Abstract
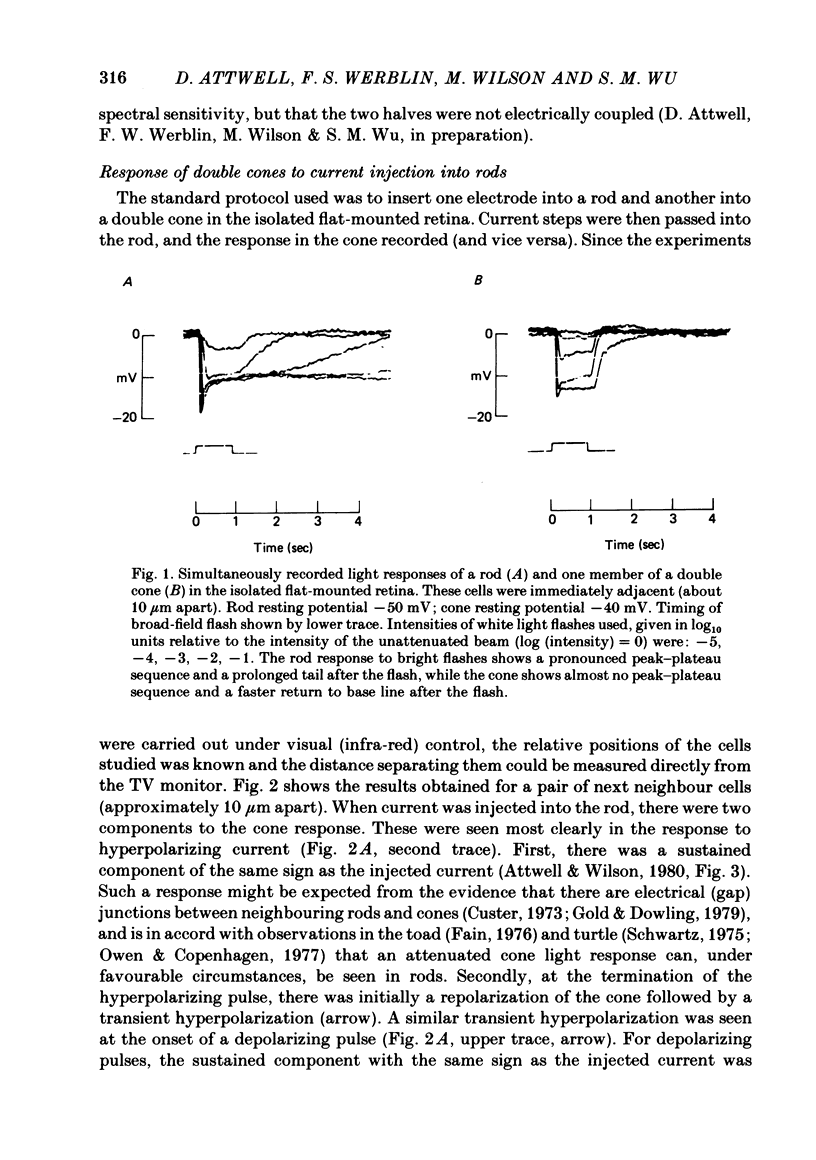
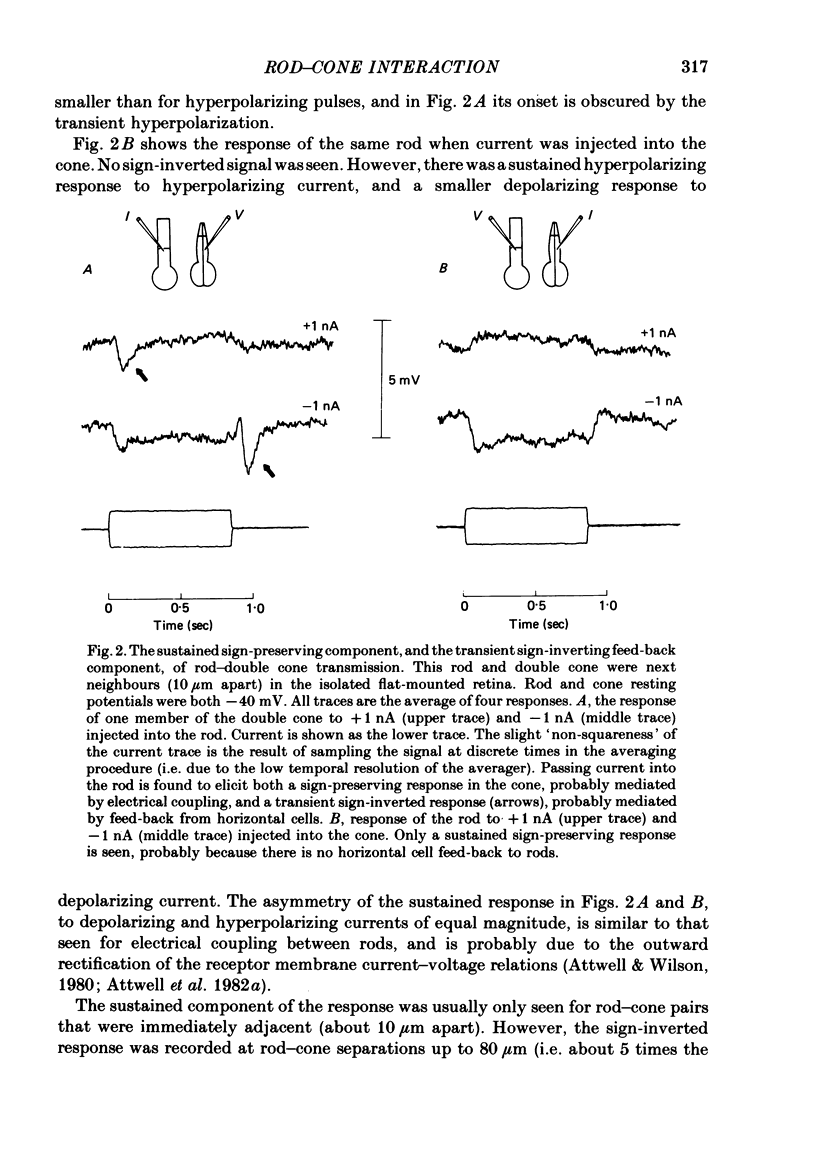
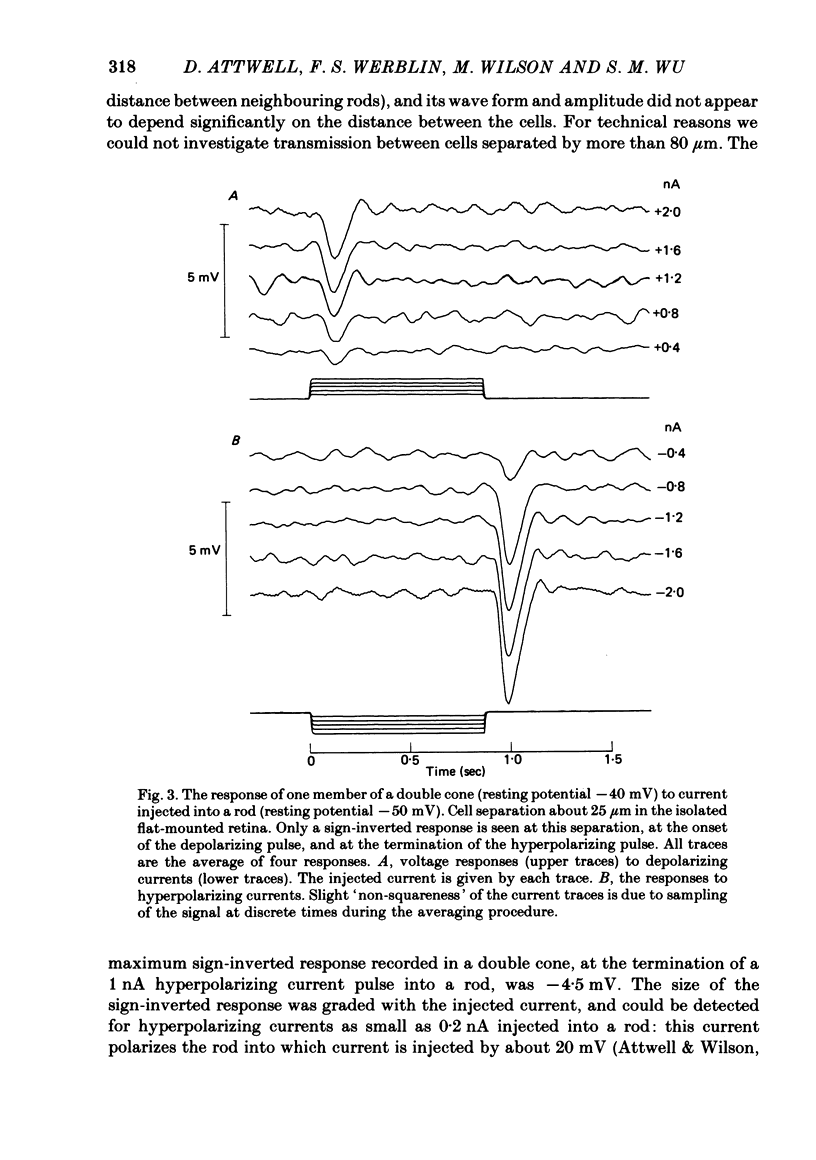
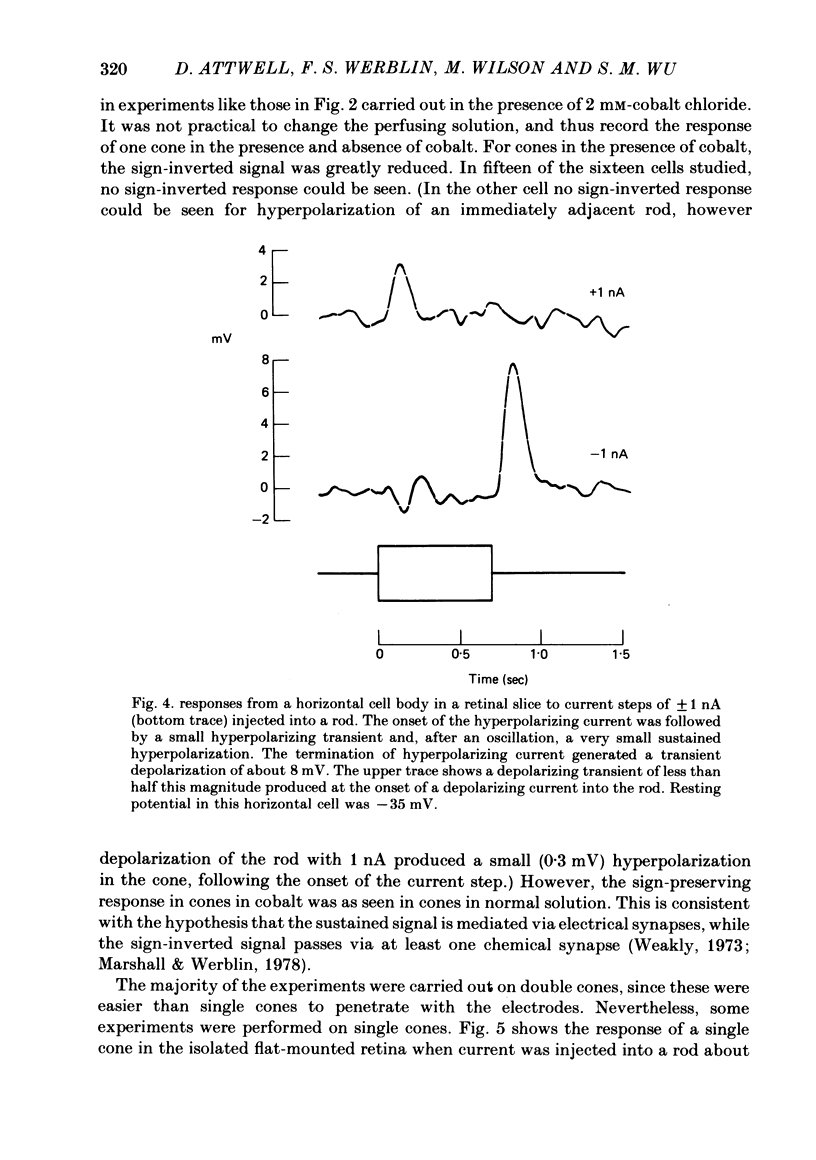
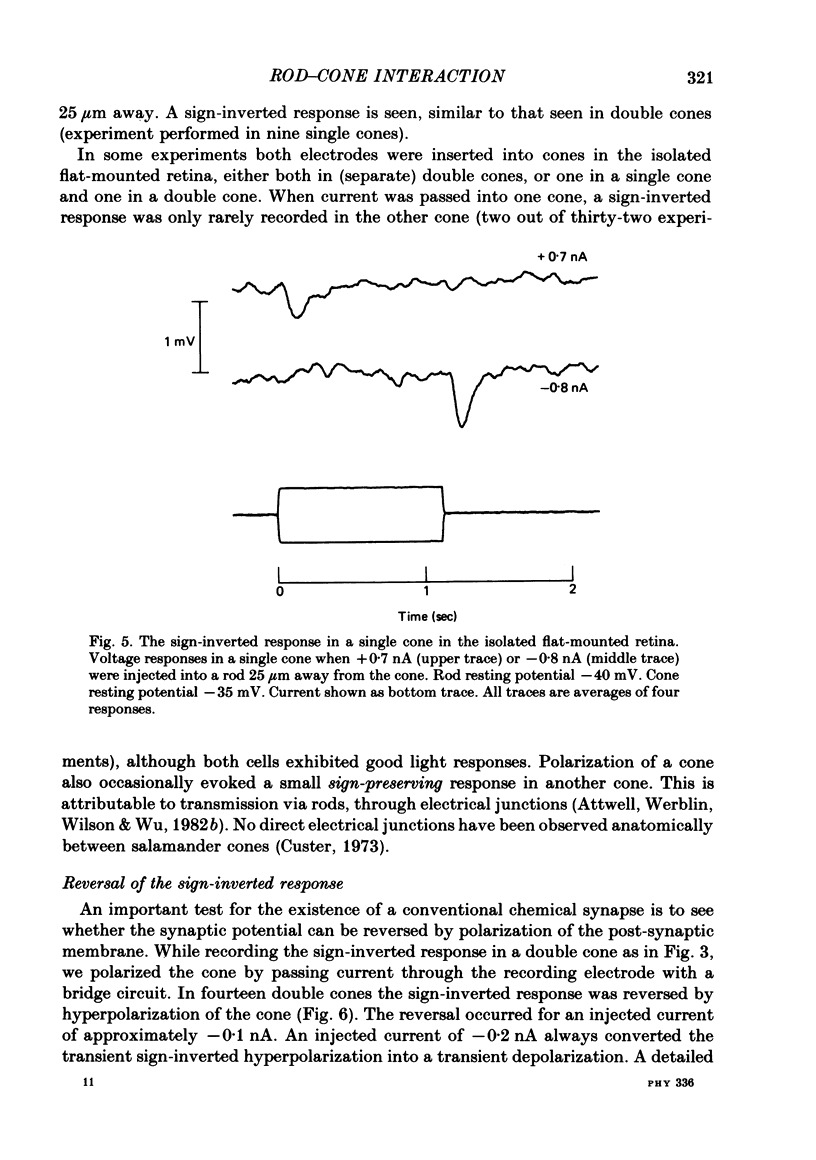
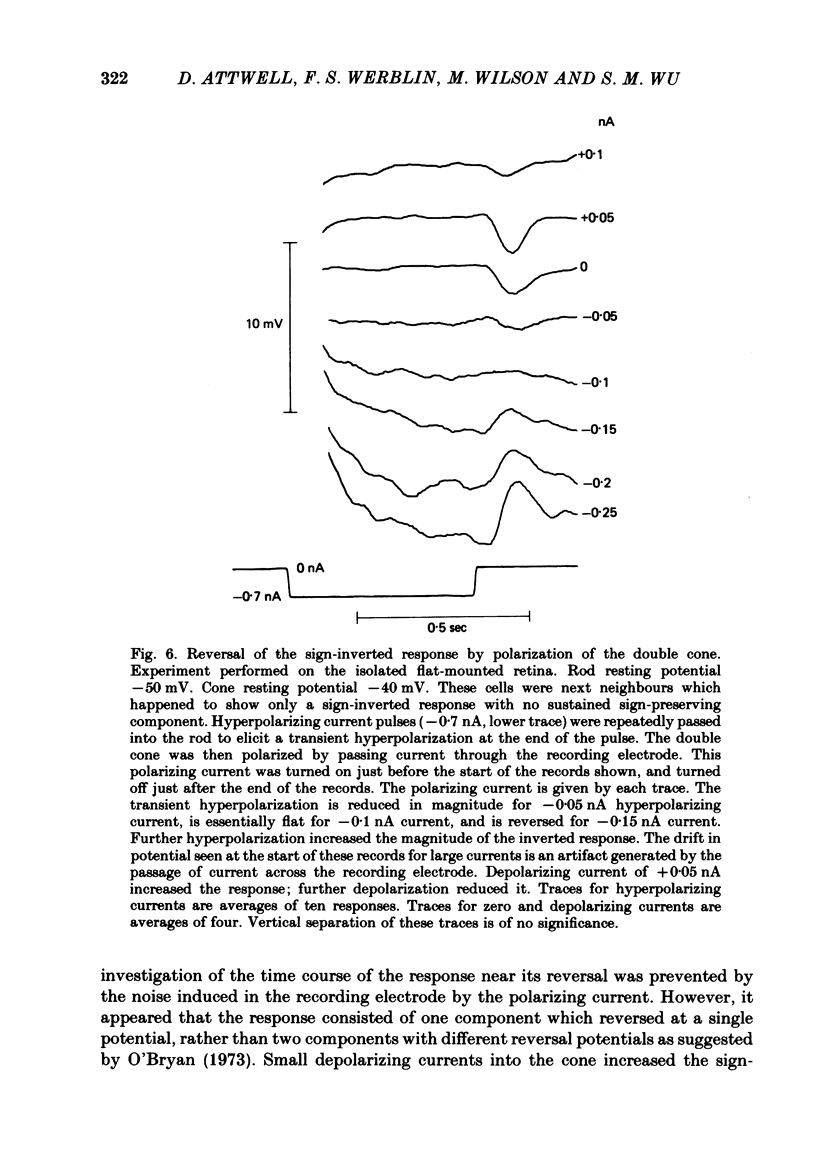
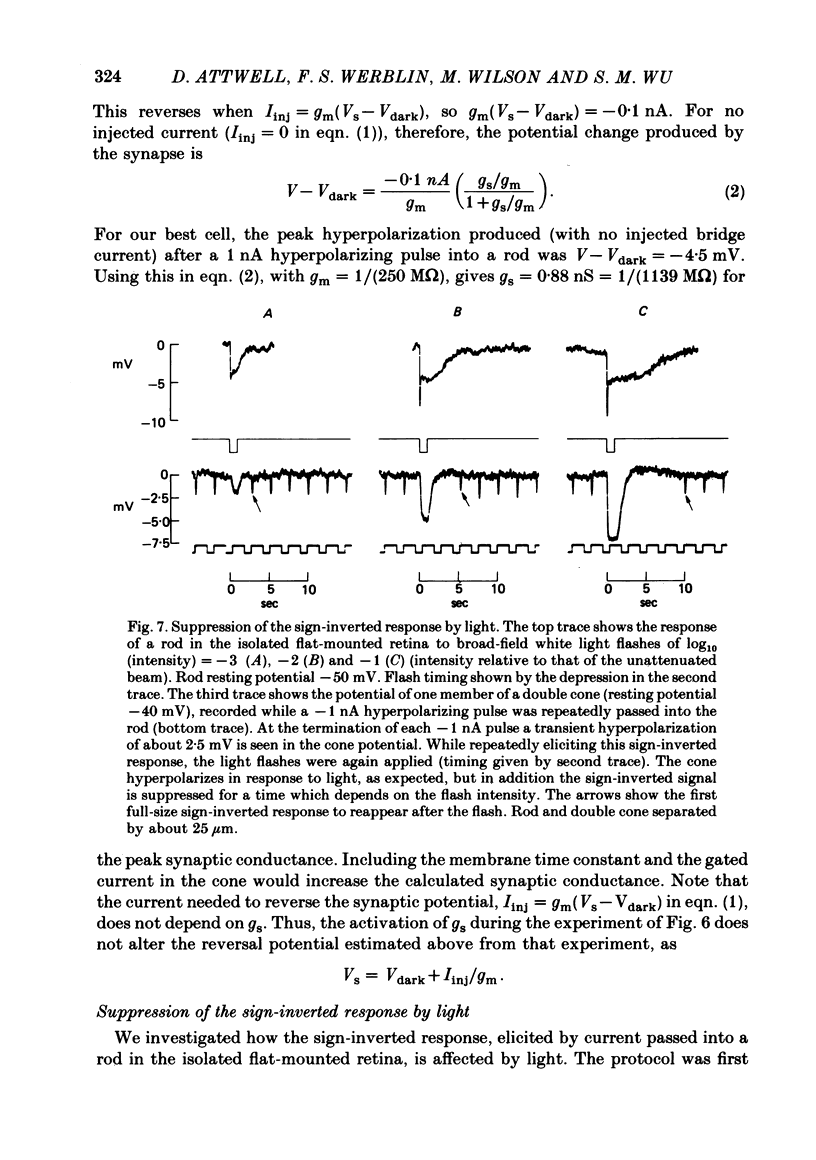
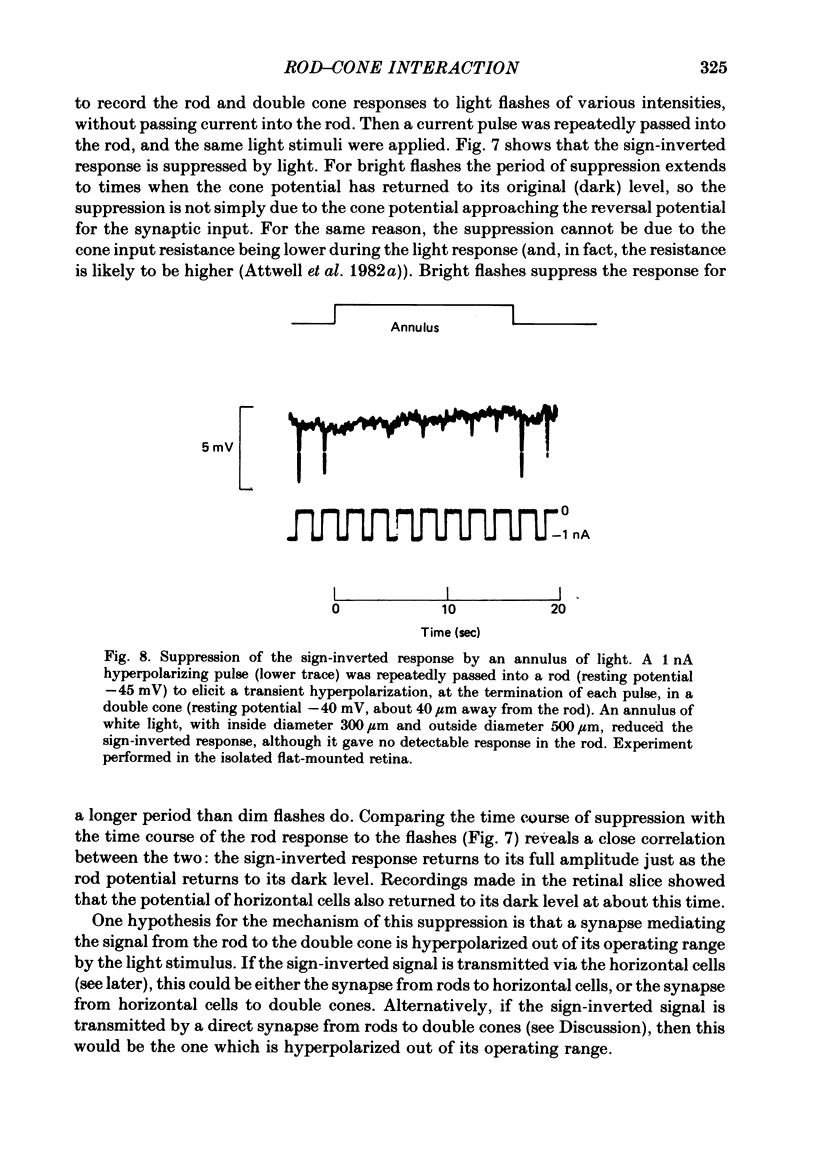
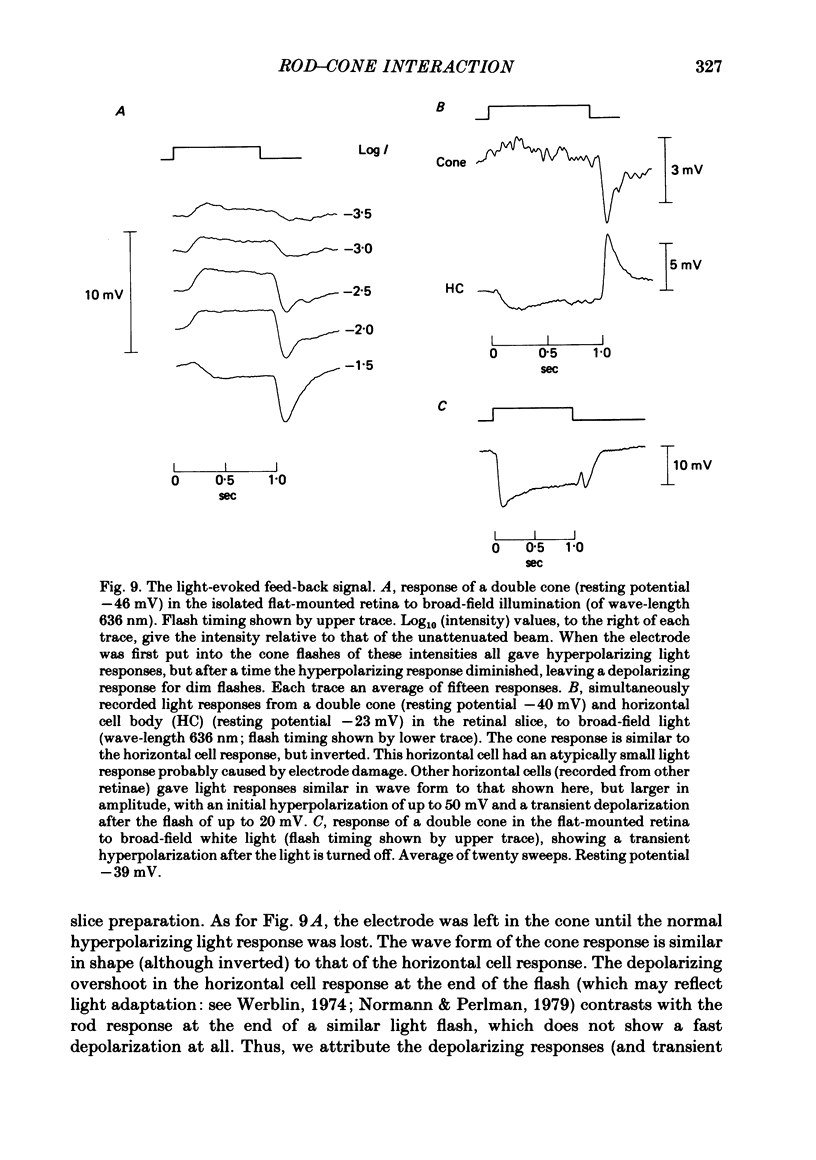
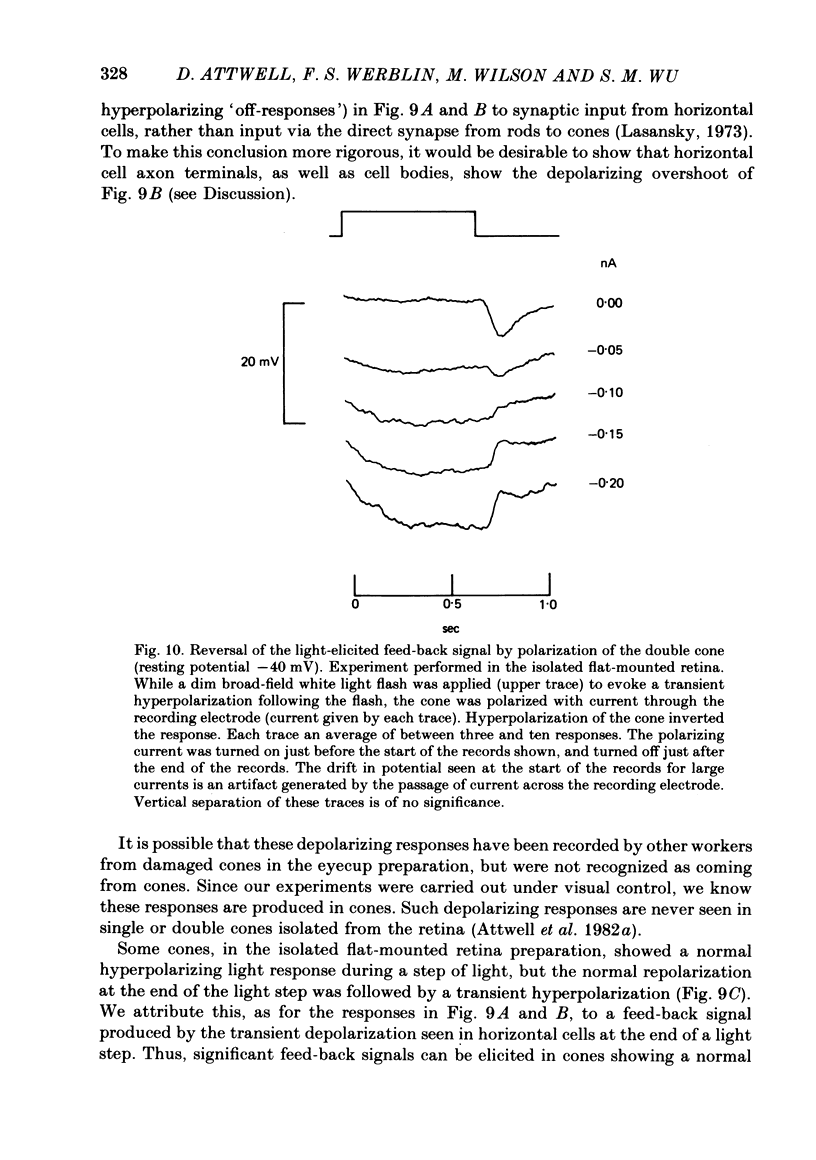
Signal transmission between rods and cones was studied by passing current into a rod and recording the voltage response in a nearby double or single cone and vice versa. Two types of rod-cone interaction were found. Between immediately adjacent rods and cones, passage of current into either receptor elicited in the other receptor a sustained voltage response of the same sign as the injected current. These signals were still seen in the presence of Co2+, and are probably mediated by the electrical synapses which have been seen anatomically between adjacent rods and cones. In addition to this short-range sign-preserving interaction, passing current into a rod elicited a transient sign-inverted signal in cones up to at least 80 micron from the injected rod. No such response was seen in rods for current injection into cones. This signal was greatly reduced by Co2+ ions. Hyperpolarization of the cone to about -65 mV, with about 0.1 nA current, reversed this signal, which is presumed to be mediated by a chemical synaptic input to cones. Light flashes suppressed the sign-inverted signal for a period which was longer for brighter flashes. The time of reappearance of the signal was correlated with the return of the rod and horizontal cell potentials to their dark levels. This suppression could also be produced by an annulus of light which produced no light response in the receptors at the centre of the annulus, but which did polarize horizontal cells under the centre of the annulus. The wave form of the sign-inverted signal was similar to that produced in horizontal cells by current injection into rods, but of opposite sign. If an electrode was left in a cone for some time, the normal hyperpolarizing light response diminished, leaving a depolarizing response produced, presumably, by feed-back from horizontal cells. This signal was reversed when the cone was hyperpolarized with about 0.1 nA current. These data suggest that the sign-inverted response is mediated by feed-back from horizontal cells and, assuming that depolarization increases the rate of release of horizontal cell synaptic transmitter, then the feed-back transmitter opens channels in the cone membrane whose currents have a reversal potential around -65 mV.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Werblin F. S., Wilson M. The properties of single cones isolated from the tiger salamander retina. J Physiol. 1982 Jul;328:259–283. doi: 10.1113/jphysiol.1982.sp014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980 Dec;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt D. A. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977 Jan;40(1):53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Custer N. V. Structurally specialized contacts between the photoreceptors of the retina of the axolotl. J Comp Neurol. 1973 Sep 1;151(1):35–56. doi: 10.1002/cne.901510104. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G. H., Dowling J. E. Photoreceptor coupling in retina of the toad, Bufo marinus. I. Anatomy. J Neurophysiol. 1979 Jan;42(1 Pt 1):292–310. doi: 10.1152/jn.1979.42.1.292. [DOI] [PubMed] [Google Scholar]
- Hanani M., Vallerga S. Rod and cone signals in the horizontal cells of the tiger salamander retina. J Physiol. 1980 Jan;298:397–405. doi: 10.1113/jphysiol.1980.sp013089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Shimazaki H. Effects of external ions on the synaptic transmission from photorecptors to horizontal cells in the carp retina. J Physiol. 1975 Nov;252(2):509–522. doi: 10.1113/jphysiol.1975.sp011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond B Biol Sci. 1973;265(872):471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Synaptic action mediating cone responses to annular illumination in the retina of the larval tiger salamander. J Physiol. 1981 Jan;310:205–214. doi: 10.1113/jphysiol.1981.sp013544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L. M., Werblin F. S. Synaptic transmission to the horizontal cells in the retina of the larval tiger salamander. J Physiol. 1978 Jun;279:321–346. doi: 10.1113/jphysiol.1978.sp012347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R. A., Perlman I. Signal transmission from red cones to horizontal cells in the turtle retina. J Physiol. 1979 Jan;286:509–524. doi: 10.1113/jphysiol.1979.sp012634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan P. M. Properties of the depolarizing synaptic potential evoked by peripheral illumination in cones of the turtle retina. J Physiol. 1973 Nov;235(1):207–223. doi: 10.1113/jphysiol.1973.sp010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Gerschenfeld H. M. Activation of a regenerative calcium conductance in turtle cones by peripheral stimulation. Proc R Soc Lond B Biol Sci. 1978 May 16;201(1144):309–315. doi: 10.1098/rspb.1978.0048. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. Center-surround antagonistic organization in small-field luminosity horizontal cells of turtle retina. J Neurophysiol. 1981 Mar;45(3):363–375. doi: 10.1152/jn.1981.45.3.363. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Cones excite rods in the retina of the turtle. J Physiol. 1975 Apr;246(3):639–651. doi: 10.1113/jphysiol.1975.sp010908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantz M., Naka K. The bipolar cell. Vision Res. 1976;16(12):1517–1519. doi: 10.1016/0042-6989(76)90175-9. [DOI] [PubMed] [Google Scholar]
- Stell W. K., Lightfood D. O., Wheeler T. G., Leeper H. F. Goldfish retina: functional polarization of cone horizontal cell dendrites and synapses. Science. 1975 Dec 5;190(4218):989–990. doi: 10.1126/science.1188380. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Control of retinal sensitivity. II. Lateral interactions at the outer plexi form layer. J Gen Physiol. 1974 Jan;63(1):62–87. doi: 10.1085/jgp.63.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978 Jul;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]



