Abstract
1. Two mechanisms proposed for the quantal release of acetylcholine (ACh) are: (i) that the quanta are pre-packaged in vesicles and released by exocytosis and (ii) that the ACh is released from the cytoplasm of the nerve terminal by the opening of an ACh channel. Our experiments were designed to test aspects of these hypotheses.
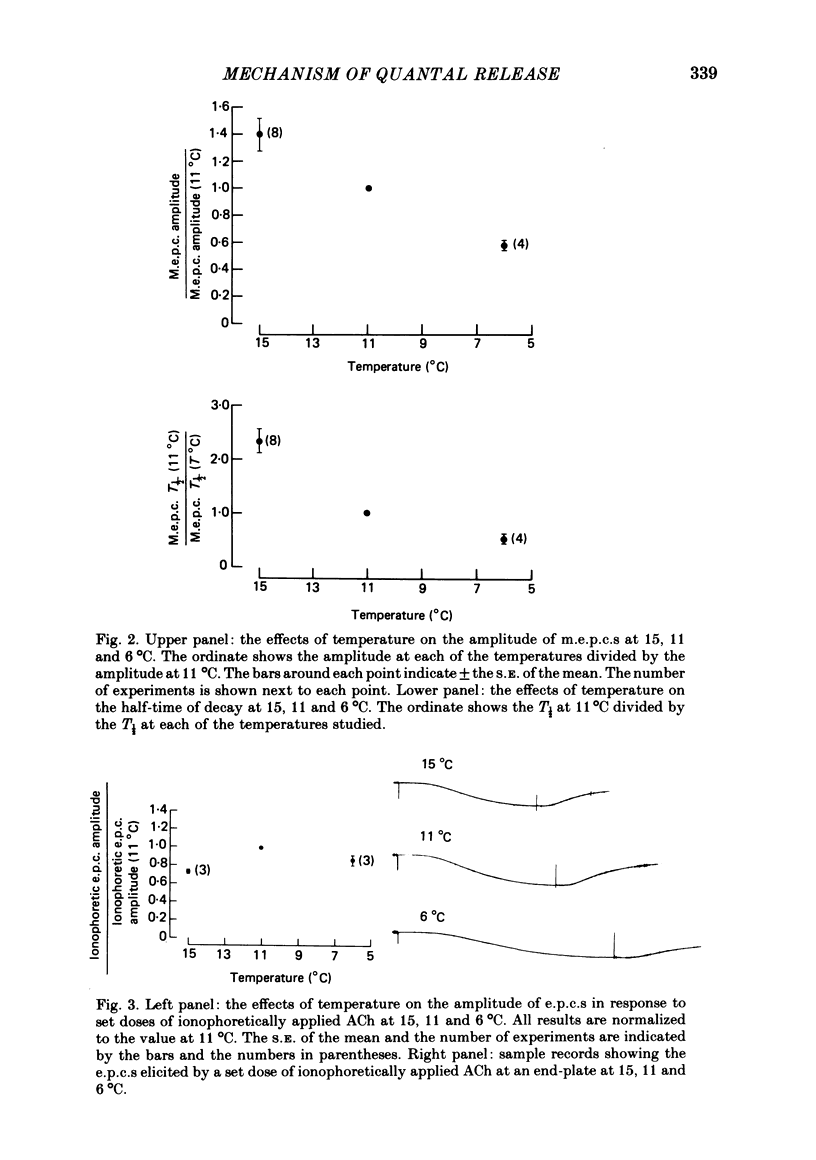
2. Miniature end-plate currents (m.e.p.c.s) were reversibly decreased in amplitude and increased in duration as the temperature was decreased between 15 and 6 °C. The amplitude decreased with a Q10 of 2·4 between 15 and 11 °C, and then with a Q10 of 3 between 11 and 6 °C. The half-decay time increased with a Q10 between 4 and 5 over the entire temperature range.
3. The effect of temperature on the end-plate current (e.p.c.) in response to ionophoretically applied ACh was also studied. The e.p.c. in response to a set, sustained dose of ACh was 30% larger at 11 °C than at 15 °C, and about 10% larger at 6 °C than at 15 °C.
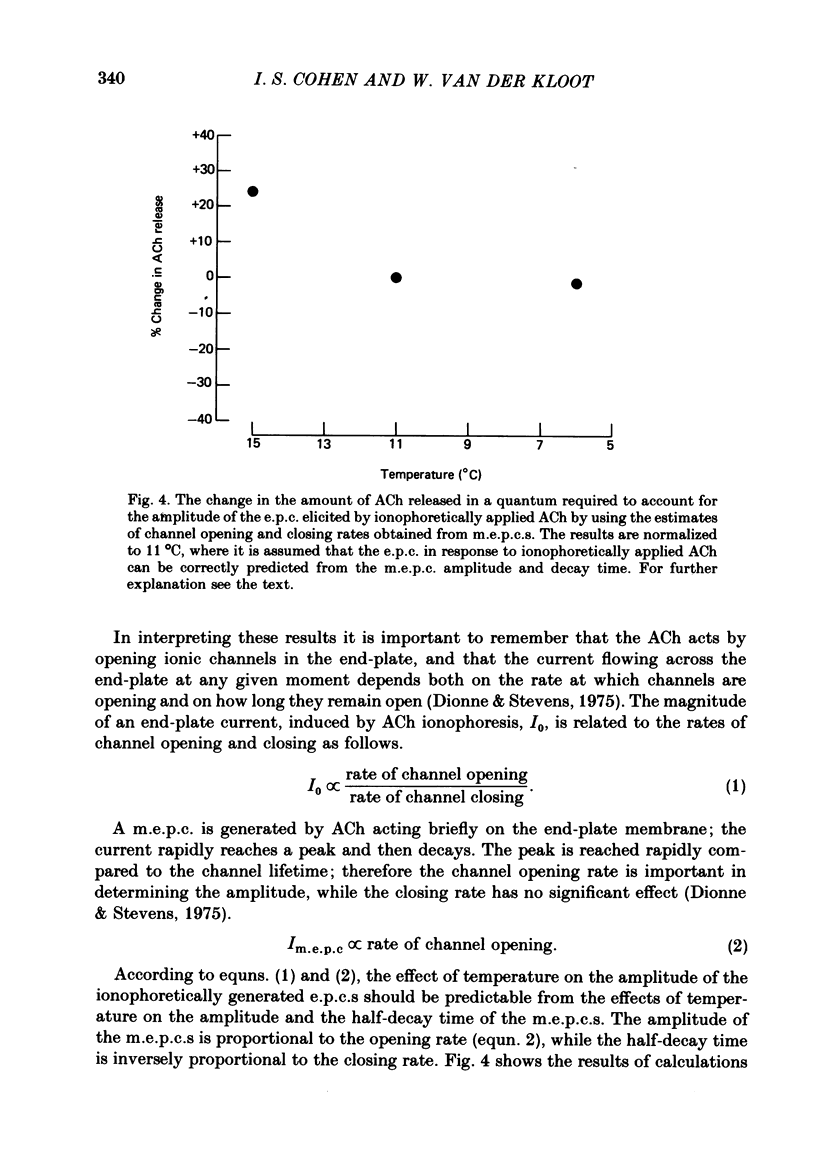
4. The difference in the end-plate response to brief pulses of ACh (m.e.p.c.s) and to sustained application of ACh was analysed by Dionne & Stevens (1975). The amplitude of the sustained response depends on both the number of channels opened in the end-plate, and the length of time they stay open. When our temperature data are analysed in this way, it appears that the amount of ACh/quanta acting on the end-plate is altered by less than 25% over the temperature range 15-6 °C.
5. From the experiments in which the nerve terminal membrane potential was shifted by external currents it was concluded that miniature end-plate potential amplitude was independent of the terminal membrane potential (Cooke & Quastel, 1973). This conclusion was confirmed by measuring m.e.p.c.s in low Ca2+ Ringer solution containing 2·0 and 22·0 mM-KCl; there was no consistent change in m.e.p.c. amplitude.
6. The effects of both temperature and terminal membrane potential are more readily interpreted by the vesicle than by the channel hypothesis, since for the channel hypothesis the duration of channel opening should be temperature-sensitive and the efflux of ACh+ through the terminal membrane should be potential-dependent.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colméus C., Gomez S., Molgó J., Thesleff S. Discrepancies between spontaneous and evoked synaptic potentials at normal, regenerating and botulinum toxin poisoned mammalian neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1982 Apr 22;215(1198):63–74. doi: 10.1098/rspb.1982.0028. [DOI] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. On the elementary conductance event produced by L-glutamate and quanta of the natural transmitter at the neuromuscular junctions of Maia squinado. J Physiol. 1976 Jun;258(1):205–225. doi: 10.1113/jphysiol.1976.sp011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y., Jones G. J., Loctin F. Acetylcholine measured at short time intervals during transmission of nerve impulses in the electric organ of Torpedo. J Physiol. 1982 Apr;325:441–460. doi: 10.1113/jphysiol.1982.sp014161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., MOORE L. E. THE EFFECT OF TEMPERATURE ON THE SODIUM AND POTASSIUM PERMEABILITY CHANGES IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:431–437. doi: 10.1113/jphysiol.1963.sp007269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Van Helden D. Effects of permeant monovalent cations on end-plate channels. J Physiol. 1979 Mar;288:509–528. [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M., Dunant Y., Manaranche R. The present status of the vesicular hypothesis. Prog Neurobiol. 1979;13(3):237–275. doi: 10.1016/0301-0082(79)90017-0. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden T. E., Citron M. C., Pierantoni R. The jet stream microbeveler: an inexpensive way to bevel ultrafine glass micropipettes. Science. 1978 Aug 4;201(4354):469–470. doi: 10.1126/science.663670. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L. Quantitative simulation of endplate currents at neuromuscular junctions based on the reaction of acetylcholine with acetylcholine receptor and acetylcholinesterase. Biophys J. 1979 May;26(2):263–289. doi: 10.1016/S0006-3495(79)85249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. Quantal size is not altered by abrupt changes in nerve terminal volume. Nature. 1978 Feb 9;271(5645):561–562. doi: 10.1038/271561a0. [DOI] [PubMed] [Google Scholar]
- Wathey J. C., Nass M. M., Lester H. A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979 Jul;27(1):145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]



