Abstract
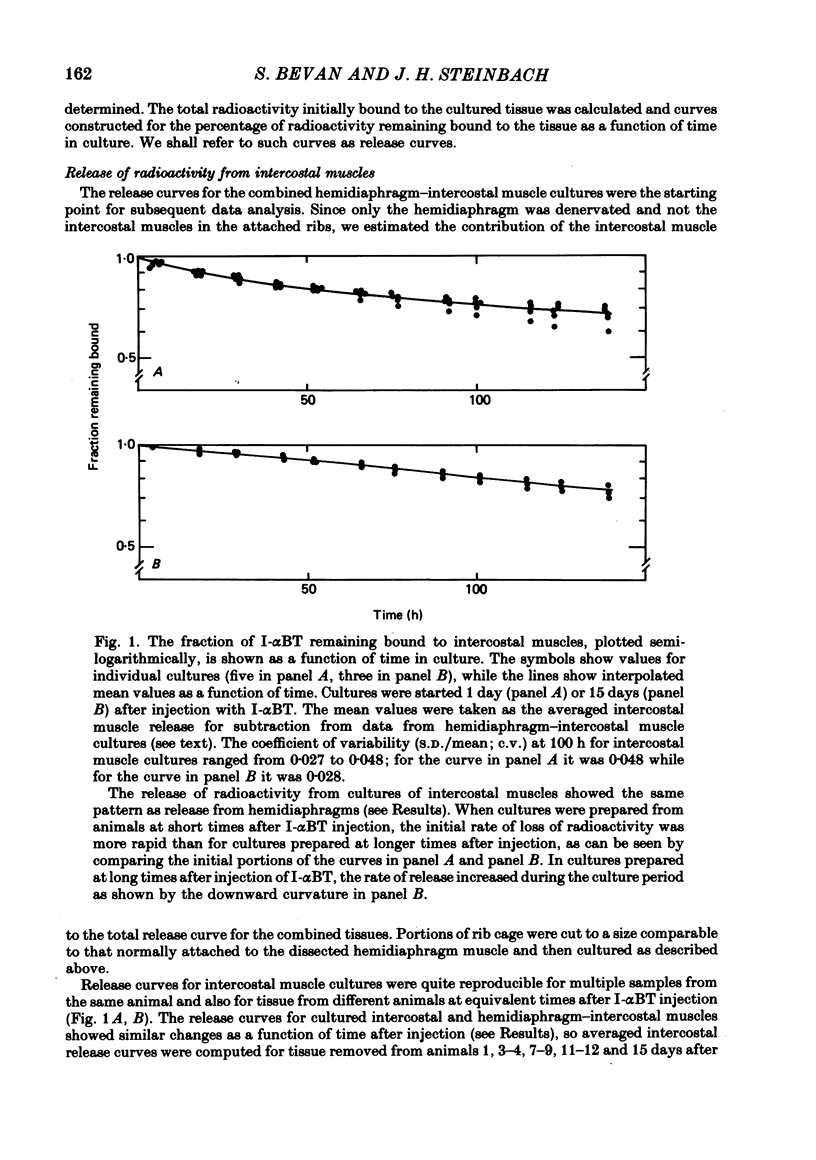
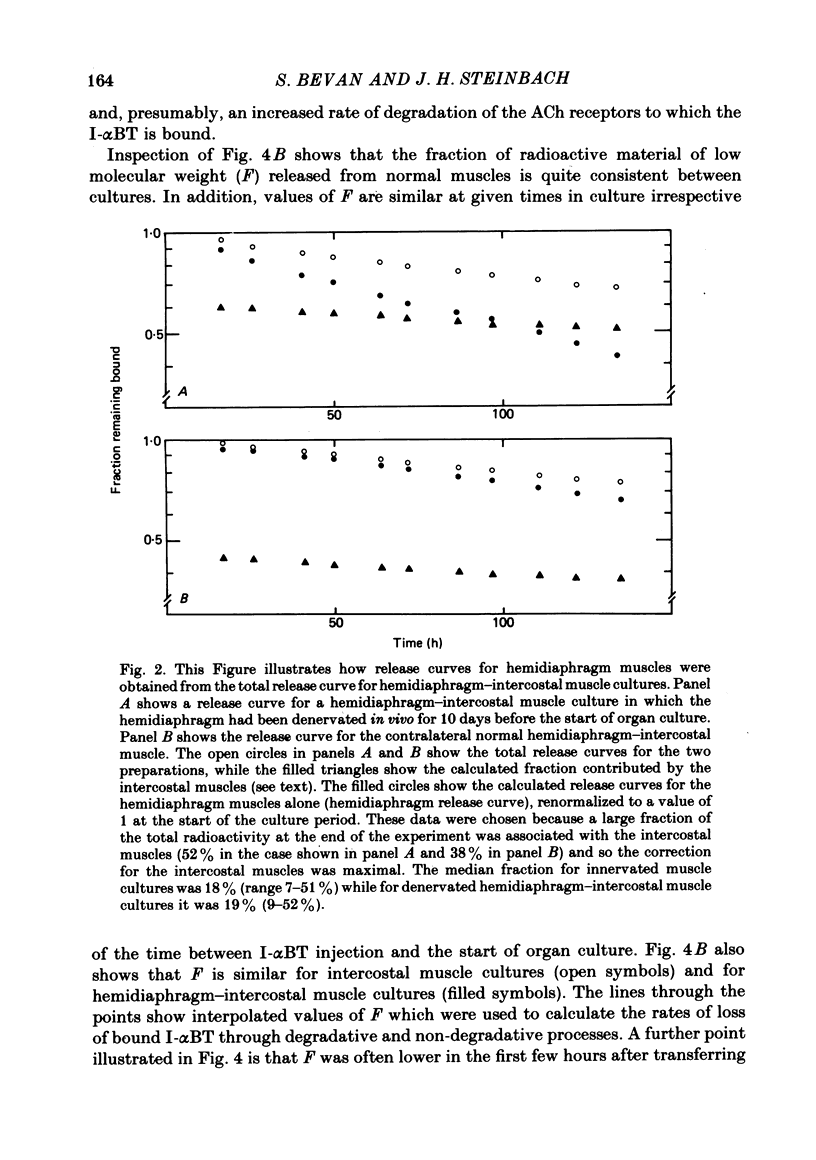
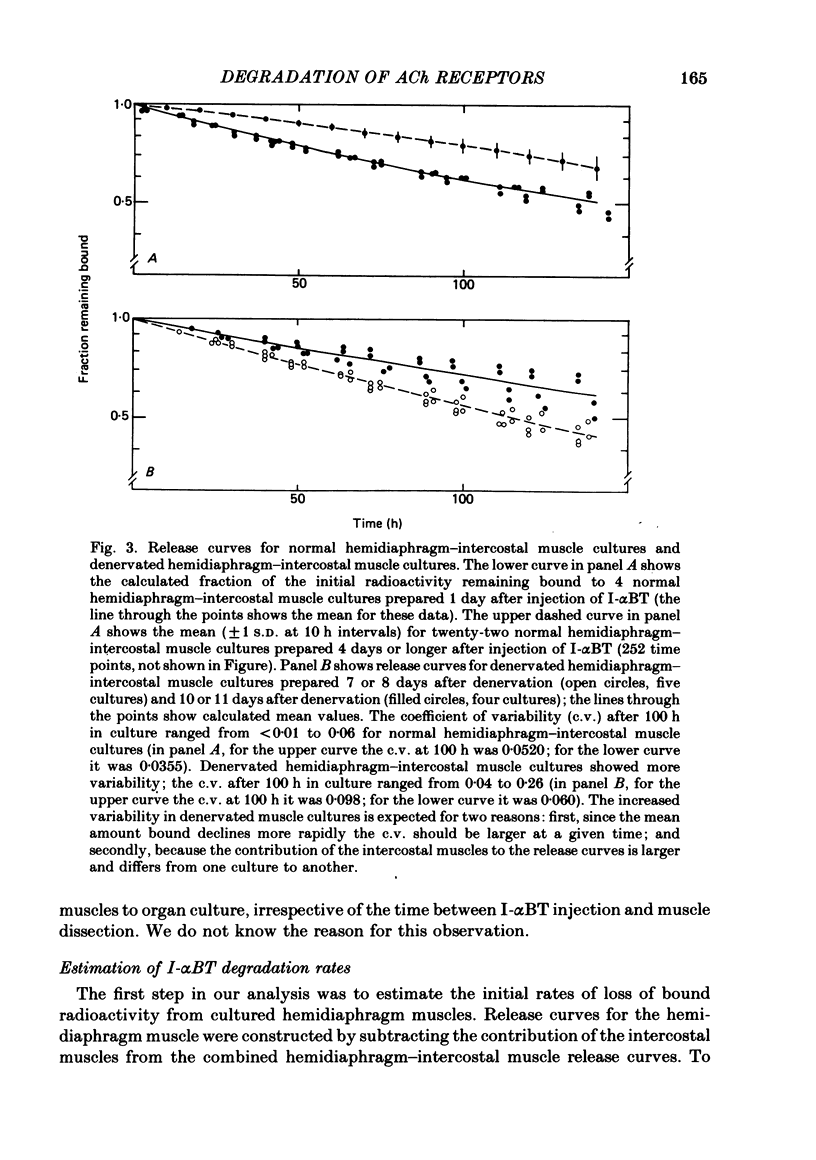
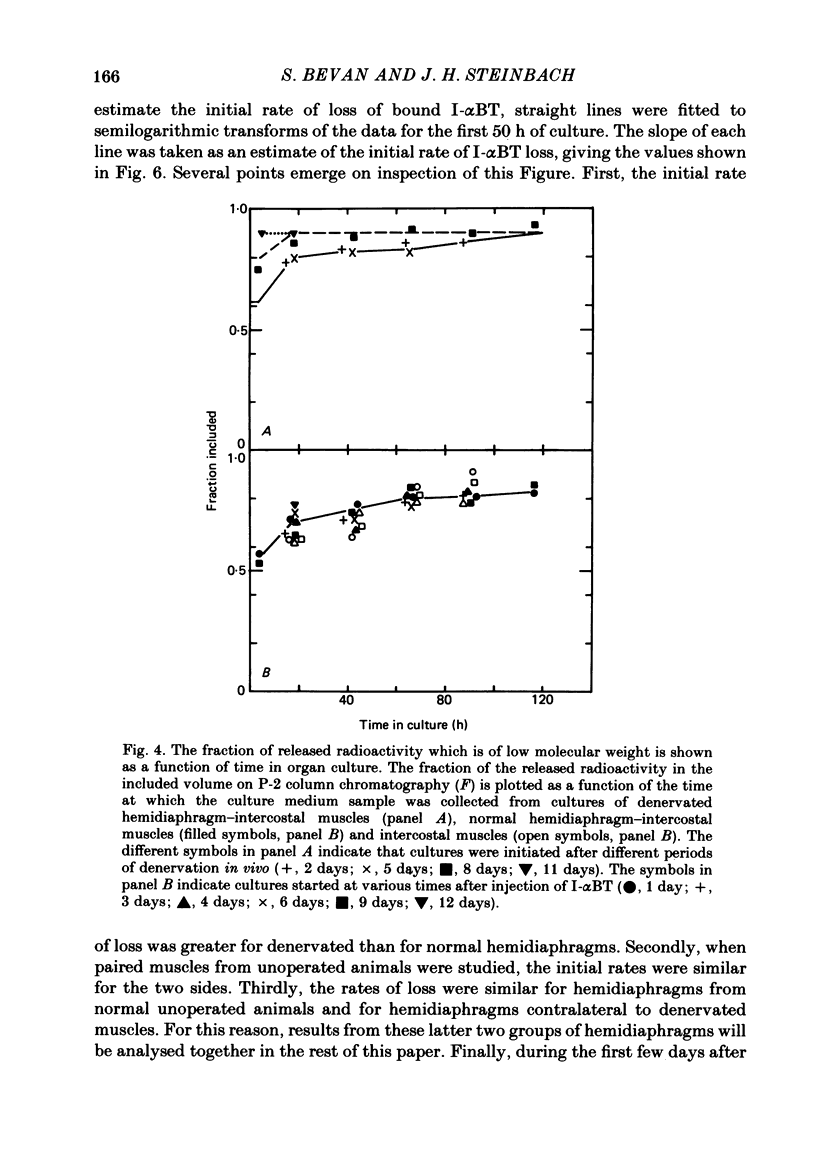
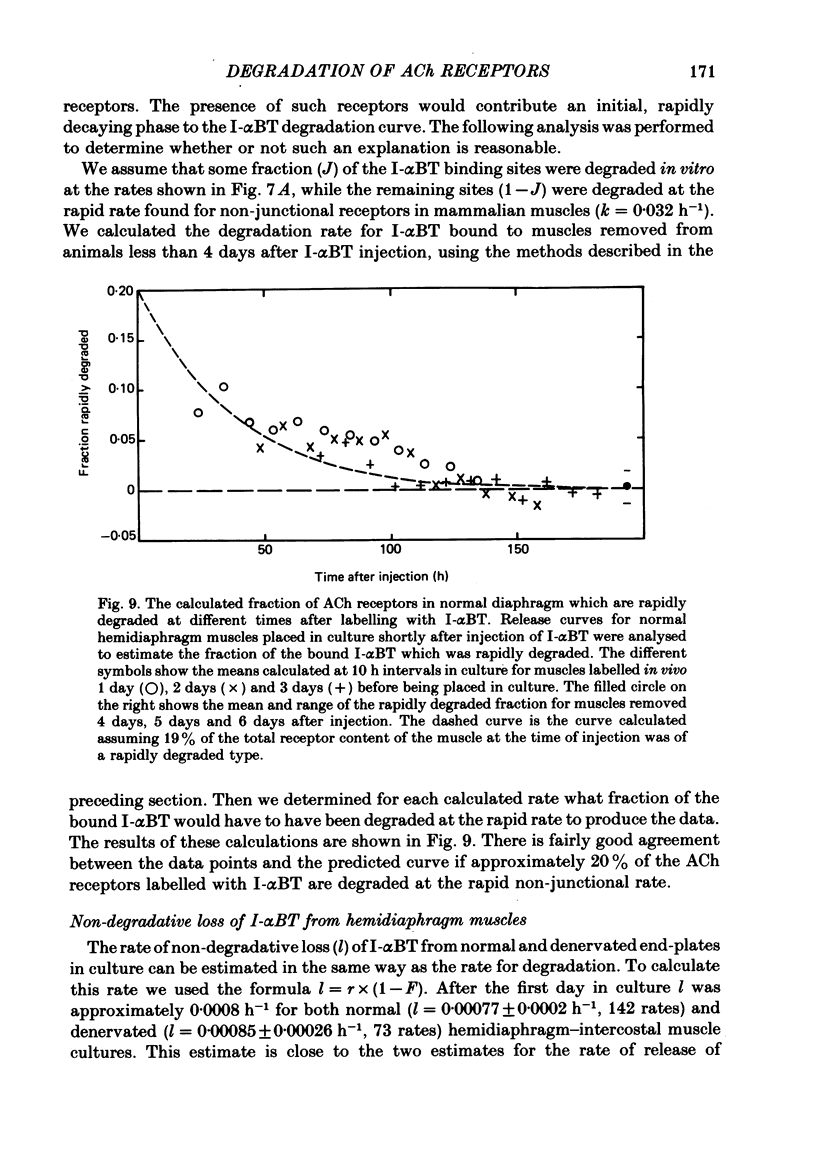
We have studied the effect of denervation on the degradation of the existing junctional acetylcholine (ACh) receptors at end-plates in rat muscles. ACh receptors were labelled by injecting animals with iodinated alpha-bungarotoxin (I-alpha BT); 1 day later the left hemidiaphragm was denervated. The degradation of bound I-alpha BT in normal and denervated muscles was examined in organ culture, beginning at various times after denervation in vivo. The original, pre-labelled end-plate ACh receptors are degraded more rapidly after denervation. The rate of degradation begins to increase shortly after the nerve is cut and reaches a maximum value at about 9 days of denervation. Muscles denervated only on transfer to organ culture also show an increase in the degradation rate of bound I-alpha BT with increasing time of denervation (time in culture). In normal diaphragm muscles, the initial rate of degradation of functional ACh receptors, after correcting for non-degradative loss of I-alpha BT, is 0.0018 h-1 (t1/2 = 383 h). The maximal rate at denervated end-plates is 0.0073 h-1 (t1/2 = 94 h). For soleus, sternomastoid, plantaris and intercostal innervated muscles the apparent rate of ACh receptor degradation either in vitro or in vivo ranged from 0.0005 h-1 to 0.002 h-1. The rate of loss of bound I-alpha BT in vivo is more rapid at denervated end-plates than at innervated end-plates. For diaphragm muscles, the rates of I-alpha BT degradation measured in organ culture are able to describe the relative rates of loss of I-alpha BT from innervated and denervated muscles in vivo. At short times after labelling, a fraction (10-20%) of the I-alpha BT bound to innervated muscles is degraded more rapidly than the remaining toxin. The possibility that these I-alpha BT binding sites are degraded at the rate characteristic of extrajunctional receptors on denervated muscle fibres is discussed.
Full text
PDF



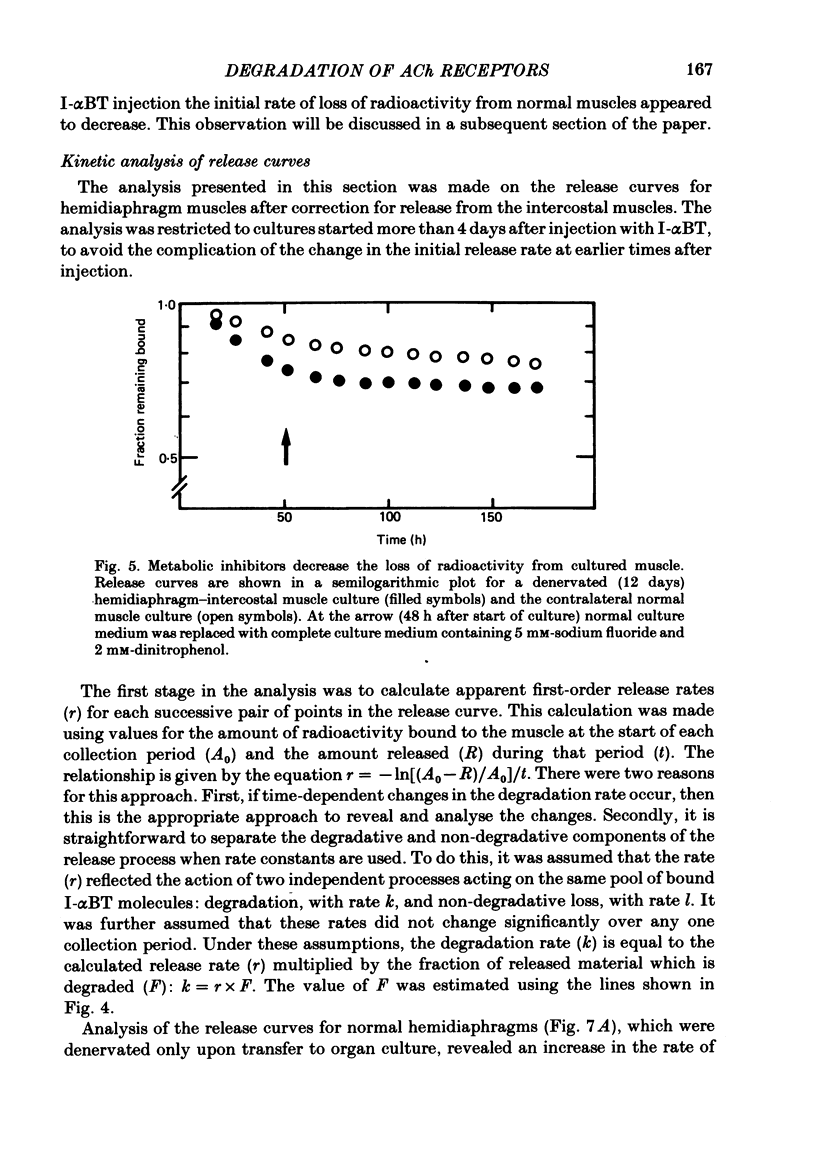
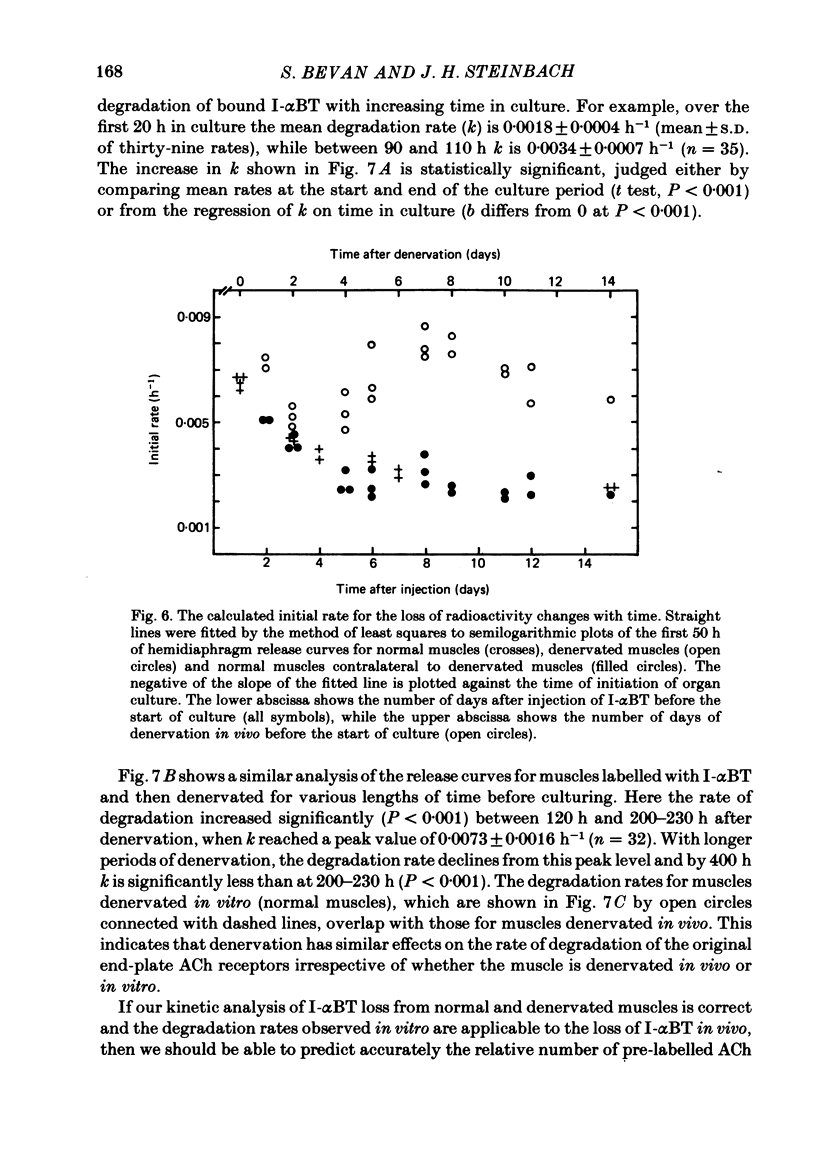
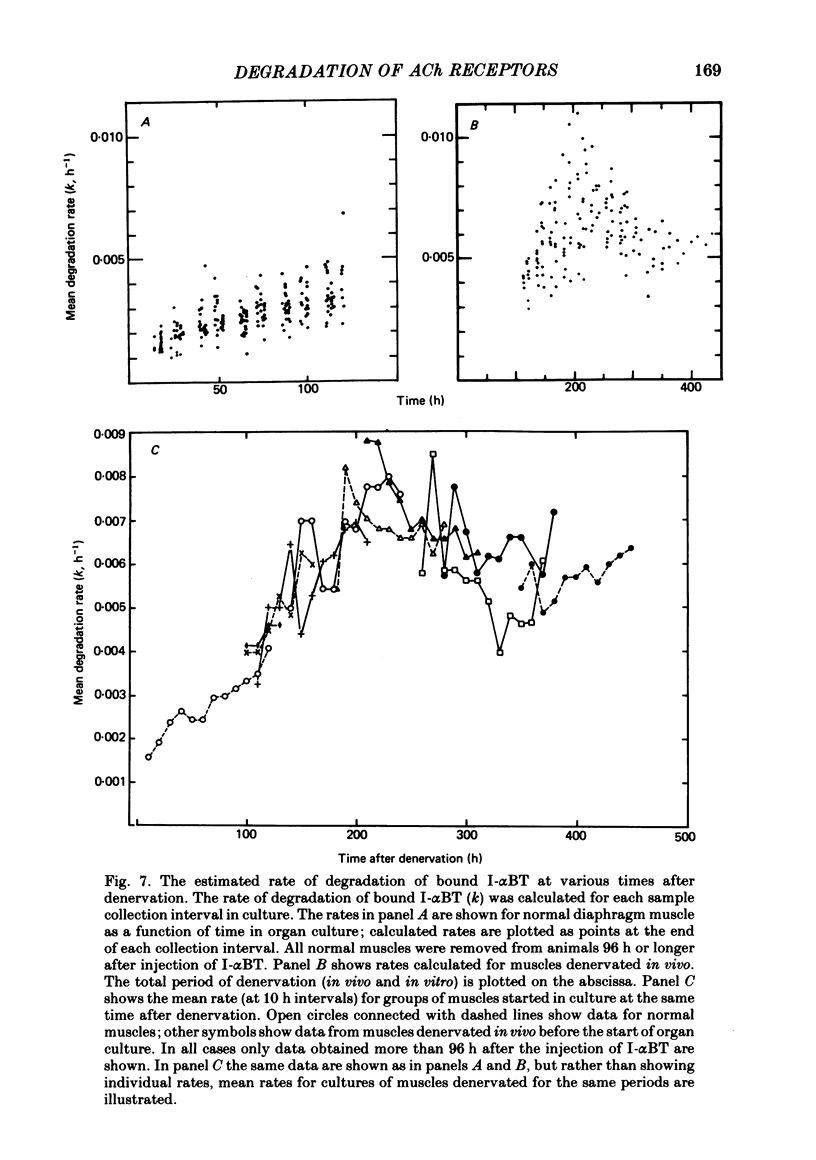
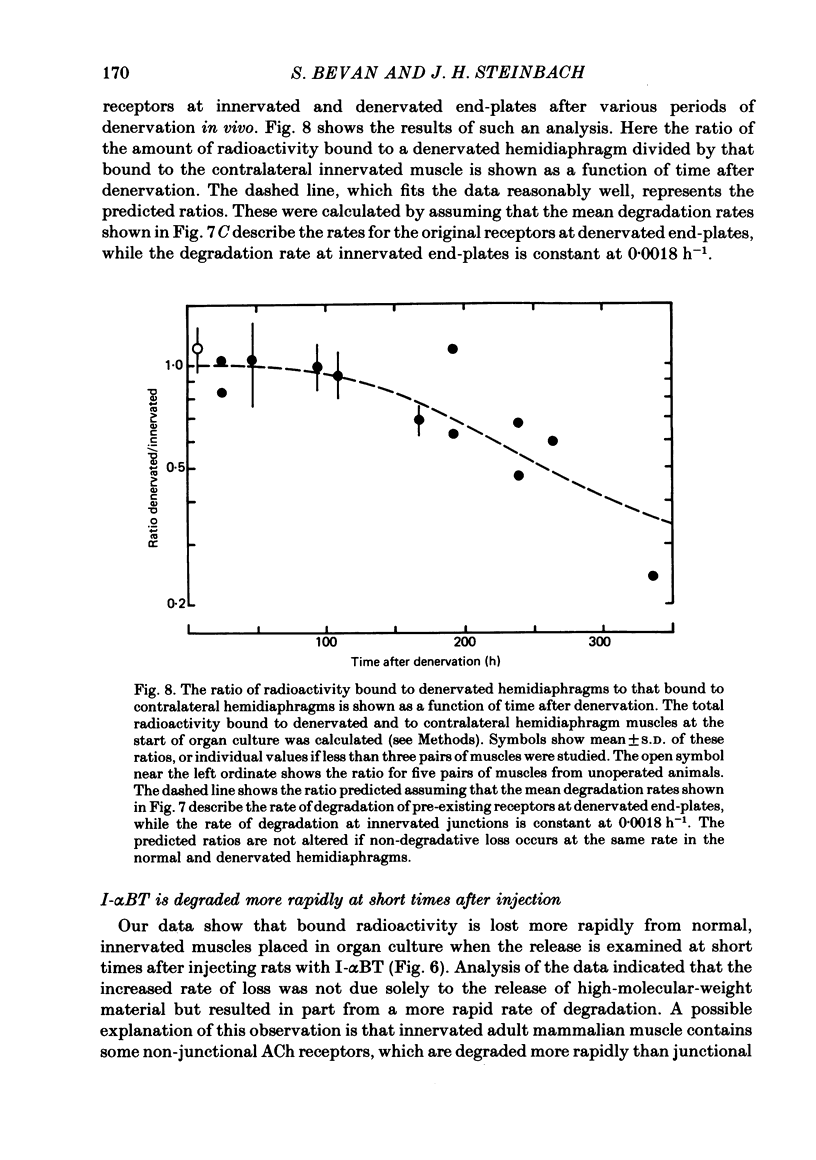





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader D. Density and distribution of alpha-bungarotoxin-binding sites in postsynaptic structures of regenerated rat skeletal muscle. J Cell Biol. 1981 Feb;88(2):338–345. doi: 10.1083/jcb.88.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Loss of alpha-bungarotoxin from junctional and extrajunctional acetylcholine receptors in rat diaphragm muscle in vivo and in organ culture. J Physiol. 1975 Nov;252(3):771–789. doi: 10.1113/jphysiol.1975.sp011169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S. J., Sargent P. B., McMahan U. J. Acetylcholine receptors in regenerating muscle accumulate at original synaptic sites in the absence of the nerve. J Cell Biol. 1979 Aug;82(2):412–425. doi: 10.1083/jcb.82.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Huang M. C. Turnover of junctional and extrajunctional acetylcholine receptors of the rat diaphragm. Nature. 1975 Feb 20;253(5493):643–644. doi: 10.1038/253643a0. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine receptors. Revised estimates of extrajunctional receptor density in denervated rat diaphragm. J Gen Physiol. 1974 Oct;64(4):468–472. doi: 10.1085/jgp.64.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M., Hartzell H. C. Acetylcholine receptors: number and distribution at neuromuscular junctions in rat diaphragm. Science. 1972 Apr 14;176(4031):189–191. doi: 10.1126/science.176.4031.189. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Gautvik K., Sommerschild H. Cholinergic receptors at denervated mammalian motor end-plates. Acta Physiol Scand. 1975 Sep;95(1):66–76. doi: 10.1111/j.1748-1716.1975.tb10026.x. [DOI] [PubMed] [Google Scholar]
- Gardner J. M., Fambrough D. M. Acetylcholine receptor degradation measured by density labeling: effects of cholinergic ligands and evidence against recycling. Cell. 1979 Mar;16(3):661–674. doi: 10.1016/0092-8674(79)90039-4. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Levitt T. A., Loring R. H., Salpeter M. M. Neuronal control of acetylcholine receptor turnover rate at a vertebrate neuromuscular junction. Science. 1980 Oct 31;210(4469):550–551. doi: 10.1126/science.7423205. [DOI] [PubMed] [Google Scholar]
- Levitt T. A., Salpeter M. M. Denervated endplates have a dual population of junctional acetylcholine receptors. Nature. 1981 May 21;291(5812):239–241. doi: 10.1038/291239a0. [DOI] [PubMed] [Google Scholar]
- Linden D. C., Fambrough D. M. Biosynthesis and degradation of acetylcholine receptors in rat skeletal muscles. Effects of electrical stimulation. Neuroscience. 1979;4(4):527–538. doi: 10.1016/0306-4522(79)90129-5. [DOI] [PubMed] [Google Scholar]
- Loring R. H., Salpeter M. M. Denervation increases turnover rate of junctional acetylcholine receptors. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2293–2297. doi: 10.1073/pnas.77.4.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Changeux J. P., Gros F. Acetylcholine receptor degradation measured by pulse chase labelling. Nature. 1976 Nov 4;264(5581):74–76. doi: 10.1038/264074a0. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Heinemann S., Lindstrom J. M. Acetylcholine receptor degradation in adult rat diaphragms in organ culture and the effect of anti-acetylcholine receptor antibodies. J Biol Chem. 1979 Jul 25;254(14):6320–6327. [PubMed] [Google Scholar]
- Michler A., Sakmann B. Receptor stability and channel conversion in the subsynaptic membrane of the developing mammalian neuromuscular junction. Dev Biol. 1980 Nov;80(1):1–17. doi: 10.1016/0012-1606(80)90494-7. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M., Hall Z. W. Subunit structure and peptide mapping of junctional and extrajunctional acetylcholine receptors from rat muscle. Biochemistry. 1979 Jul 24;18(15):3392–3401. doi: 10.1021/bi00582a028. [DOI] [PubMed] [Google Scholar]
- Stanley E. F., Drachman D. B. Denervation accelerates the degradation of junctional acetylcholine receptors. Exp Neurol. 1981 Aug;73(2):390–396. doi: 10.1016/0014-4886(81)90274-0. [DOI] [PubMed] [Google Scholar]
- Steinbach J. H., Merlie J., Heinemann S., Bloch R. Degradation of junctional and extrajunctional acetylcholine receptors by developing rat skeletal muscle. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3547–3551. doi: 10.1073/pnas.76.7.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H. Neuromuscular junctions and alpha-bungarotoxin-binding sites in denervated and contralateral cat skeletal muscles. J Physiol. 1981;313:513–528. doi: 10.1113/jphysiol.1981.sp013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Steps in the neoplastic transformation of hamster embryo cells by polyoma virus. Proc Natl Acad Sci U S A. 1963 Feb 15;49:171–179. doi: 10.1073/pnas.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigny M., Koenig J., Rieger F. The motor end-plate specific form of acetylcholinesterase: appearance during embryogenesis and re-innervation of rat muscle. J Neurochem. 1976 Dec;27(6):1347–1353. doi: 10.1111/j.1471-4159.1976.tb02614.x. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Junctional form of acetylcholinesterase restored at nerve-free endplates. Dev Biol. 1979 Feb;68(2):631–635. doi: 10.1016/0012-1606(79)90233-1. [DOI] [PubMed] [Google Scholar]



