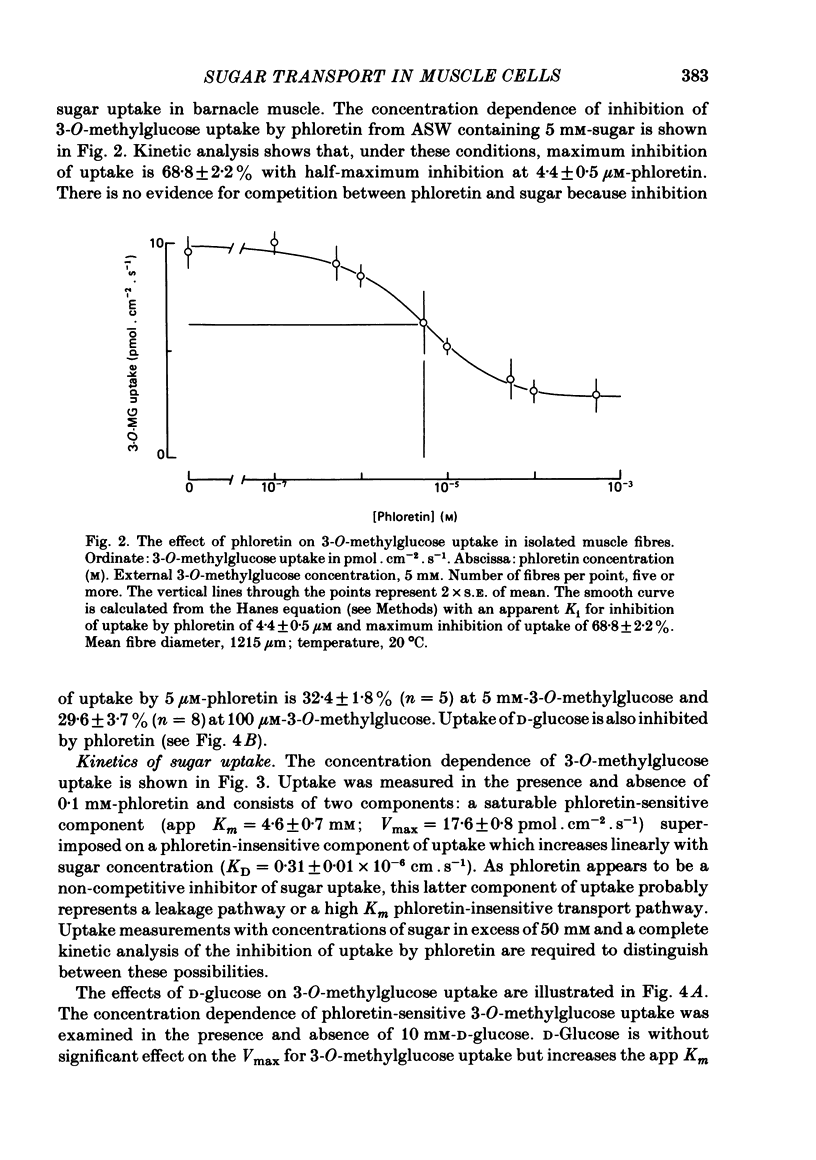
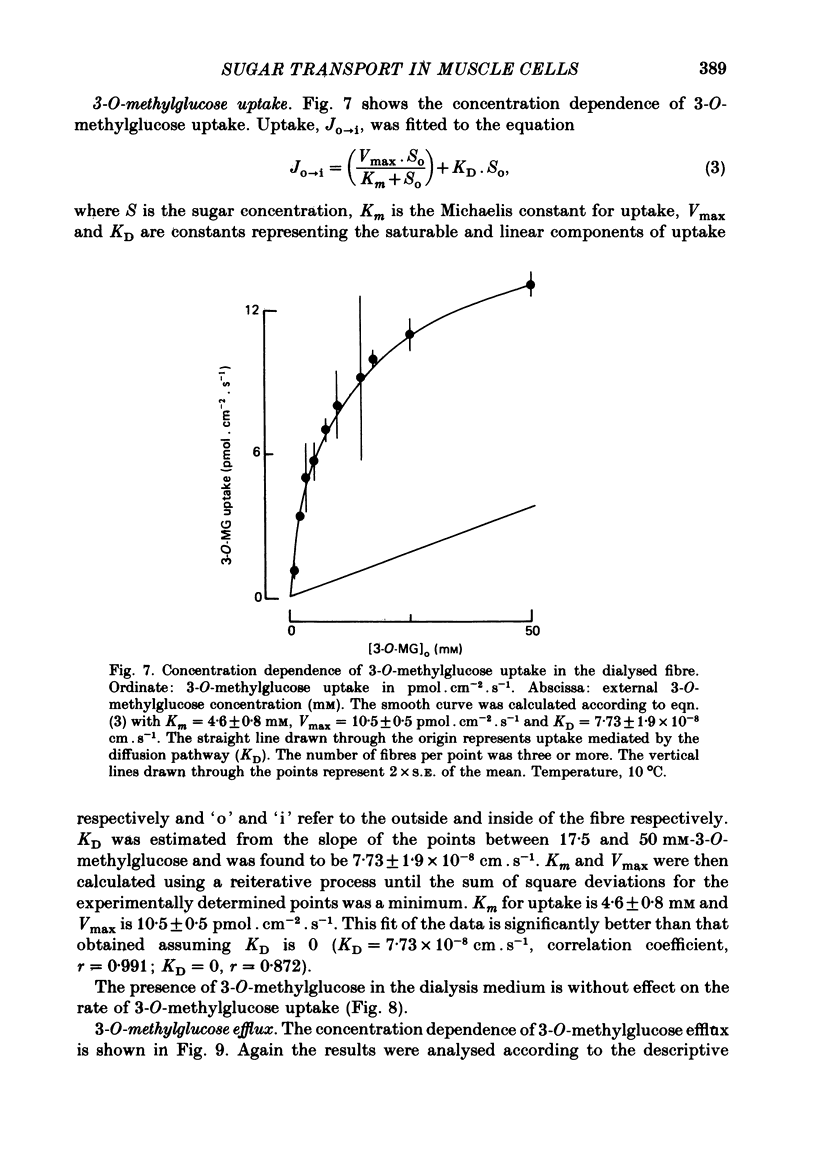
Abstract
The kinetics of 3-O-methylglucose transport in the giant muscle cells of Balanus nubilus have been studied both in intact fibres and in fibres subjected to intracellular solute control using internal dialysis. 3-O-methylglucose is not metabolized by barnacle muscle and at equilibrium the 3-O-methylglucose space of the tissue does not differ significantly from the water content of the muscle. These results indicate that 3-O-methylglucose transfer in barnacle muscle is mediated by a passive process. 3-O-methylglucose transport is facilitated by a saturable, symmetric transfer mechanism inhibited by cis but not trans sugars and by low concentrations of phloretin and cytochalasin B. The kinetic constants for uptake and exit are identical. These features indicate that sugar transport in barnacle muscle is mediated by a limited number of membrane transport sites. The number of sugar-displaceable cytochalasin B binding sites in barnacle muscle is 3 X 10(13) cm-2. Indirect kinetic estimates indicate that the number of sugar transport sites is in the order of 1.6 X 10(12) cm-2. This passive, facilitated, selective, saturable transport system is consistent with both symmetric mobile carrier (one-site) and symmetric simultaneous carrier (two-site) models for transport.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Lea T. J. Calcium fluxes in single muscle fibres measured with a glass scintillator probe. J Physiol. 1978 Sep;282:307–331. doi: 10.1113/jphysiol.1978.sp012465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATTAGLIA F. C., RANDLE P. J. Regulation of glucose uptake by muscle. 4. The specificity of monosaccharide-transport systems in rat-diaphragm muscle. Biochem J. 1960 May;75:408–416. doi: 10.1042/bj0750408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITTON H. G. PERMEABILITY OF THE HUMAN RED CELL TO LABELLED GLUCOSE. J Physiol. 1964 Jan;170:1–20. doi: 10.1113/jphysiol.1964.sp007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker G. F., Widdas W. F. The asymmetry of the facilitated transfer system for hexoses in human red cells and the simple kinetics of a two component model. J Physiol. 1973 May;231(1):143–165. doi: 10.1113/jphysiol.1973.sp010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Carruthers A. 3-O-methylglucose transport in internally dialysed giant axons of Loligo. J Physiol. 1981 Jul;316:503–525. doi: 10.1113/jphysiol.1981.sp013803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Carruthers A. Insulin regulation of sugar transport in giant muscle fibres of the barnacle. J Physiol. 1983 Mar;336:397–431. doi: 10.1113/jphysiol.1983.sp014588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Carruthers A. Insulin stimulates sugar transport in giant muscle fibres of the barnacle. Nature. 1980 Jul 17;286(5770):276–279. doi: 10.1038/286276a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Carruthers A. Sugar transport in giant axons of Loligo. J Physiol. 1981 Jul;316:481–502. doi: 10.1113/jphysiol.1981.sp013802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Stone A. J. A kinetic method for investigating hypothetical models of the sodium pump. Biochim Biophys Acta. 1966 Oct 10;126(2):321–329. doi: 10.1016/0926-6585(66)90069-0. [DOI] [PubMed] [Google Scholar]
- Basketter D. A., Widdas W. F. Asymmetry of the hexose transfer system in human erythrocytes. Comparison of the effects of cytochalasin B, phloretin and maltose as competitive inhibitors. J Physiol. 1978 May;278:389–401. doi: 10.1113/jphysiol.1978.sp012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Schultz R., Harkness C. Influence of insulin on sodium efflux in barnacle muscle fibers. J Membr Biol. 1977 Jun 6;34(2-3):203–222. doi: 10.1007/BF01870300. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2303–2331. doi: 10.1085/jgp.50.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. C., Lea T. J. Use of an intercellular glass scintillator for the continuous measurement of the uptake of 14C-labelled glycine into squid giant axons. J Physiol. 1973 Jul;232(1):4P–5P. [PubMed] [Google Scholar]
- Carruthers A. Internal dialysis as a method for studying sugar transport in large nerve and muscle fibres [proceedings]. J Physiol. 1979 Feb;287:5P–6P. [PubMed] [Google Scholar]
- Craik J. D., Elliott K. R. Kinetics of 3-O-methyl-D-glucose transport in isolated rat hepatocytes. Biochem J. 1979 Aug 15;182(2):503–508. doi: 10.1042/bj1820503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. B., LINDSAY D. B. The action of insulin on the penetration of sugars into the perfused heart. J Physiol. 1956 Mar 28;131(3):526–541. doi: 10.1113/jphysiol.1956.sp005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE G., SMYTH T., Jr NEUROMUSCULAR PHYSIOLOGY OF GIANT MUSCLE FIBERS OF A BARNACLE, BALANUS NUBILUS DARWIN. Comp Biochem Physiol. 1963 Dec;10:291–314. doi: 10.1016/0010-406x(63)90229-9. [DOI] [PubMed] [Google Scholar]
- Hoyle G., McNeill P. A., Selverston A. I. Ultrastructure of barnacle giant muscle fibers. J Cell Biol. 1973 Jan;56(1):74–91. doi: 10.1083/jcb.56.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. Y. Evidence of high stability of the glucose transport carrier function in human red cell ghosts extensively washed in various media. Arch Biochem Biophys. 1971 Sep;146(1):215–226. doi: 10.1016/s0003-9861(71)80058-9. [DOI] [PubMed] [Google Scholar]
- Kipnis D. M., Parrish J. E. Role of Na+ and K+ on sugar (2-deoxyglucose) and amino acid (alpha-aminoisobutyric acid) transport in striated muscle. Fed Proc. 1965 Sep-Oct;24(5):1051–1059. [PubMed] [Google Scholar]
- Kohn P. G., Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. VI. The effect of insulin, ouabain, and metabolic inhibitors on the transport of 3-O-methylglucose and glucose in rat soleus muscles. Biochim Biophys Acta. 1971 Feb 2;225(2):277–290. doi: 10.1016/0005-2736(71)90221-5. [DOI] [PubMed] [Google Scholar]
- Krupka R. M., Devés R. An experimental test for cyclic versus linear transport models. The mechanisms of glucose and choline transport in erythrocytes. J Biol Chem. 1981 Jun 10;256(11):5410–5416. [PubMed] [Google Scholar]
- Levine M., Oxender D. L., Stein W. D. The substrate-facilitated transport of the glucose carrier across the human erythrocyte membrane. Biochim Biophys Acta. 1965 Sep 27;109(1):151–163. doi: 10.1016/0926-6585(65)90099-3. [DOI] [PubMed] [Google Scholar]
- Lidgard G. P., Jones M. N. D-glucose permeability of black lipid membranes modified by human erythrocyte membrane fractions. J Membr Biol. 1975 Apr 23;21(1-2):1–10. doi: 10.1007/BF01941058. [DOI] [PubMed] [Google Scholar]
- Lin S., Spudich J. A. Biochemical studies on the mode of action of cytochalasin B. Cytochalasin B binding to red cell membrane in relation to glucose transport. J Biol Chem. 1974 Sep 25;249(18):5778–5783. [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P. Studies of tissue permeability. IX. The effect of insulin on the penetration of 3-methylglucose-H3 in frog muscle. J Biol Chem. 1963 Jan;238:40–49. [PubMed] [Google Scholar]
- REGEN D. M., MORGAN H. E. STUDIES OF THE GLUCOSE-TRANSPORT SYSTEM IN THE RABBIT ERYTHROCYTE. Biochim Biophys Acta. 1964 Jan 27;79:151–166. doi: 10.1016/0926-6577(64)90048-8. [DOI] [PubMed] [Google Scholar]
- WIDDAS W. F. Inability of diffusion to account for placental glucose transfer in the sheep and consideration of the kinetics of a possible carrier transfer. J Physiol. 1952 Sep;118(1):23–39. doi: 10.1113/jphysiol.1952.sp004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


