) |
| 128 | 0 (0) | 42 (19)* | 36 (20) | 6 (15) |
| 256 | 0 (0) | 30 (14)* | 23 (13) | 7 (17) |
| ≥512 | 0 (0) | 31 (14)* | 26 (15) | 5 (13) |
*, statistically significant difference (P < 0.05 by two-tailed χ2 test) between CHD patients (pooled or individual UA and AMI data) and controls.
Detection of anti-HSP60 and anti-OMP2 antibodies by ELISA with R-proteins.
Antibodies against chlamydial HSP60 and OMP2 in serum samples were sought by ELISA with the R-proteins as antigens. Neither IgM nor IgA antibodies to the R-antigens were detected in the study population (data not shown). However, IgG antibodies to R-OMP2 were consistently detected in both control and CHD sera, but at a significantly higher frequency in the latter than in the former (Table 4). Importantly, none of the control sera but 99.1% of the CHD sera contained IgG antibodies against R-HSP60. In line with the MIF results, no difference was found in the seropositivity to either R-protein between UA and AMI subjects (Tables 3 and 4).
TABLE 4.
ELISA positivity for anti-R-HSP60 and anti-R-OMP2 IgG antibodies in sera of CHD patients and healthy controls
| Subject category | No. positive/total (% positive)a
|
|
|---|---|---|
| Anti-R-HSP60 | Anti-R-OMP2 | |
| Control | 0/100 (0) | 27/100 (27.0) |
| CHD | 217/219 (99.1)* | 156/219 (71.2)* |
| UA | 178/179 (99.5)* | 129/179 (72.0)* |
| AMI | 39/40 (97.5)* | 27/40 (67.5)* |
For positivity definition, see Materials and Methods. *, statistically significant difference (P < 0.05 by two-tailed χ2 test) between CHD patients (pooled or individual UA and AMI data) and controls.
MIF-ELISA correlations.
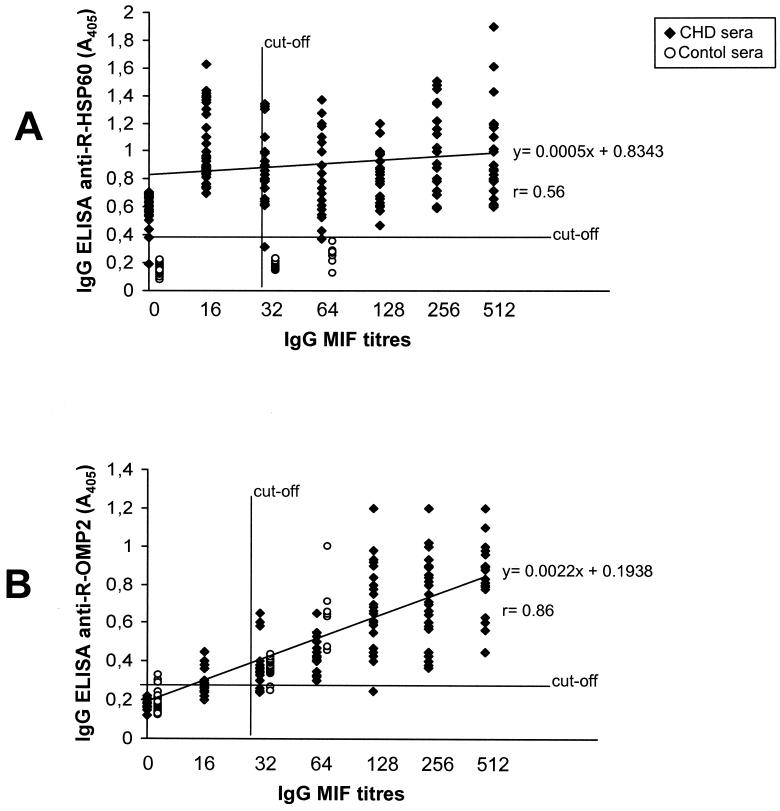
An attempt was made to search for a correlation between MIF and ELISA with the two R-antigens. A rather poor correlation (r = 0.56) was observed between MIF titers and A405 values in the anti-R-HSP60 ELISA, resulting from the large proportion (31%) of MIF-negative sera which gave a positive reaction in the IgG ELISA with R-HSP60 (Fig. 2A). In contrast, a positive correlation (r = 0.86) was observed between the IgG MIF assay and the IgG ELISA with R-OMP2 (Fig. 2B). In addition, all of the IgA-positive sera in the MIF assay were also positive in IgG ELISA against the R-OMP2 antigen.
FIG. 2.
Correlation between IgG seroreactivity to recombinant chlamydial antigens R-HSP60 (A) and R-OMP2 (B) and MIF titers. The assays were conducted with a total of 319 serum specimens, including samples from CHD patients (n = 219) and healthy controls (n = 100). The A405 values are averages from duplicate assays, with SDs of <15% of the mean. A computer-interpolated regression line and the corresponding linear equation with the coefficient of correlation (r) are shown in each panel.
To focus on the discrepancy observed between the MIF data and the ELISA reactivity to the R-antigens, we examined all sera for the presence of anti-C. trachomatis and anti-C. psittaci IgG antibodies (as revealed by seroreactivity with specific control antigens in the commercial C. pneumoniae MIF assay). Cross-reacting antibodies were detected in only 10 serum specimens, which had all tested positive in the C. pneumoniae MIF assay. All of them were reactive to R-HSP60, and eight also recognized R-OMP2.
Specificity and sensitivity of the ELISA with R-HSP60 and R-OMP2.
The results of the ELISAs with the R-proteins were assessed for specificity, sensitivity, reproducibility, and positive and negative predictive values, according to standard definitions and taking the MIF assay as the reference standard. The corresponding values are shown in Table 5. For both R-antigens there was high sensitivity, but only for R-OMP2 was there sufficient specificity for a good diagnostic value. Moreover, the reproducibility for 30 samples analyzed was within the acceptability limit (CV < 0.20) for both R-proteins.
TABLE 5.
Sensitivity, specificity, reroducibility, and positive and negative predictive values for anti-R-HSP60 and anti-R-OMP2 IgG ELISAsa
| R-antigen | Sensitivity (%) | Specificity (%) | CVb | PPV (%)c | NPV (%)d |
|---|---|---|---|---|---|
| R-HSP60 | 98.7 | 3.0 | 0.17 | 68.0 | 50.0 |
| R-OMP2 | 98.7 | 87.0 | 0.13 | 94.0 | 96.8 |
Relative to the IgG MIF assay as a reference standard.
For definition of CV, see Materials and Methods.
PPV, positive predictive value.
NPV, negative predictive value.
Immunoblot analysis of antibodies to C. pneumoniae R-proteins.
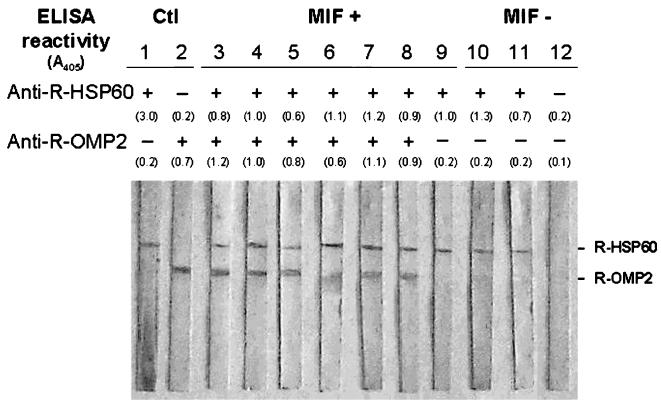
The immune response to chlamydial R-antigens was confirmed by immunoblots with CHD and control sera. The immunoreactivity observed in Western blots fully agreed with the ELISA results obtained with R-OMP2 and R-HSP60 as antigens. An example is provided in Fig. 3, in which representative sera from MIF-positive and -negative individuals were studied in detail. As a rule, recognition of individual R-protein bands was consistently associated with elevated IgG seroreactivity to the corresponding antigen in ELISA, independent of whether or not the subject had CHD. Likewise, sera which had been regarded as negative in the ELISA with either or both R-proteins showed similar behavior when tested by immunoblotting. These results not only confirm the specificity of our ELISA test but also validate the cutoff values defined for IgG seroreactivity in the ELISA with R-protein antigens (see Materials and Methods).
FIG. 3.
Immunoblots for the detection of IgG antibodies to R-HSP60 and R-OMP2 proteins in representative sera from CHD patients. Lanes 1 and 2, positive controls (Ctl) with mouse anti-R-HSP60 and anti-R-OMP2 hyperimmune sera, respectively; lanes 3 to 9, MIF-positive sera; lanes 10 to 12, MIF-negative sera. The IgG ELISA results with R-HSP60 and R-OMP2 antigens are shown at the top, and individual seroreactivity values are reported as A405 readings in parentheses.
DISCUSSION
C. pneumoniae is presently considered a possible cofactor in atherosclerosis and atheroma development, based on various epidemiological, pathological, and clinical studies (17, 21, 23, 28, 32). In particular, persistent infection with C. pneumoniae has been suggested to induce a chronic immune response orchestrated by cytokines, which may result in direct damage to the endothelium, increased synthesis of acute-phase proteins, or changes in the plasma lipid profile. Activation of monocytes may also lead to increased expression of procoagulant factors such as tissue factor, which increase the risk of thrombus formation (16).
C. pneumoniae is a common infectious agent as revealed by seroprevalence studies by the standard MIF assay (22). However, differences in the threshold for seropositivity by MIF have been regarded as the main source of discrepancy in various seroepidemological studies linking C. pneumoniae to atherosclerosis (10, 14, 15, 20).
In this study, we measured levels of serum antibodies against two highly immunogenic, recombinant chlamydial proteins, namely, R-HSP60 and R-OMP2, in patients with CHD and in healthy subjects. We also attempted to correlate these responses to IgM, IgG, and IgA anti-C. pneumoniae antibodies detected by a commercial MIF assay. We set our IgG titer limit for MIF seropositivity at ≥32 and observed that 30% of the healthy subject group were positive for C. pneumoniae, consistent with previously published results for similar study groups from the same geographic area (5, 26). In agreement with a number of previous reports (10, 14, 15, 20, 26, 27, 28, 32), we also found evidence of a significant association between CHD and anti-C. pneumoniae antibodies, as inferred from the standard MIF assay. This association was confirmed here by ELISA measurement of IgG antibodies against R-HSP60 and R-OMP2, although within the CHD group of patients no significant differences were found in MIF or ELISA seroreactivity between UA and AMI subjects.
In particular, our investigation provides compelling evidence for a link between the presence of anti-HSP60 IgG antibodies and CHD, as none out of 100 and 217 out of 219 sera from control and CHD subjects, respectively, contained antibodies to this protein. This finding confirms that an IgG response to HSP60 can be regarded as a prominent serological feature of individuals with CHD, although it cannot define progressive stages of the disease or be used as a specific marker of C. pneumoniae infection, as also shown by the rather large proportion of HSP60-positive, MIF-negative sera of CHD patients.
HSPs have been suggested to play a role in the pathogenesis of both atherosclerosis and chlamydial infections. Amino acid sequence homologies between chlamydial HSP60 and its bacterial and human counterparts have been detected, and antigenic epitopes were shown to cross-react extensively in both bacterial and human homologues (12, 13, 24, 28). On this basis, it was assumed that chronic infection with C. pneumoniae and other bacteria might represent a persistent antigenic stimulus capable of intensifying a generalized response to the HSP60 protein family and, possibly, autoimmunity (42). Thus, immune reactions to HSPs (in particular, production of antibodies and cytokines) have been advocated to link infection to atherosclerosis (25). Interestingly, circulating HSP60 antigen is elevated in subjects with atherosclerosis (42), and CD14 and Toll-like receptor 4 are used as receptors of HSP60, as they are used by lipopolysaccharide, a classical proinflammatory and immunostimulatory agent from all gram-negative bacteria (33). Because of the extensive cross-reactivity within the HSP60/65 protein family, it is logical to expect that some of the anti-HSP60 antibody detected in this study was not elicited by C. pneumoniae HSP60. The poor correlation between MIF and HSP60 ELISA indirectly strengthens this suggestion. Studies are now in progress to assess the reactivity of our sera with HSP60 from other sources, as well as to dissect HSP60 epitope sequences more specific for C. pneumoniae.
Antibodies to chlamydial HSP60 and other chlamydial proteins were previously shown to be nondiscriminatory between seropositive patients with and without CHD (18). This discrepancy was previously attributed to the absence of conformational epitopes in a denatured HSP60 preparation (18). Interestingly, our R-HSP60 was generated under native conditions, and a clear correlation was found between anti-HSP60 antibody and CHD. Yet, no correlation was found here between MIF titers and anti-HSP60 antibodies. Compared with the MIF assay, the R-HSP60 ELISA showed high sensitivity but very low specificity, probably resulting, as mentioned above, from the extensive cross-reactivity among HSP60 homologues from prokaryotic and eukaryotic sources. In conclusion, for all of the reasons discussed above, seropositivity to HSP60 cannot be a marker of C. pneumoniae infection even when purified, conformation-preserved HSP60 of this organism is used as the antigen.
In contrast to the results of Christiansen et al. (9) we found that OMP2 is a primary target of the IgG-mediated humoral response to C. pneumoniae. Our results with this antigen appear to be of major significance inasmuch as antibody titers to R-OMP2 were highly concordant with results of MIF serology, different from the case for the R-HSP60-based ELISA. This was confirmed by immunoblot assay in which R-OMP2 was exclusively recognized by MIF-positive sera, while R-HSP60 was also recognized by a minor proportion of MIF-negative sera.
Overall, besides the previously discussed correlation between anti-HSP60 antibodies and CHD, our data would also stand in favor of the association between C. pneumoniae infection and CHD if high IgA and IgG MIF titers or anti-OMP2 IgG levels are considered. However, our data do not discriminate between CHD and AMI, as is the case for C. pneumoniae detection within general blood monocytes (24) as well as in other serological studies (6, 34).
Surprisingly, the ELISAs with either R-HSP60 or R-OMP2 were unable to detect specific IgA antibodies, although these were present at MIF titers of ≥32 in 45% of the CHD patients. Failure to detect IgA antibodies could not be attributed to technical problems with our in-house ELISA, since the IgA MIF-positive sera also were IgA ELISA positive upon plate coating with whole C. pneumoniae elementary bodies (A. Ciervo, A. Petrucca, F. Blasi, A. Cassone, and P. Visca, unpublished data). IgA seroreactivity has been regarded as a useful criterion for the differentiation between acute and chronic or persistent C. pneumoniae infection (38), and elevated IgA titers have been associated with CHD in this and previous studies (37). While the antigens which induce such reactivity have not yet been identified, our results suggest that neither HSP60 nor OMP2 is a main target of the IgA-mediated humoral response in C. pneumoniae-seropositive patients. Our data reflect the substantial uncertainty in IgA-based serology, depending on the antigen used for antibody detection. This issue was recently addressed by Shumacher and coworkers (34), who reported 59% concordance between commercial MIF assay and ELISA with chlamydial lipopolysaccharide. Comparable poor agreement was observed when IgA levels were measured by ELISA with either LPS or a crude C. pneumoniae antigenic preparation (34). Whether these divergent results are related to differences in immunodominance of the different antigens used for IgA detection, antibody persistence, individual immunological response, or reaction methodology is still an open issue.
As recently emphasized (29, 40), it would be convenient to replace MIF, a substantially subjective and nonquantitative assay, with a standardizable immunoenzymatic assay. However, useful chlamydial R-proteins to be used as antigens for this purpose have not yet been identified. Here we provide evidence that antibodies to R-OMP2 in its nondenatured state are present and very well detectable by ELISA. The high correlation between this objective and reliable immunoenzymatic assay and MIF in our study populations indicates the need for further studies with expanded and well-characterized populations for a possible validation of an R-OMP2-based ELISA for the detection of anti-C. pneumoniae antibodies and the assessment of their significance in CHD.
Acknowledgments
We are grateful to P. Saikku for the generous gift of C. pneumoniae strain Parola and to Valentina Quintiliani for technical assistance.
This study was supported in part by a grant from a special project, “Role of Infectious Agents in Cardiovascular and Cerebrovascular Diseases,” Istituto Superiore di Sanità-Ministero della Sanità, Italy, contract no. 98/YA.
REFERENCES
- 1.Allen, J. E., and R. S. Stephens. 1993. An intermolecular mechanism of T cell help for the production of antibodies to the bacterial pathogen, Chlamydia trachomatis. Eur. J. Immunol. 23:1169–7112. [DOI] [PubMed] [Google Scholar]
- 2.Bachmaier, K., N. Neu, L. M. de la Maza, S. Pal, A. Hessel, and J. M. Penninger. 1999. Chlamydia infections and heart disease linked through antigenic mimicry. Science 283:1335–1339. [DOI] [PubMed] [Google Scholar]
- 3.Beatty, W. L., G. I. Byrne, and R. P. Morrison. 1993. Morphologic and antigenic characterization of interferon-gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. USA 90:3998–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betsou, F., J. M. Soeur, and J. Orfila. 1999. Serological investigation of Chlamydia pneumoniae heat shock protein 10. Infect. Immun. 67:5243–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasi, F., R. Cosentini, R. Raccanelli, F. M. Massari, C. Arosio, P. Tarsia, and L. Allegra. 1997. A possible association of Chlamydia pneumoniae infection and acute myocardial infarction in patients younger than 65 years of age. Chest 112:309–312. [DOI] [PubMed] [Google Scholar]
- 6.Boman, J., S. Soderberg, J. Forsberg, L. S. Birgander, A. Allard, K. Persson, E. Jidell, U. Kumlin, P. Juto, A. Waldenström, and G. Wadell. 1998. High prevalence of Chlamydia pneumoniae DNA in peripheral blood mononuclear cells in patients with cardiovascular disease and in middle-aged blood donors. J. Infect. Dis. 178:274–277. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254. [DOI] [PubMed] [Google Scholar]
- 8.Caligiuri, G., G. Paulsson, A. Nicoletti, A. Maseri, and G. K. Hansson. 2000. Evidence for antigen-driven T-cell response in unstable angina. Circulation 102:1114–1119. [DOI] [PubMed] [Google Scholar]
- 9.Christiansen, G., A. S. Pedersen, K. Hjernø, B. Vandahl, and S. Birkelund. 2000. Potential relevance of Chlamydia pneumoniae surface proteins to an effective vaccine. J. Infect. Dis. 181:S528–S537. [DOI] [PubMed] [Google Scholar]
- 10.Cook, P. J., D. Honeybourne, G. Y. H. Lip, D. G. Beevers, R. Wise, and P. Davies. 1998. Chlamydia pneumoniae antibody titers are significantly associated with acute stroke and trancient cerebral ischaemia: the West Birmingham Stroke Project. Stroke 29:404–410. [DOI] [PubMed] [Google Scholar]
- 11.Danesh, J., R. Collins, and R. Peto. 1997. Chronic infections and coronary heart disease: is there a link? Lancet 350:430–436. [DOI] [PubMed] [Google Scholar]
- 12.Friedank, H. M., A. Clad, A. S. Herr, M. Wiedmann-Al-Ahmad, and B. Jung. 1995. Immune responses to Chlamydia trachomatis heat shock protein in infertile female patients and influence of Chlamydia pneumoniae antibodies. Eur. J. Clin. Microbiol. Infect. Dis. 14:1063–1069. [DOI] [PubMed] [Google Scholar]
- 13.Gaston, J. S. H., A. J. Curry, I. Portig, J. C. Goodall, and P. J. Kirkpatrick. 2000. Immune response to chlamydial antigens in atherosclerosis. Herz 25:73–78. [DOI] [PubMed] [Google Scholar]
- 14.Grayston, J. T., C. C. Kuo, A. S. Coulson, L. A. Campbell, R. D. Lawrence, M. J. Lee, E. D. Strandness, and S. Wang. 1995. Chlamydia pneumoniae (TWAR) in atherosclerosis of the carotid artery. Circulation 92:3397–3400. [DOI] [PubMed] [Google Scholar]
- 15.Grayston, J. T., C. C. Kuo, L. A. Campbell, and E. P. Benditt. 1993. Chlamydia pneumoniae, strain TWAR and atherosclerosis. Eur. Heart J. 14:66–71. [PubMed] [Google Scholar]
- 16.Gupta, S., and A. J. Camm. 1997. Chlamydia pneumoniae in coronary heart disease. Br. Med. J. 314:1778–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammerschlag, M. R. 1996. Diagnostic methods for intracellular pathogens. Clin. Microbiol. Infect. 1:S3–S8. [DOI] [PubMed] [Google Scholar]
- 18.Jantos, C. A., C. Krombach, F. N. Wuppermann, A. Gardemann, S. Bepler, H. Asslan, J. H. Hegemann, and W. Haberbosch. 2000. Antibody response to the 60-kDa heat-shock protein of Chlamydia pneumoniae in patients with coronary artery disease. J. Infect. Dis. 181:1700–1705. [DOI] [PubMed] [Google Scholar]
- 19.Kol, A., G. K. Sulkhova, A. H. Lichtman, and P. Libby. 1998. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-α and matrix metalloproteinase expression. Circulation 98:300–307. [DOI] [PubMed] [Google Scholar]
- 20.Körner, I., R. Blatz, I. Wittig, D. Pleiffer, and C. Rühlmann. 1999. Serological evidence of Chlamydia pneumoniae lipopolysaccharide antibodies in atherosclerosis of various vascular regions. Vasa 28:259–263. [DOI] [PubMed] [Google Scholar]
- 21.Kuo, C. C., L. A. Jackson, L. A. Campebell, and J. T. Grayston. 1995. Chlamydia pneumoniae (TWAR). Clin. Microbiol. Rev. 8:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo, C. C., A. Shor, L. A. Campbell, H. Fukushi, D. L. Patton, and J. T. Grayston. 1993. Demostration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J. Infect. Dis. 167:841–849. [DOI] [PubMed] [Google Scholar]
- 23.Maass, M., J. Gieffers, E. Krause, P. M. Engel, C. Bartels, and W. Solbach. 1998. Poor correlation between microimmunofluorescence serology and polymerase chain reaction for detection of vascular Chlamydia pneumoniae infection in coronary artery disease patients. Med. Microbiol. Immunol. 187:103–106. [DOI] [PubMed] [Google Scholar]
- 24.Maass, M., J. Jahn, J. Gieffers, K. Dalhoff, H. A. Katus, and W. Solbach. 2000. Detection of Chlamydia pneumoniae within peripheral blood monocytes of patients with unstable angina or myocardial infarction. J. Infect. Dis. 181:S449–S451. [DOI] [PubMed] [Google Scholar]
- 25.Mayr, M., B. Metzler, S. Kiechl, J. Willeit, G. Schett, Q. Xu, and G. Wick. 1999. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation 99:1560–1566. [DOI] [PubMed] [Google Scholar]
- 26.Mazzoli, S., N. Tofani, A. Fantini, F. Semplici, F. Bandini, A. Salvi, and R. Vergassola. 1998. Chlamydia pneumoniae antibody response in patients with acute myocardial infarction and their follow-up. Am. Heart J. 135:15–20. [DOI] [PubMed] [Google Scholar]
- 27.Mendall, M. A., D. Carrington, D. Strachan, P. Patel, N. Molineaux, J. Levi, T. Toosey, A. J. Camm, and T. C. Northfield. 1995. Chlamydia pneumoniae risk factors for seropositivity and association with coronary heart disease. J. Infect. 30:121–128. [DOI] [PubMed] [Google Scholar]
- 28.Orfila, J. J. 1998. Seroepidemiological evidence for an association between C. pneumoniae and atherosclerosis. Atherosclerosis 140:S11–S15. [DOI] [PubMed] [Google Scholar]
- 29.Peeling, R. W., S. P. Wang, J. T. Grayston, F. Blasi, J. Boman, A. Clad, H. Freidank, C. A. Gaydos, J. Gnarpe, T. Hagiwara, R. B. Jones, J. Orfila, K. Persson, M. Puolakkainen, P. Saikku, and J. Schachter. 2000. Chlamydia pneumoniae serology: interlaboratory variation in microimmunofluorescence assay results. J. Infect. Dis. 181:S426–S429. [DOI] [PubMed] [Google Scholar]
- 30.Penttila, T., J. M. Vuola, V. Puurula, M. Anttila, M. Sarvas, N. Rautonen, P. H. Makela, and M. Puolakkainen. 2000. Immunity to Chlamydia pneumoniae induced by vaccination with DNA vectors expressing a cytoplasmic protein (Hsp60) or outer membrane proteins (MOMP and Omp2). Vaccine 8:1256–1265. [DOI] [PubMed] [Google Scholar]
- 31.Ross, R. 1999. Atherosclerosis an inflammatory disease. N. Engl. J. Med. 340:115–126. [DOI] [PubMed] [Google Scholar]
- 32.Saikku, P., M. Leinonnen, K. Mattila, M. R. Ekman, M. S. Nieminen, P. H. Makela, J. K. Huttunen, and V. Valtonen. 1988. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet ii:983–986. [DOI] [PubMed] [Google Scholar]
- 33.Sasu, S., D. La Verda, N. Qureshi, D. T. Golenbock, and D. Beasley. 2001. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ. Res. 89:244–250. [DOI] [PubMed] [Google Scholar]
- 34.Schumacher, A., A. B. Lerkerød, I. Seljeflot, L. Sommervoll, I. Holme, J. E. Otterstad, and H. Arnesen. 2001. Chlamydia pneumoniae serology: importance of methodology in patients with coronary heart disease and healthy individuals. J. Clin. Microbiol. 39:1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stüber, D., H. Matile, and G. Garotta. 1990. System for high level production in E. coli and rapid purification of recombinant proteins: application to epitope mapping preparation of antibodies and structure function analysis, p. 121–152. In I. Lafkovitz and B. Pernis (ed.), Immunological methods, vol. 4. Academic Press, Inc., Orlando, Fla.
- 36.Umezawa, E. S., S. F. Bastos, M. E. Camargo, L. M. Yamauchi, M. R. Santos, A. Gonzalez, B. Zingales, M. J. Levin, O. Sousa, R. Rangel-Aldao, and J. F. Da Silveira. 1999. Evaluation of recombinant antigens for serodiagnosis of Chagas’ disease in South and Central America. J. Clin. Microbiol. 37:1554–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Hertzen, L., H. Alakärppä, R. Koskinen, K. Liippo, H. M. Surcel, M. Leinonen, and P. Saikku. 1997. Chlamydia pneumoniae infection in patients with chronic obstructive pulmonary disease. Epidemiol. Infect. 118:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Hertzen, L., M. Leinonen, H. M. Surcel, J. Karjalainen, and P. Saikku. 1995. Measurement of sputum antibodies in the diagnosis of acute and chronic respiratory infections associated with Chlamydia pneumoniae. Clin. Diagn. Lab. Immunol. 2:454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagar, E. A., J. Schachter, P. Bavoil, and R. S. Stephens. 1990. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J. Infect. Dis. 162:922–927. [DOI] [PubMed] [Google Scholar]
- 40.Wang, S. 2000. The microimmunofluorescence test for Chlamydia pneumoniae infection: technique and interpretation. J. Infect. Dis. 181:S421–S425. [DOI] [PubMed] [Google Scholar]
- 41.Watson, M. W., P. R. Lambden, J. S. Everson, and I. N. Clarke. 1994. Immunoreactivity of the 60 kDa cysteine-rich proteins of Chlamydia trachomati, Chlamydia psittaci and Chlamydia pneumoniae expressed in Escherichia coli. Microbiology 140:2003–2011. [DOI] [PubMed] [Google Scholar]
- 42.Xu, Q., G. Schett, H. Perschinka, M. Mayr, G. Egger, F. Oberhollenzer, J. Willeit, S. Kiechl, and G. Wick. 2000. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation 102:14–20. [DOI] [PubMed] [Google Scholar]