Abstract
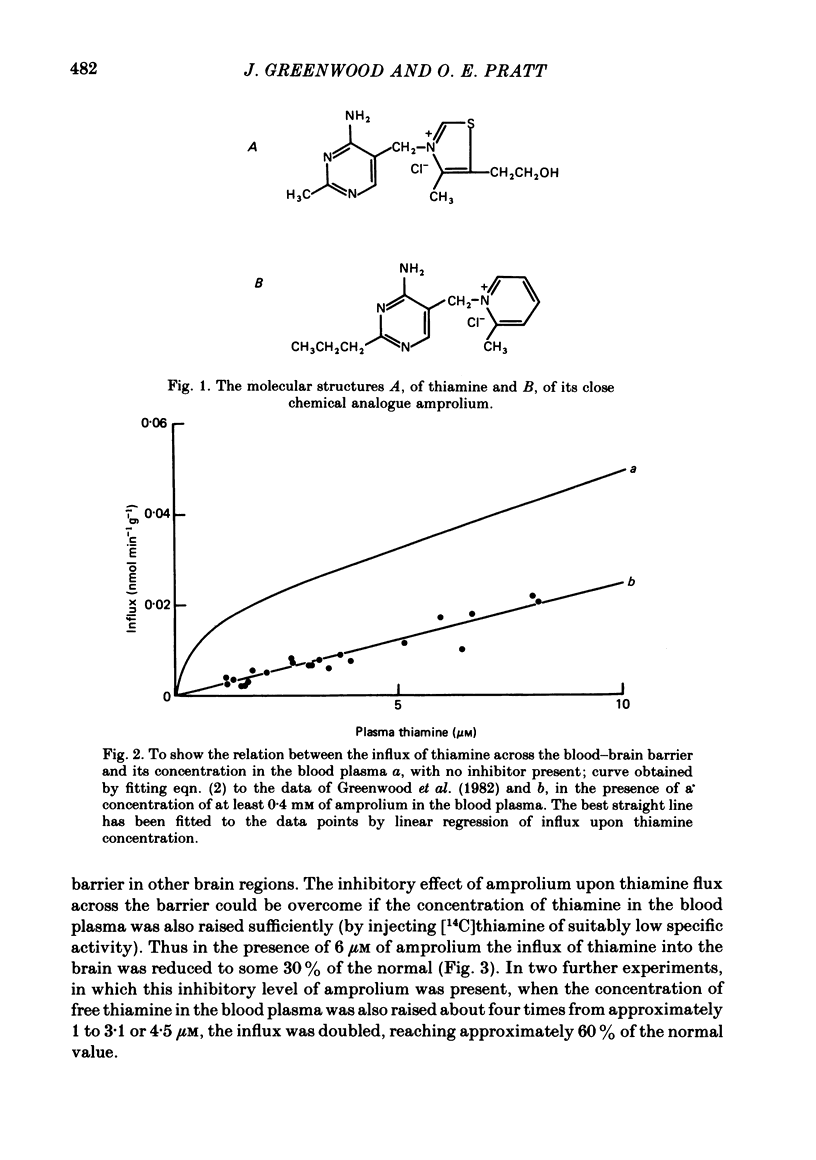
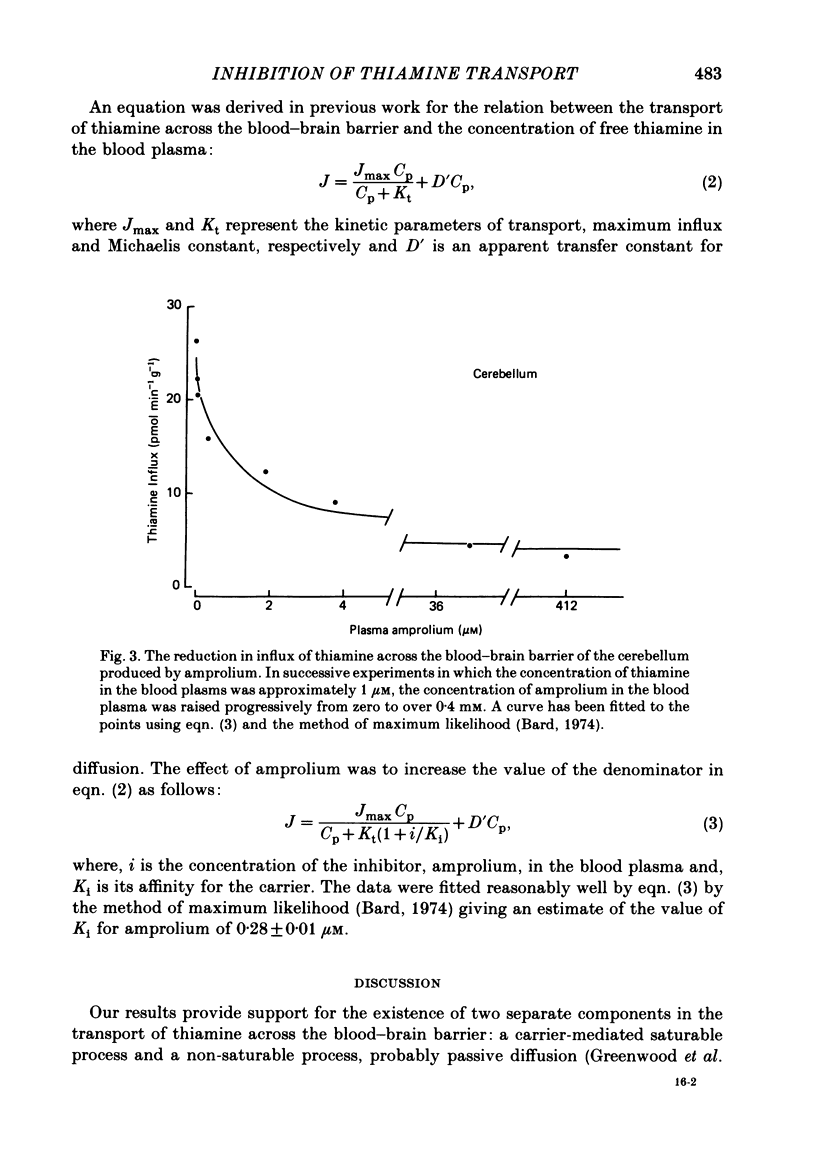
The flux of thiamine from the blood into the brain has been measured using a specially devised technique by which a steady raised level of the vitamin, with or without radioactive labelling, can be achieved rapidly and maintained in the bloodstream. This is done by a continuous injection, given at a rate which is adjusted by a pre-determined programme so as to replace the tracer at the rate at which it has been found to leave the circulation in previous experiments. A further programme was worked out to maintain, in a similar manner by a separate injection, a steady raised level in the bloodstream of a chemical analogue of thiamine, 1-[(4-amino-2-propyl-5-pyrimidinyl)methyl]-2-picolinium chloride HCl (amprolium). In the presence of a high concentration of amprolium the flux of thiamine across the blood-brain barrier was greatly reduced and no longer saturable by raising the blood thiamine concentration up to at least 10 microM. It was concluded that this analogue of thiamine inhibited the saturable component of thiamine transport across the barrier but not the non-saturable component. In a further series of experiments, progressively higher levels of thiamine were maintained in the bloodstream and the influx of the vitamin across the blood-brain barrier was measured. From kinetic analysis of the results, it was clear that the affinity of amprolium for the transport carrier was of a similar magnitude to that of thiamine itself. That the inhibition was competitive was shown by the way in which it could be overcome if the level of thiamine in the blood plasma was raised sufficiently above the normal.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniel P. M., Donaldson J., Pratt O. E. A method for injecting substances into the circulation to reach rapidly and to maintain a steady level. With examples of its application in the study of carbohydrate and amino acid metabolism. Med Biol Eng. 1975 Mar;13(2):214–227. doi: 10.1007/BF02477731. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Love E. R., Moorhouse S. R., Pratt O. E., Wilson P. A method for rapidly washing the blood out of an organ or tissue of the anaesthetized living animal. J Physiol. 1974 Mar;237(2):11P–12P. [PubMed] [Google Scholar]
- Edwin E. E., Jackman R. Ruminal thiaminase and tissue thiamine in cerebrocortical necrosis. Vet Rec. 1973 Jun 16;92(24):640–641. doi: 10.1136/vr.92.24.640. [DOI] [PubMed] [Google Scholar]
- Greenwood J., Love E. R., Pratt O. E. Kinetics of thiamine transport across the blood-brain barrier in the rat. J Physiol. 1982 Jun;327:95–103. doi: 10.1113/jphysiol.1982.sp014222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. Thiamine uptake in isolated schizonts of Eimeria tenella and the inhibitory effects of amprolium. Parasitology. 1980 Apr;80(2):313–322. doi: 10.1017/s0031182000000779. [DOI] [PubMed] [Google Scholar]
- Lumeng L., Edmondson J. W., Schenker S., Li T. K. Transport and metabolism of thiamin in isolated rat hepatocytes. J Biol Chem. 1979 Aug 10;254(15):7265–7268. [PubMed] [Google Scholar]
- Markson L. M., Edwin E. E., Lewis G., Richardson C. The production of cerebrocortical necrosis in ruminant calves by the intraruminal administration of Amprolium. Br Vet J. 1974 Jan-Feb;130(1):9–16. doi: 10.1016/s0007-1935(17)35985-7. [DOI] [PubMed] [Google Scholar]
- Markson L. M., Lewis G., Terlecki S., Edwin E. E., Ford J. E. The aetiology of cerebrocortical necrosis: the effects of administering antimetabolites of thiamine to preruminant calves. Br Vet J. 1972 Oct;128(10):488–499. doi: 10.1016/s0007-1935(17)36733-7. [DOI] [PubMed] [Google Scholar]
- POLIN D., WYNOSKY E. R., PORTER C. C. IN VIVO ABSORPTION OF AMPROLIUM AND ITS COMPETITION WITH THIAMINE. Proc Soc Exp Biol Med. 1963 Nov;114:273–277. doi: 10.3181/00379727-114-28650. [DOI] [PubMed] [Google Scholar]
- Patrini C., Rindi G. An improved method for the electrophoretic separation and fluorometric determination of thiamine and its phosphates in animal tissues. Int J Vitam Nutr Res. 1980;50(1):10–18. [PubMed] [Google Scholar]
- Pincus J. H., Solitare G. B., Cooper J. R. Thiamine triphosphate levels and histopathology. Correlation in Leigh disease. Arch Neurol. 1976 Nov;33(11):759–763. doi: 10.1001/archneur.1976.00500110027005. [DOI] [PubMed] [Google Scholar]
- Pratt O. E. Kinetics of tryptophan transport across the blood-brain barrier. J Neural Transm Suppl. 1979;(15):29–42. doi: 10.1007/978-3-7091-2243-3_3. [DOI] [PubMed] [Google Scholar]
- Rindi G., Ventura U. Thiamine intestinal transport. Physiol Rev. 1972 Oct;52(4):821–827. doi: 10.1152/physrev.1972.52.4.821. [DOI] [PubMed] [Google Scholar]
- Ryley J. F., Betts M. J. Chemotherapy of chicken coccidiosis. Adv Pharmacol Chemother. 1973;11:221–293. doi: 10.1016/s1054-3589(08)60459-7. [DOI] [PubMed] [Google Scholar]
- SHARMA S. K., QUASTEL J. H. TRANSPORT AND METABOLISM OF THIAMINE IN RAT BRAIN CORTEX IN VITRO. Biochem J. 1965 Mar;94:790–800. doi: 10.1042/bj0940790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. D. Alcohol-related structural brain changes. Br Med Bull. 1982 Jan;38(1):87–91. doi: 10.1093/oxfordjournals.bmb.a071740. [DOI] [PubMed] [Google Scholar]
- Thomson A. D., Baker H., Leevy C. M. Patterns of 35S-thiamine hydrochloride absorption in the malnourished alcoholic patient. J Lab Clin Med. 1970 Jul;76(1):34–45. [PubMed] [Google Scholar]



