Abstract
1. Sugar transport in the giant muscle cells of Balanus nubilus is accelerated during contractile activity and exposure to porcine insulin. The characteristics of hexose-transfer regulation in the giant muscle cells have been examined by studying the transport of 3-O-methylglucose (a non-metabolized sugar) in both intact giant fibres and fibres subjected to internal solute control by internal dialysis.
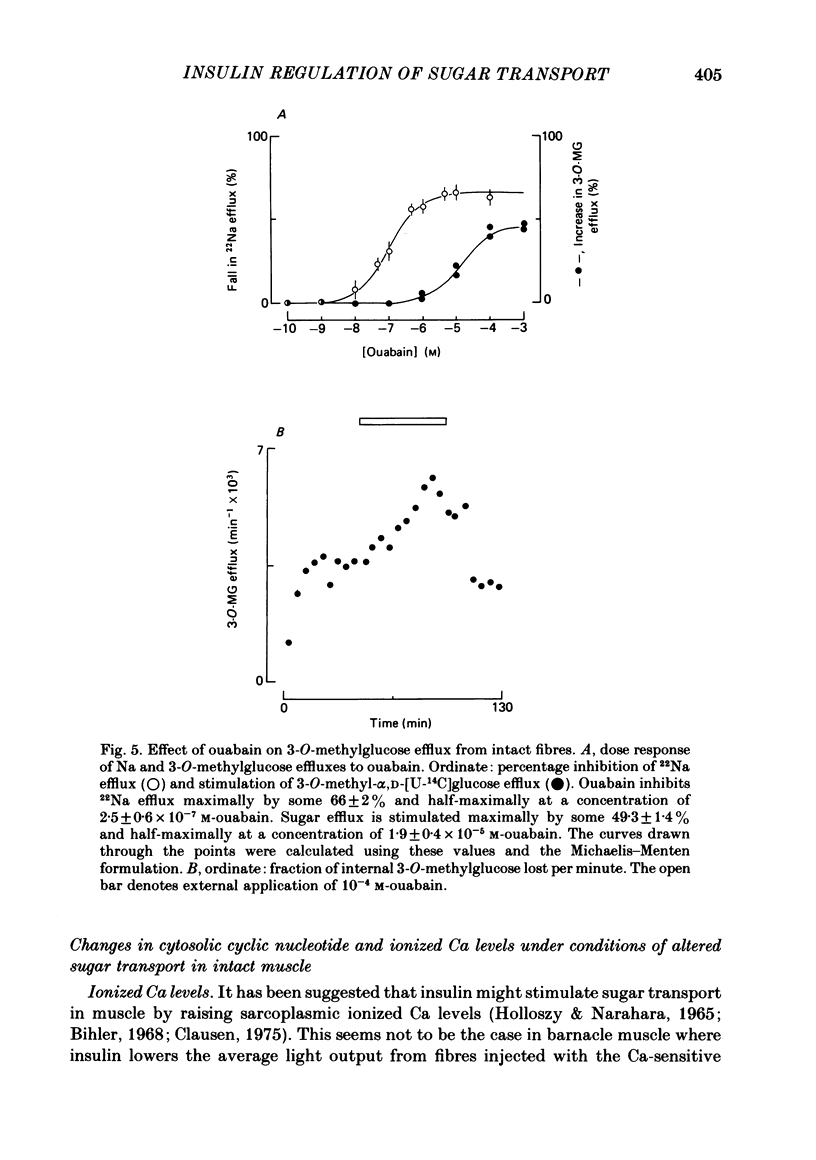
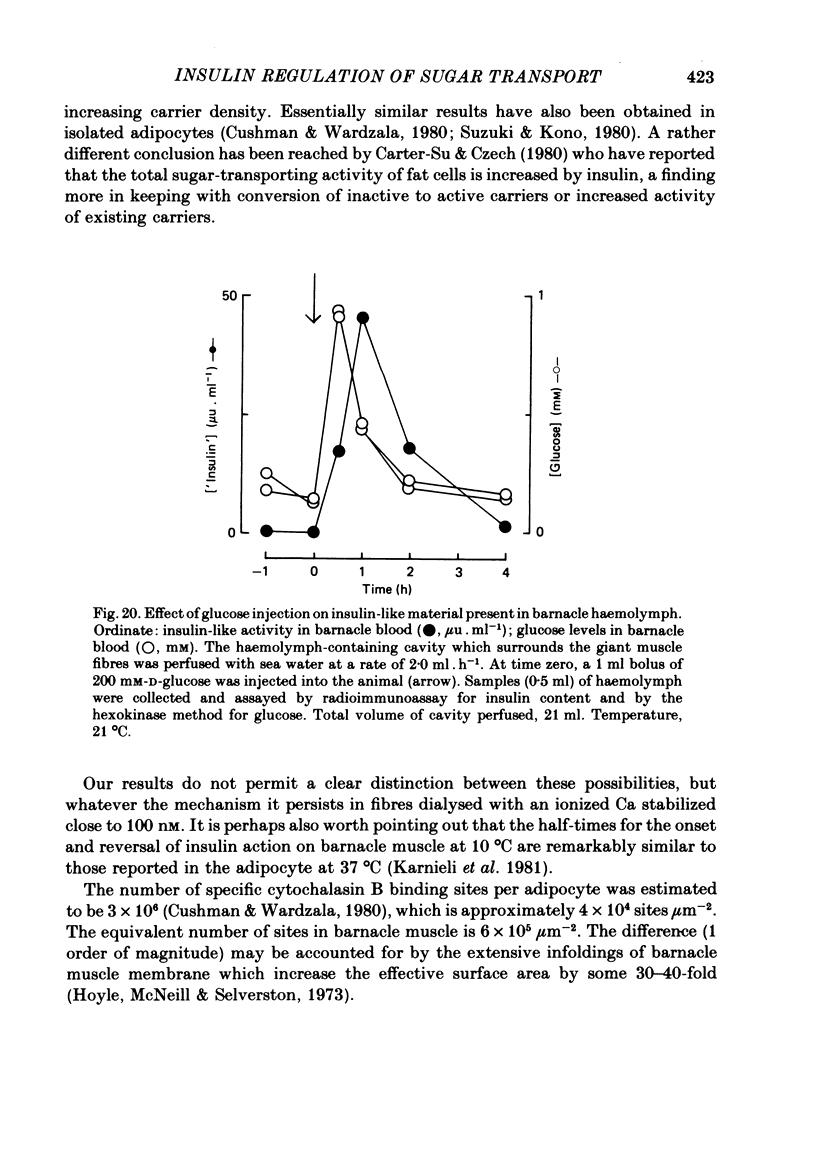
2. Sugar transport in barnacle muscle is mediated by a saturable process which is inhibited by both phloretin and cytochalasin B. Insulin increases the capacity of the transport system with little effect on its apparent affinity for sugar. Under the same conditions insulin increases 3-O-methylglucose-displaceable cytochalasin B binding. The effects of insulin on transport are half-maximal at 5 μM-insulin and are abolished by both insulin antibody and phloretin. The intact barnacle releases an insulin-like material in response to a rise in blood glucose levels.
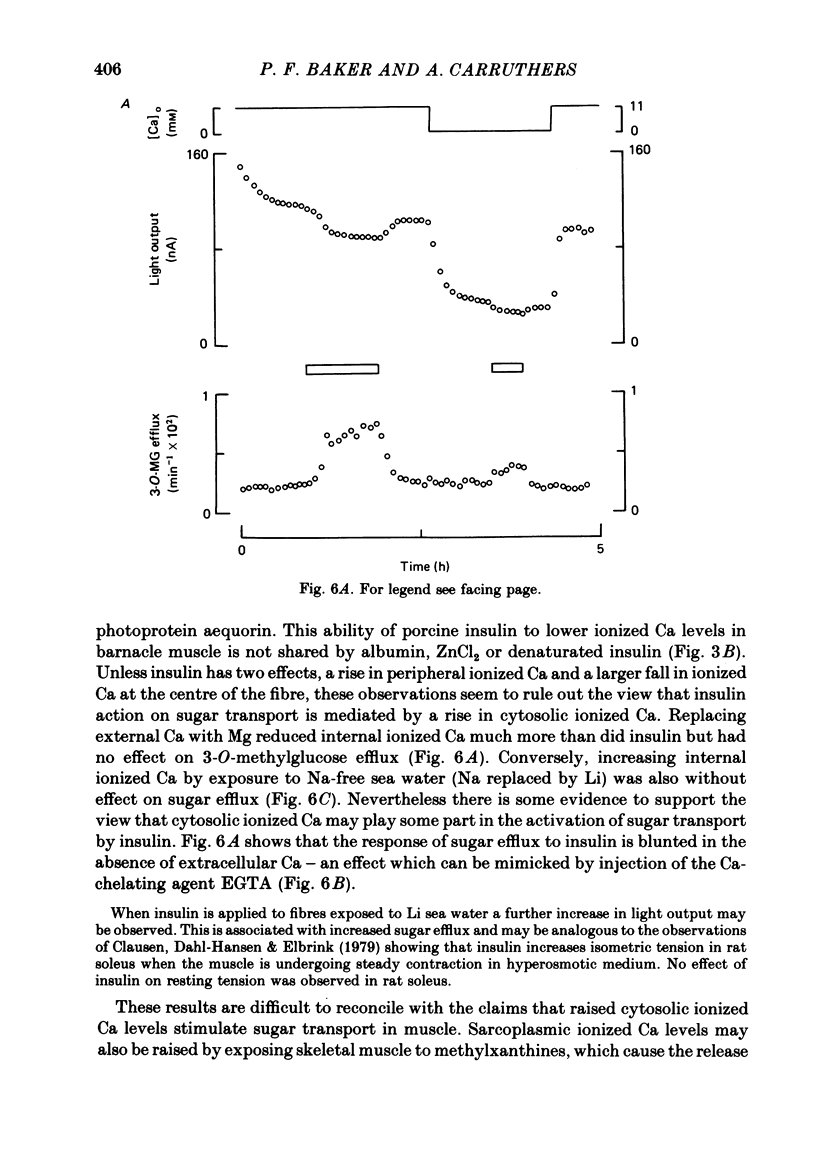
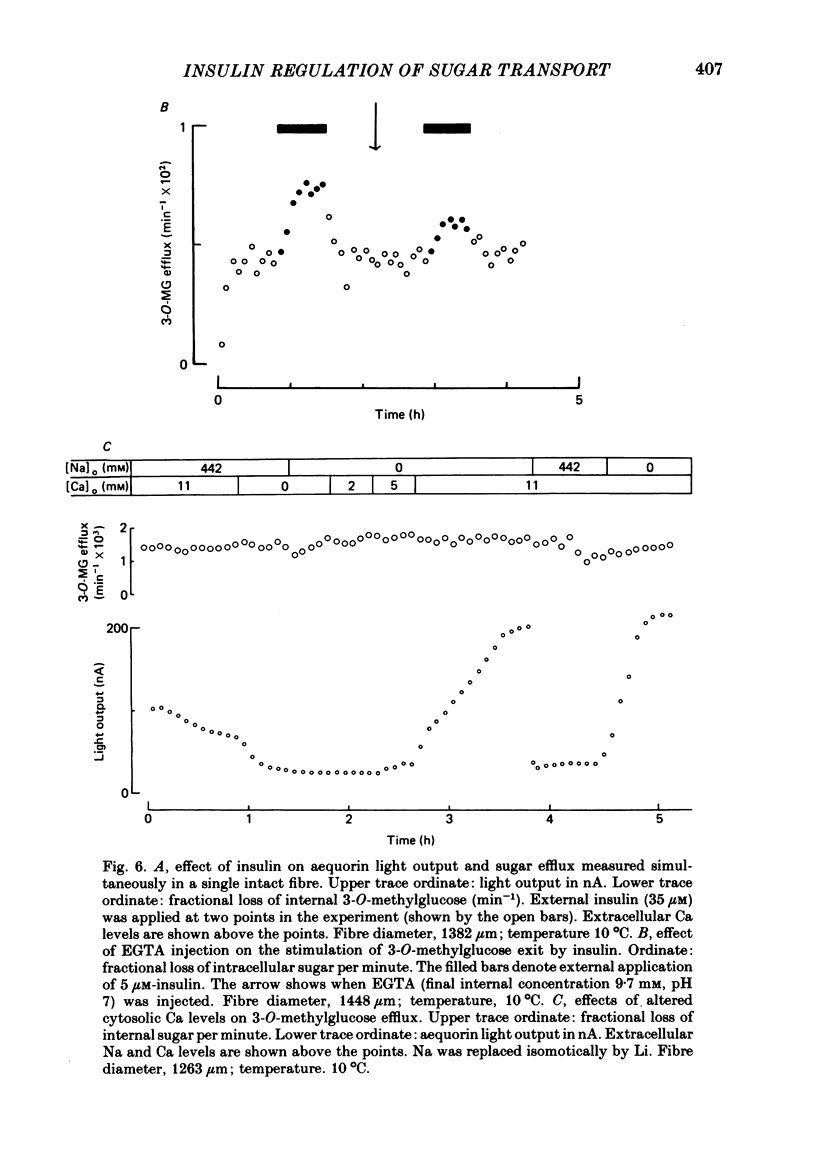
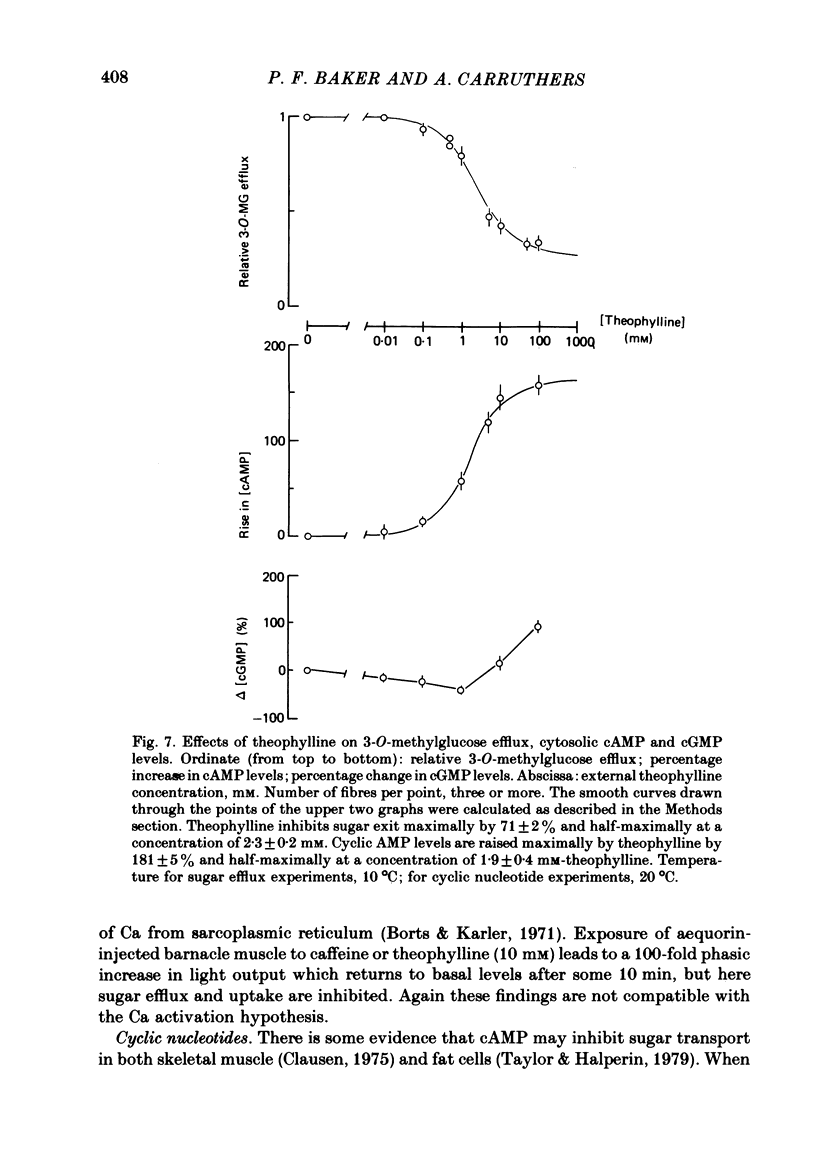
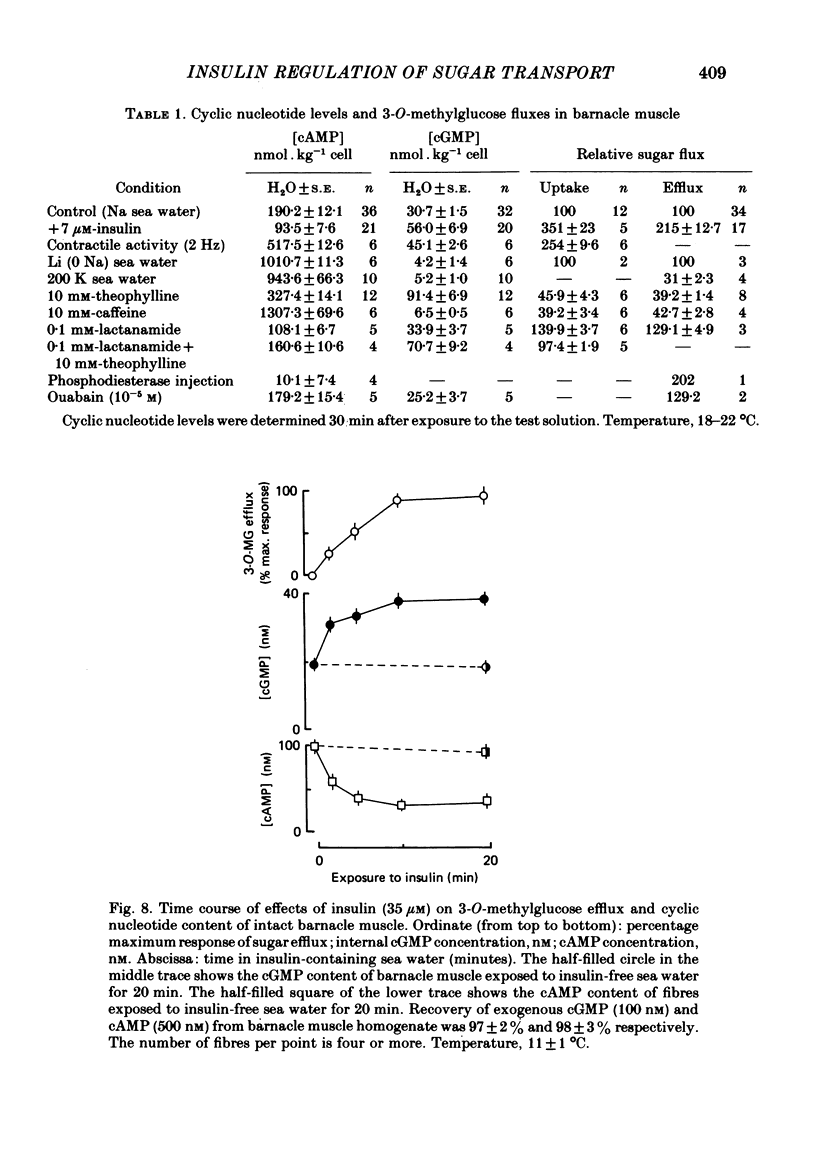
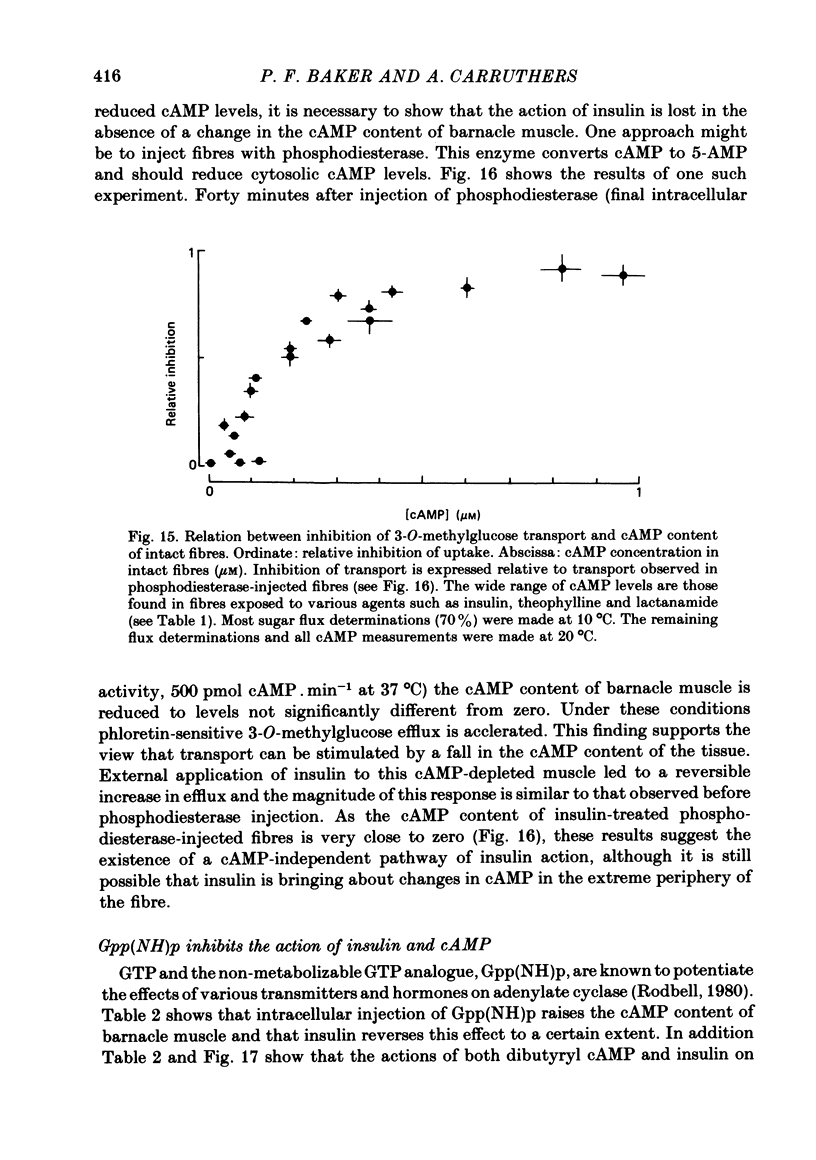
3. Insulin increases the cyclic GMP (cGMP) content and reduces the cyclic AMP (cAMP) content of barnacle muscle. Experiments with fibres injected with aequorin show that insulin also lowers cytosolic ionized Ca levels. The changes in cyclic nucleotide levels induced by insulin precede the effects on sugar transport and cytosolic ionized Ca. During repetitive contractile activity, cAMP, cGMP and ionized Ca levels are raised.
4. Agents which raise the cAMP content of barnacle muscle normally inhibit sugar transport. Dibutyryl cAMP also inhibits transport. Alterations in cytosolic ionized Ca levels in intact fibres are without effect on sugar transport. Nevertheless, stimulation of transport by insulin is blunted when cytosolic ionized Ca is lowered by intracellular injection of the Ca-chelating agent, EGTA.
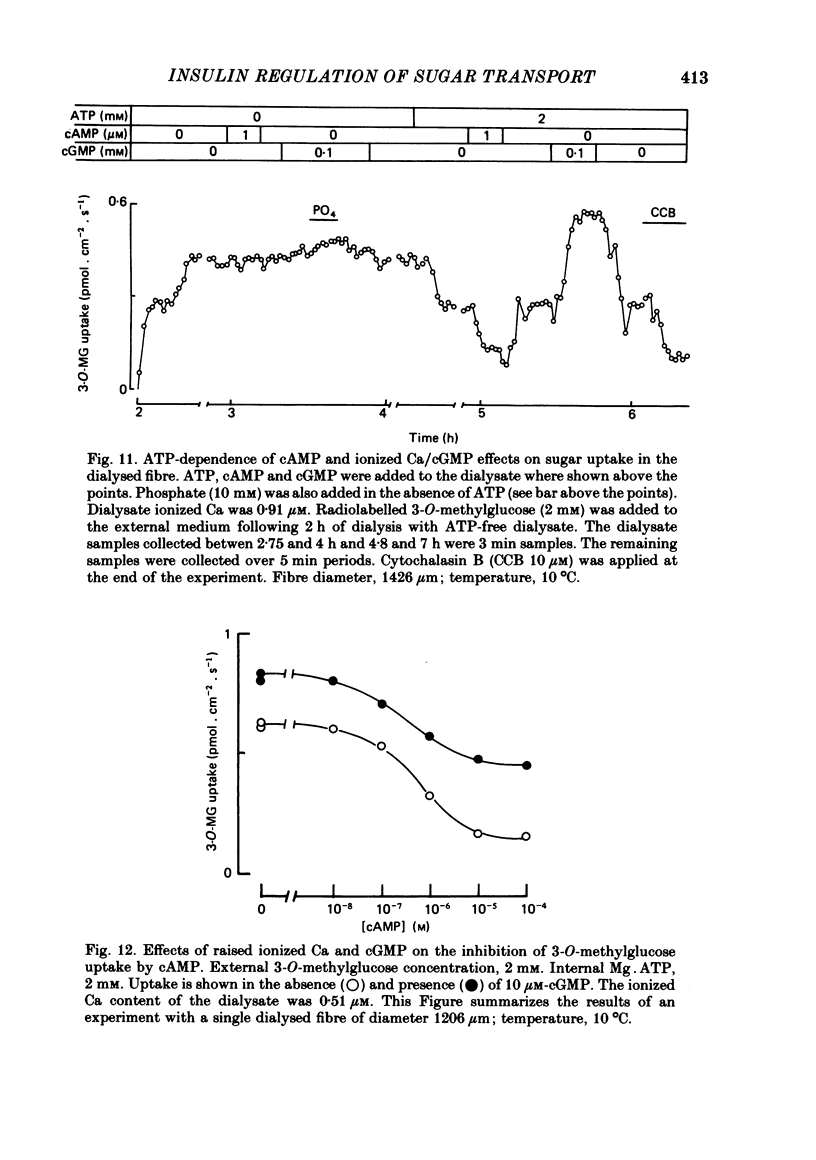
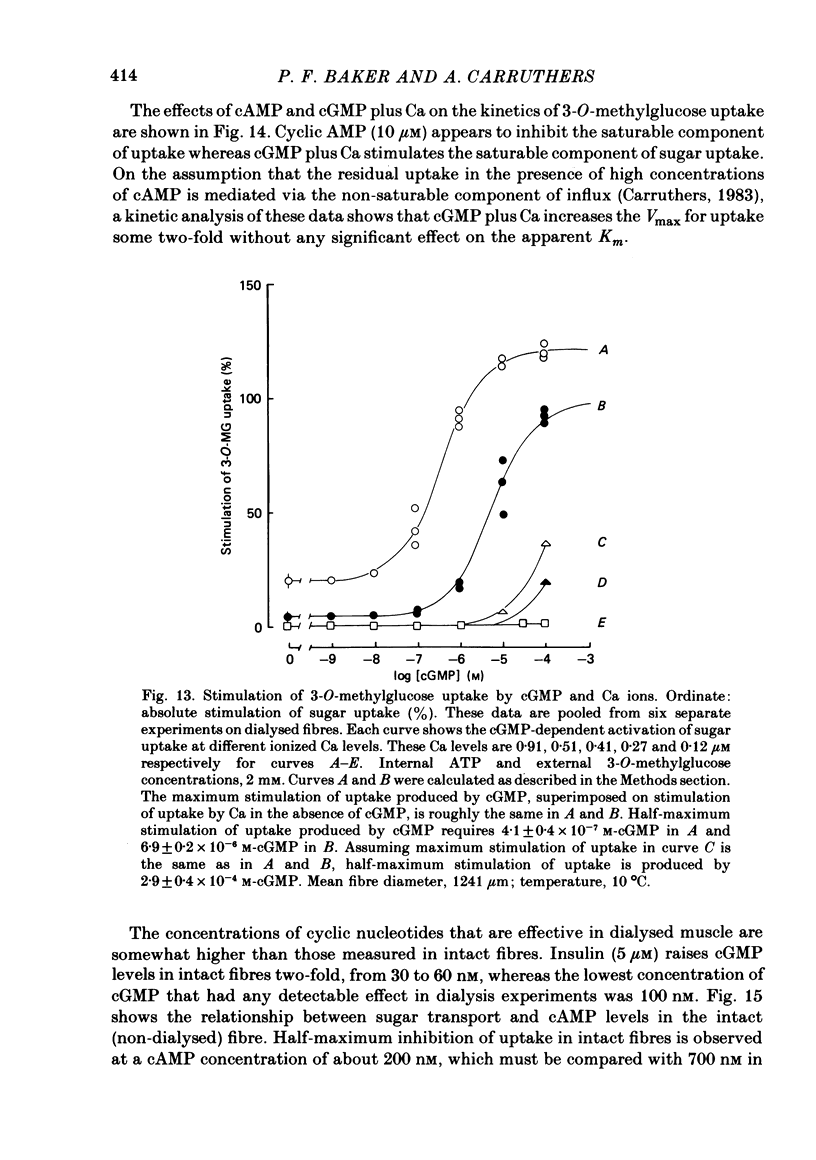
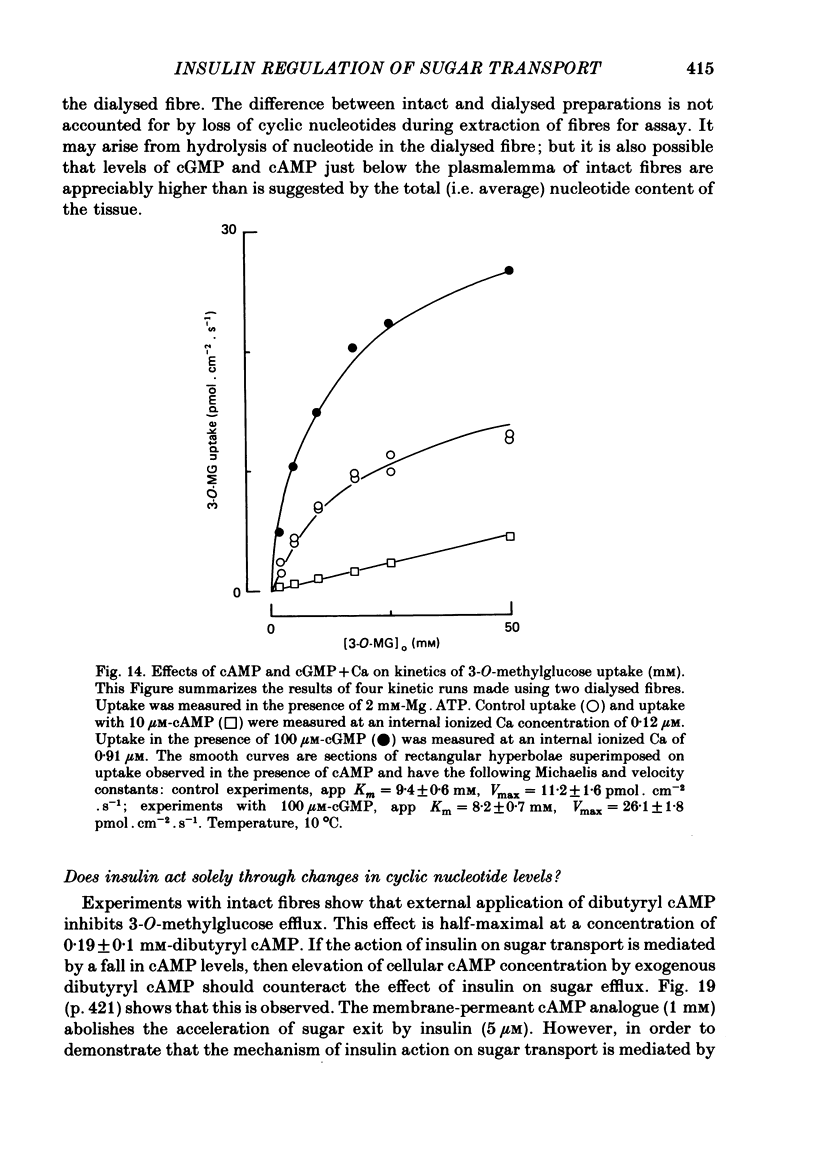
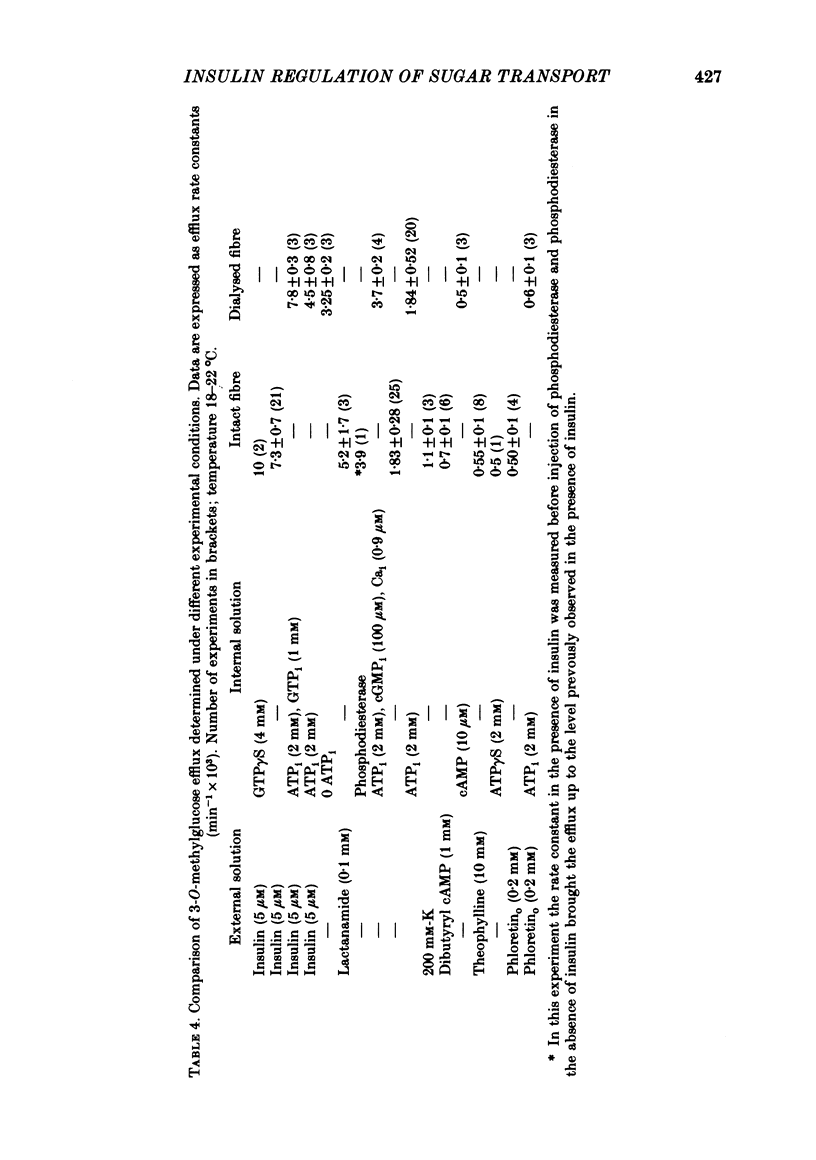
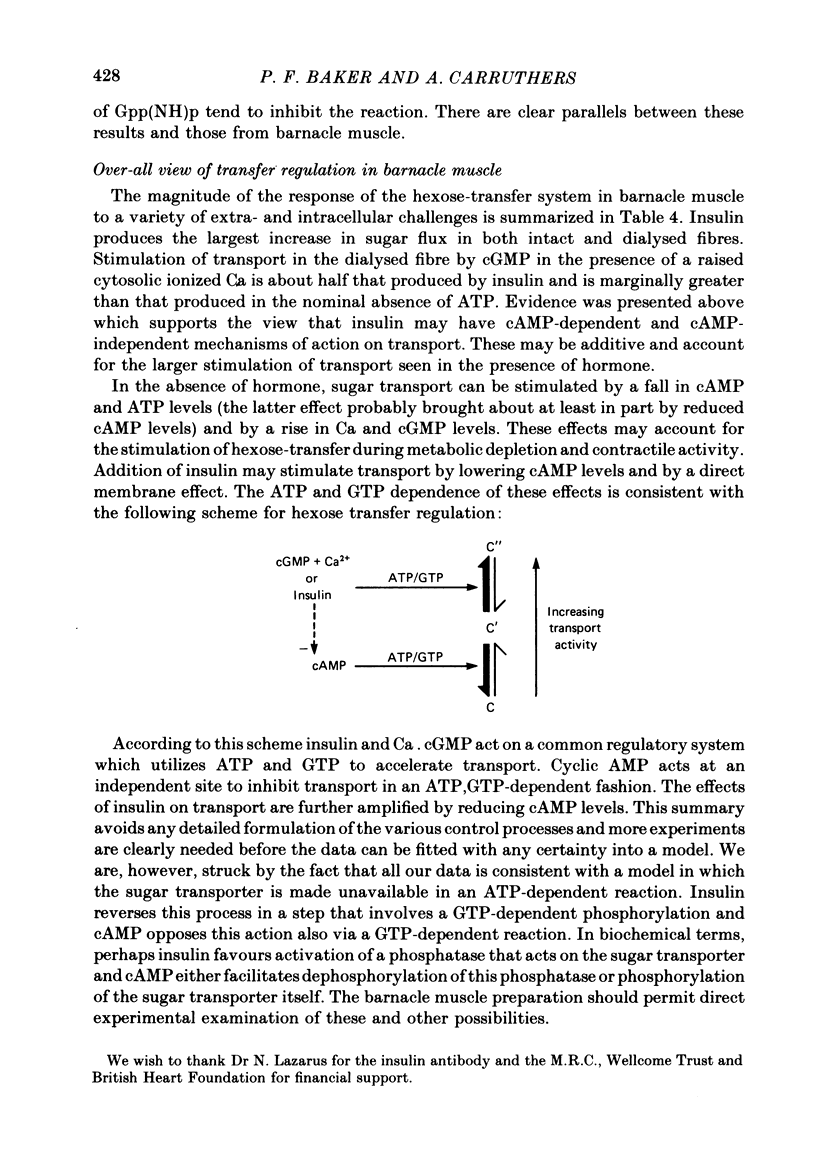
5. Sugar uptake in the internally dialysed fibre is inhibited by intracellular application of cAMP. Internal application of Ca and cGMP stimulate sugar uptake in the dialysed fibre. Cyclic AMP reduces the capacity of the transport system whereas Ca and cGMP increase the capacity of the saturable transfer system. Cyclic AMP and cGMP act at kinetically independent sites. Internal ATP (2 mM) inhibits sugar uptake in the dialysed fibre by some 40%, possibly through the production of cAMP.
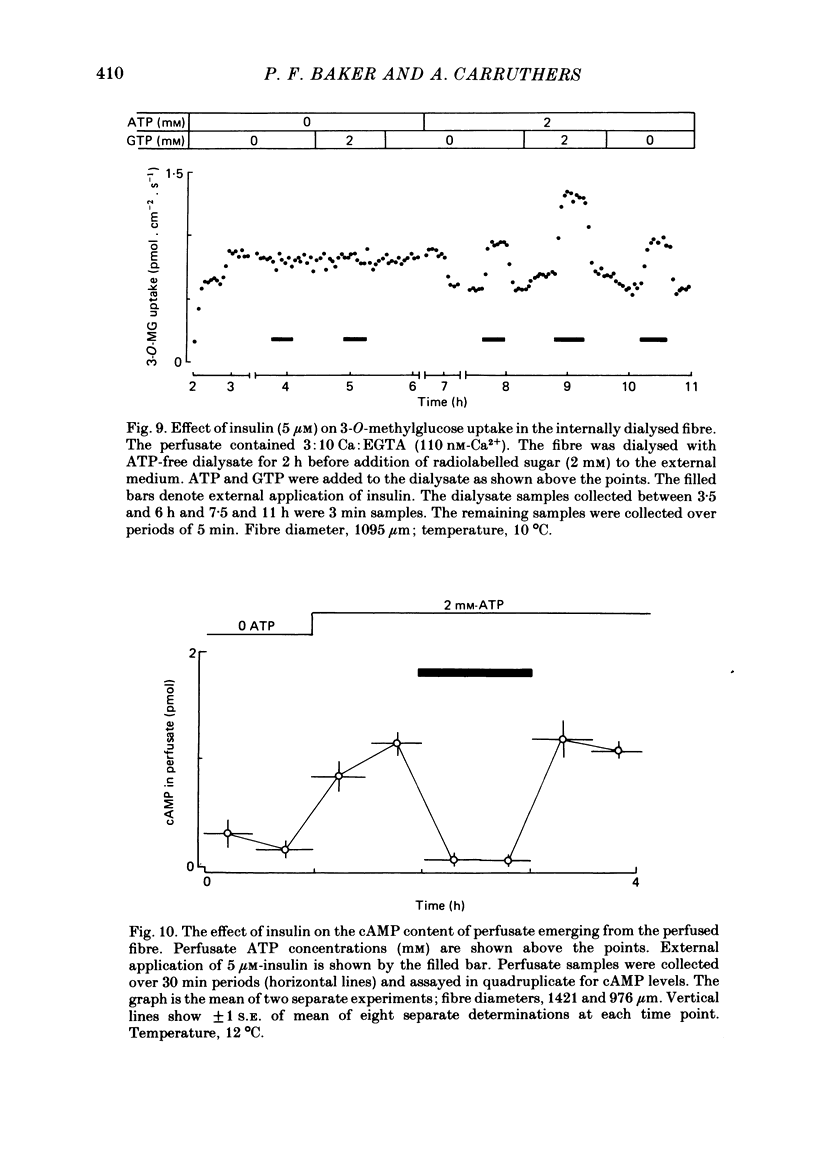
6. External insulin stimulates sugar uptake in the dialysed fibre even when ionized Ca levels are buffered using EGTA. Stimulation by insulin requires the presence of cytosolic ATP and is potentiated by internal application of 1 mM-GTP. In the dialysed fibre stimulation of transport by insulin is greater than that brought about by Ca and cGMP.
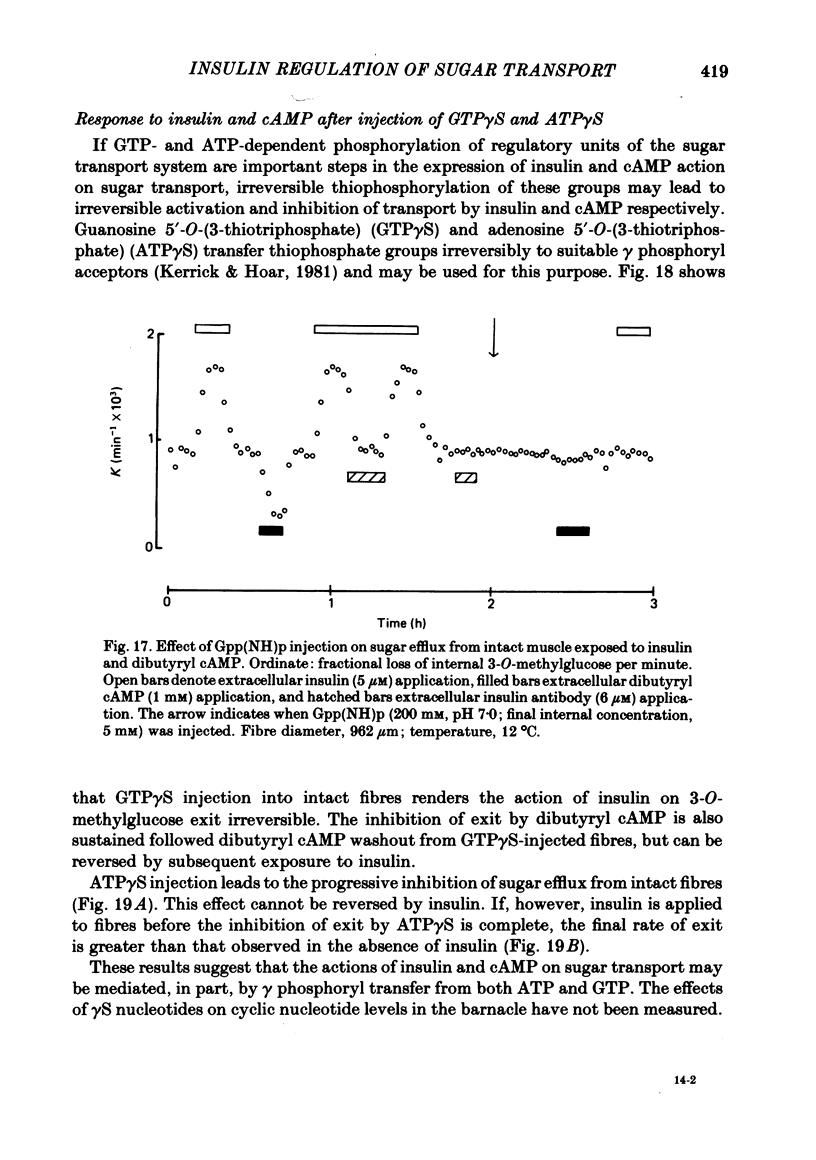
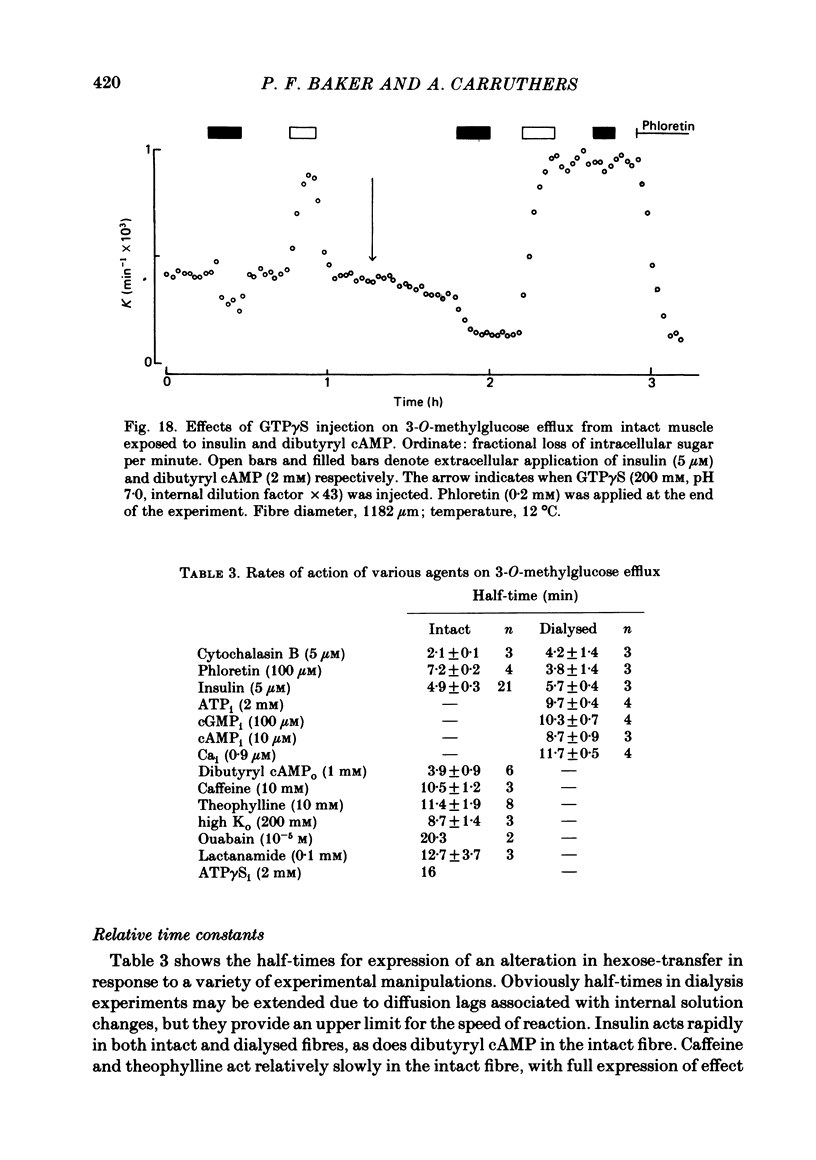
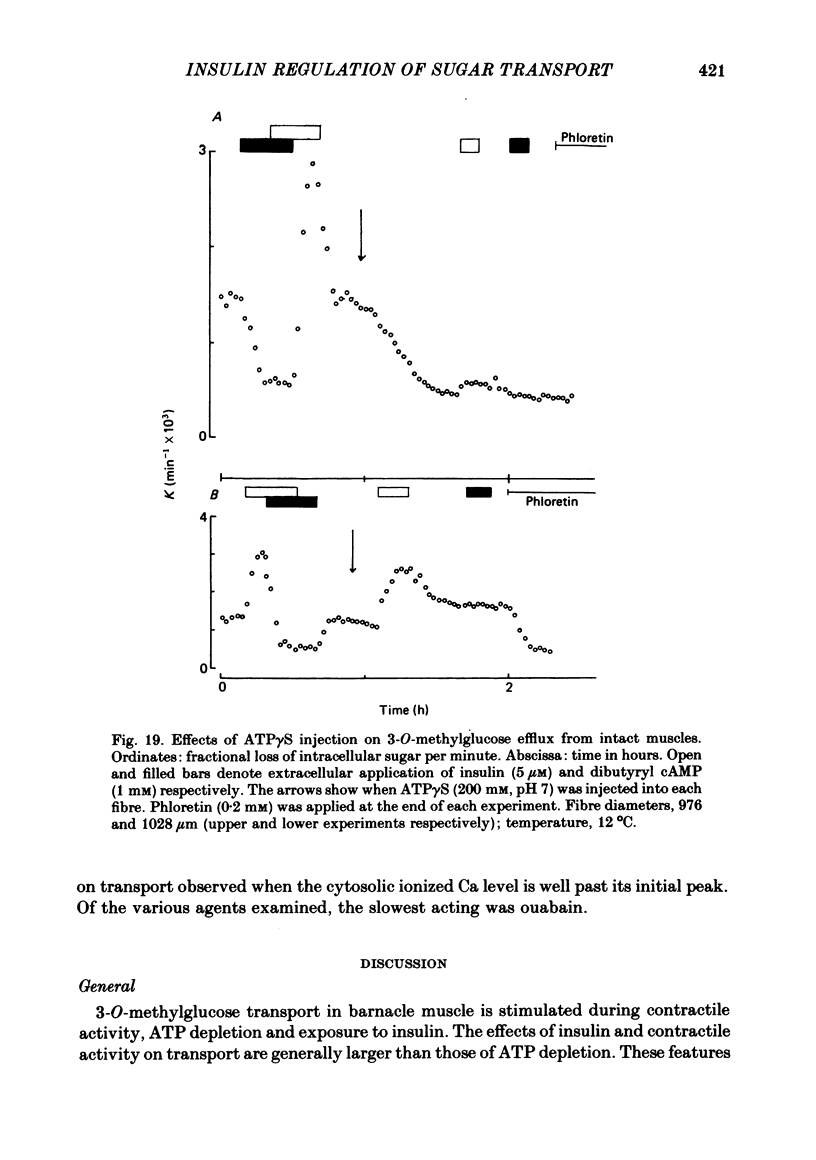
7. The stimulation of transport by insulin in the intact fibre and its inhibition by dibutyryl cAMP are abolished by intracellular injection of Gpp(NH)p. Injection of intact fibres with GTPγS potentiates the stimulation of transport by insulin and renders insulin-activation of transport irreversible. Injection of intact fibres with ATPγS leads to the irreversible inhibition of transport.
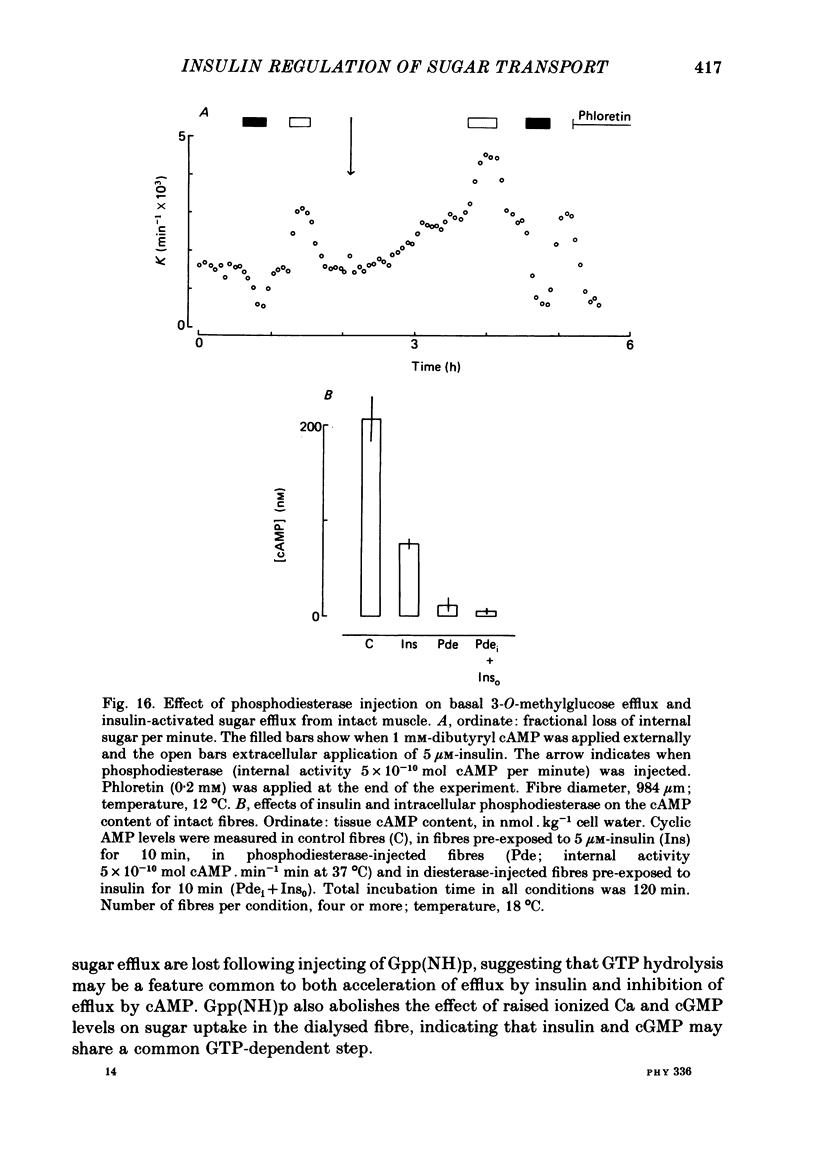
8. Injection of intact fibres with cAMP phosphodiesterase lowers cAMP levels close to zero and stimulates sugar transport. Application of insulin to diesterase-injected fibres still stimulates transport in the absence of altered cytosolic cAMP.
Full text
PDF


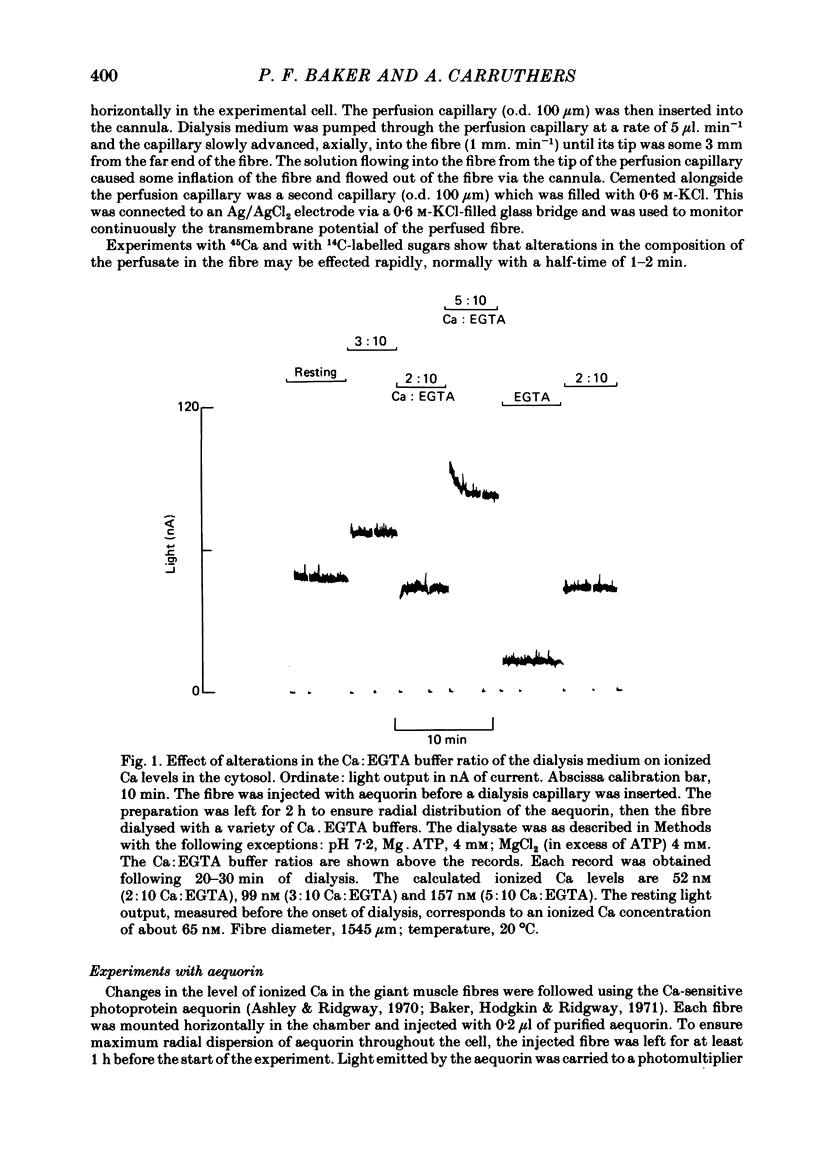

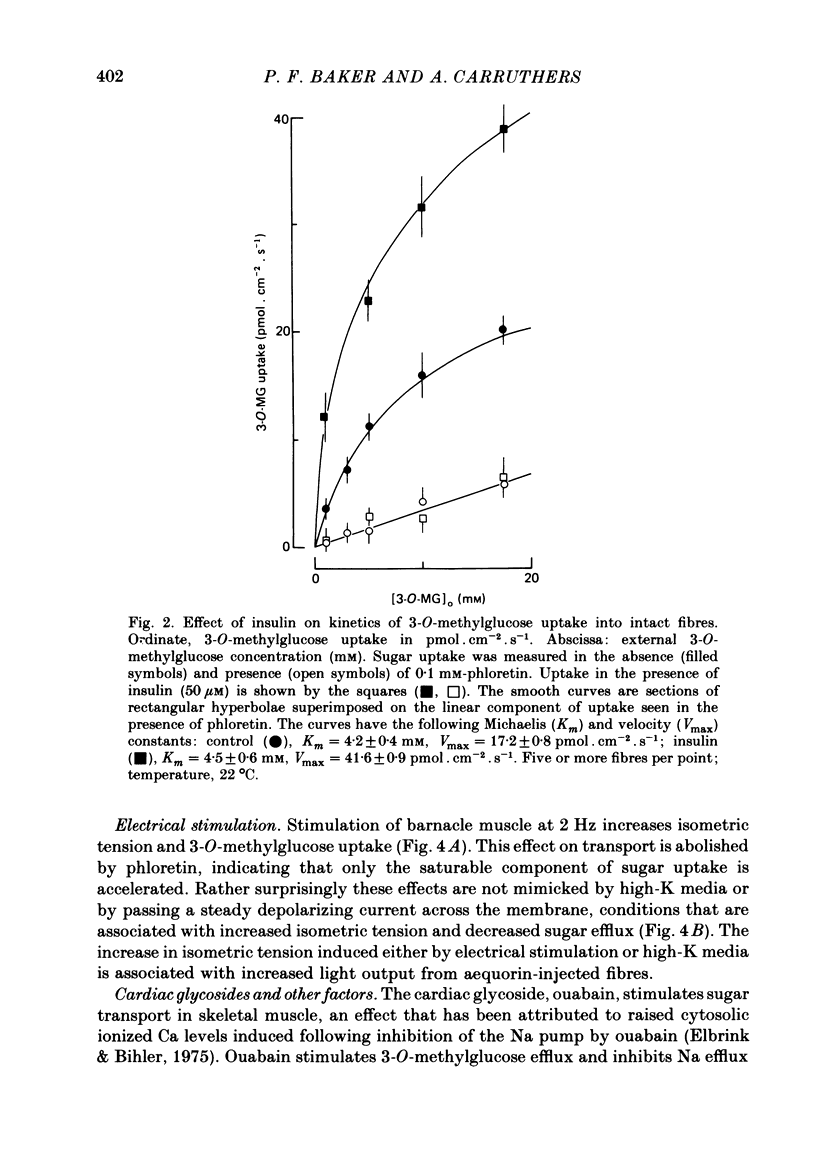
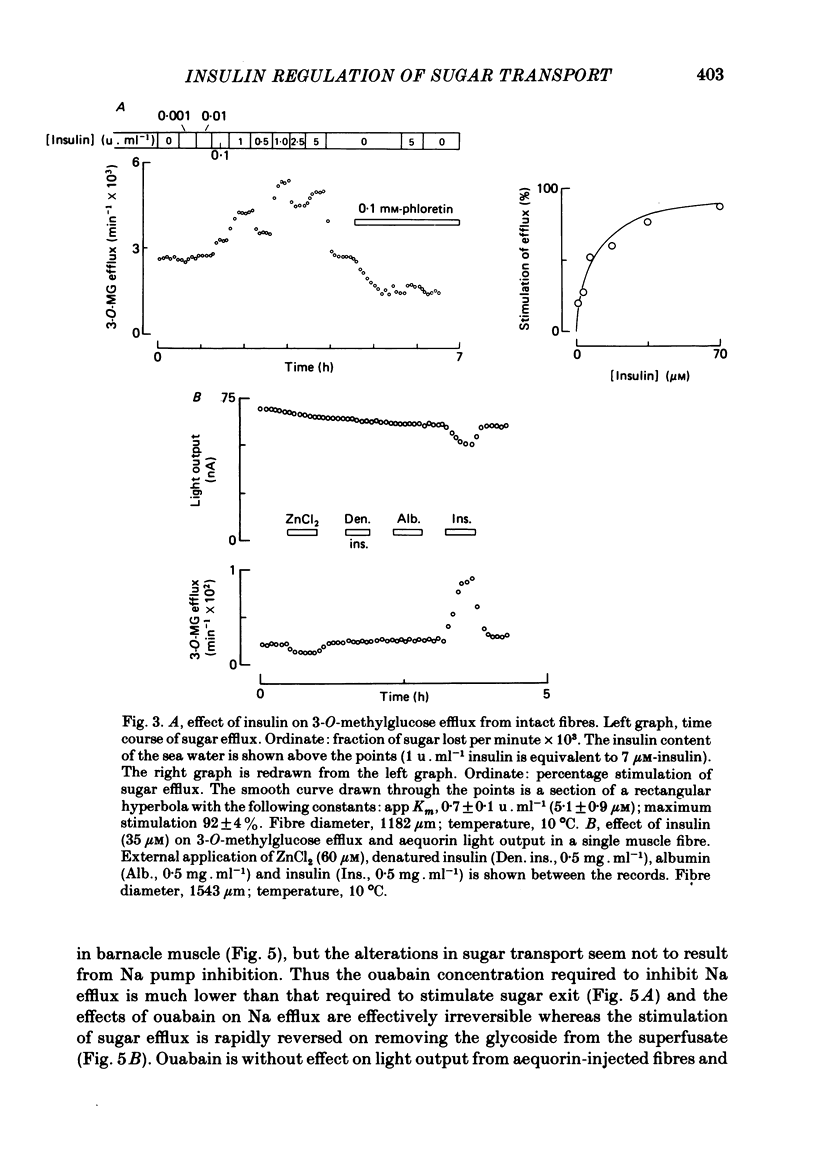
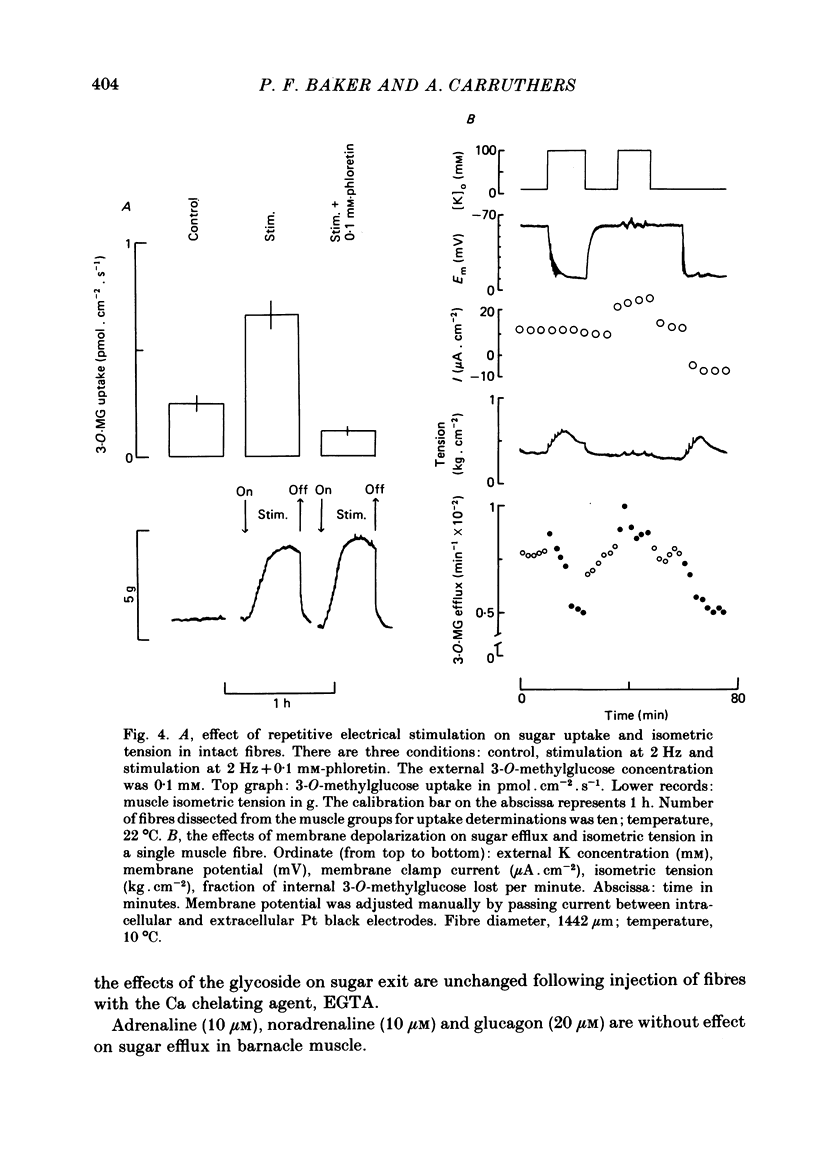










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Carruthers A. 3-O-methylglucose transport in internally dialysed giant axons of Loligo. J Physiol. 1981 Jul;316:503–525. doi: 10.1113/jphysiol.1981.sp013803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Carruthers A. Insulin stimulates sugar transport in giant muscle fibres of the barnacle. Nature. 1980 Jul 17;286(5770):276–279. doi: 10.1038/286276a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham G. J., Denton R. M., Tanner M. J. Use of a novel rapid preparation of fat-cell plasma membranes employing Percoll to investigate the effects of insulin and adrenaline on membrane protein phosphorylation within intact fat-cells. Biochem J. 1980 Nov 15;192(2):457–467. doi: 10.1042/bj1920457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihler I. The action of cardiotonic steroids on sugar-transport in muscle, in vitro. Biochim Biophys Acta. 1968 Nov 5;163(3):401–410. doi: 10.1016/0005-2736(68)90125-9. [DOI] [PubMed] [Google Scholar]
- Borys H. K., Karler R. Effects of caffeine on the intracellular distribution of calcium in frog sartorius muscle. J Cell Physiol. 1971 Dec;78(3):387–404. doi: 10.1002/jcp.1040780308. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2303–2331. doi: 10.1085/jgp.50.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Scarpa A., Tiffert T. The concentration of ionized magnesium in barnacle muscle fibres. J Physiol. 1977 Apr;266(3):545–565. doi: 10.1113/jphysiol.1977.sp011781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busuttil R. W., Paddock R. J., Fisher J. W., George W. J. Changes in cyclic nucleotide levels and contractile force in the isolated hypoxic rat heart during perfusion with glucagon. Circ Res. 1976 Mar;38(3):162–167. doi: 10.1161/01.res.38.3.162. [DOI] [PubMed] [Google Scholar]
- Carruthers A. Internal dialysis as a method for studying sugar transport in large nerve and muscle fibres [proceedings]. J Physiol. 1979 Feb;287:5P–6P. [PubMed] [Google Scholar]
- Carruthers A. Sugar transport in giant barnacle muscle fibres. J Physiol. 1983 Mar;336:377–396. doi: 10.1113/jphysiol.1983.sp014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Su C., Czech M. P. Reconstitution of D-glucose transport activity from cytoplasmic membranes. Evidence against recruitment of cytoplasmic membrane transporters into the plasma membrane as the sole action of insulin. J Biol Chem. 1980 Nov 10;255(21):10382–10386. [PubMed] [Google Scholar]
- Chambaut A. M., Eboué-Bonis D., Hanoune J., Clauser H. Antagonistic actions between dibutyryl adenosine-3',5'-cyclic monophosphate and insulin on the metabolism of the surviving rat diaphragm. Biochem Biophys Res Commun. 1969 Feb 7;34(3):283–290. doi: 10.1016/0006-291x(69)90829-8. [DOI] [PubMed] [Google Scholar]
- Clausen T., Dahl-Hansen A. B., Elbrink J. The effect of hyperosmolarity and insulin on resting tension and calcium fluxes in rat soleus muscle. J Physiol. 1979 Jul;292:505–526. doi: 10.1113/jphysiol.1979.sp012868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Elbrink J., Dahl-Hansen A. B. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. IX. The role of cellular calcium in the activation of the glucose transport system in rat soleus muscle. Biochim Biophys Acta. 1975 Jan 28;375(2):292–308. doi: 10.1016/0005-2736(75)90197-2. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Elbrink J., Bihler I. Membrane transport: its relation to cellular metabolic rates. Science. 1975 Jun 20;188(4194):1177–1184. doi: 10.1126/science.1096301. [DOI] [PubMed] [Google Scholar]
- Fisher R. B., Gilbert J. C. The effect of insulin on the kinetics of pentose permeation of the rat heart. J Physiol. 1970 Sep;210(2):297–304. doi: 10.1113/jphysiol.1970.sp009211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I., CHICHIBU S. MEMBRANE PROPERTIES OF BARNACLE MUSCLE FIBER. Science. 1964 Mar 27;143(3613):1446–1448. doi: 10.1126/science.143.3613.1446. [DOI] [PubMed] [Google Scholar]
- HOYLE G., SMYTH T., Jr NEUROMUSCULAR PHYSIOLOGY OF GIANT MUSCLE FIBERS OF A BARNACLE, BALANUS NUBILUS DARWIN. Comp Biochem Physiol. 1963 Dec;10:291–314. doi: 10.1016/0010-406x(63)90229-9. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Narahara H. T. Studies of tissue permeability. X. Changes in permeability to 3-methylglucose associated with contraction of isolated frog muscle. J Biol Chem. 1965 Sep;240(9):3493–3500. [PubMed] [Google Scholar]
- Hoyle G., McNeill P. A., Selverston A. I. Ultrastructure of barnacle giant muscle fibers. J Cell Biol. 1973 Jan;56(1):74–91. doi: 10.1083/jcb.56.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illiano G., Tell G. P., Siegel M. E., Cuatrecasas P. Guanosine 3':5'-cyclic monophosphate and the action of insulin and acetylcholine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2443–2447. doi: 10.1073/pnas.70.8.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S., Andersson R. G. Variations of cyclic nucleotide monophosphate levels during spontaneous uterine contractions. Experientia. 1975 Nov 15;31(11):1314–1315. doi: 10.1007/BF01945800. [DOI] [PubMed] [Google Scholar]
- KONO T., COLOWICK S. P. Stereospecific sugar transport caused by uncouplers and SH-inhibitors in rat diaphragm. Arch Biochem Biophys. 1961 Jun;93:514–519. doi: 10.1016/s0003-9861(61)80045-3. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Kerrick W. G., Hoar P. E. Inhibition of smooth muscle tension by cyclic AMP-dependent protein kinase. Nature. 1981 Jul 16;292(5820):253–255. doi: 10.1038/292253a0. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabchi A. E., Solomon S. S., Brush J. S. The insulin-like activity of cyclic nucleotides and their inhibition by caffeine on the isolated fat cells. Biochem Biophys Res Commun. 1970;39(6):1065–1072. doi: 10.1016/0006-291x(70)90667-4. [DOI] [PubMed] [Google Scholar]
- Kohn P. G., Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. VI. The effect of insulin, ouabain, and metabolic inhibitors on the transport of 3-O-methylglucose and glucose in rat soleus muscles. Biochim Biophys Acta. 1971 Feb 2;225(2):277–290. doi: 10.1016/0005-2736(71)90221-5. [DOI] [PubMed] [Google Scholar]
- Kohn P. G., Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. VII. The effects of extracellular Na + and K + on the transport of 3-O-methylglucose and glucose in rat soleus muscle. Biochim Biophys Acta. 1972 Mar 17;255(3):798–814. doi: 10.1016/0005-2736(72)90392-6. [DOI] [PubMed] [Google Scholar]
- Kono T., Barham F. W. Effects of insulin on the levels of adenosine 3':5'-monophosphate and lipolysis in isolated rat epididymal fat cells. J Biol Chem. 1973 Nov 10;248(21):7417–7426. [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Sarver J. A. Insulin-sensitive phosphodiesterase. Its localization, hormonal stimulation, and oxidative stabilization. J Biol Chem. 1975 Oct 10;250(19):7826–7835. [PubMed] [Google Scholar]
- LEVINE R., GOLDSTEIN M. The action of insulin on the distribution of galactose in eviscerated nephrectomized dogs. J Biol Chem. 1949 Jun;179(2):985–985. [PubMed] [Google Scholar]
- Le Roith D., Shiloach J., Roth J., Lesniak M. A. Evolutionary origins of vertebrate hormones: substances similar to mammalian insulins are native to unicellular eukaryotes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6184–6188. doi: 10.1073/pnas.77.10.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D., Shiloach J., Roth J., Lesniak M. A. Insulin or a closely related molecule is native to Escherichia coli. J Biol Chem. 1981 Jul 10;256(13):6533–6536. [PubMed] [Google Scholar]
- Lundholm L., Rall T., Vamos N. Influence of K-ions and adrenaline on the adenosine 3',-5'-monophosphate content in rat diaphragm. Acta Physiol Scand. 1967 May;70(1):127–128. doi: 10.1111/j.1748-1716.1967.tb03607.x. [DOI] [PubMed] [Google Scholar]
- MORGAN H. E., RANDLE P. J., REGEN D. M. Regulation of glucose uptake by muscle. 3. The effects of insulin, anoxia, salicylate and 2:4-dinitrophenol on membrane transport and intracellular phosphorylation of glucose in the isolated rat heart. Biochem J. 1959 Dec;73:573–579. doi: 10.1042/bj0730573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P. Studies of tissue permeability. IX. The effect of insulin on the penetration of 3-methylglucose-H3 in frog muscle. J Biol Chem. 1963 Jan;238:40–49. [PubMed] [Google Scholar]
- POST R. L., MORGAN H. E., PARK C. R. Regulation of glucose uptake in muscle. III. The interaction of membrane transport and phosphorylation in the control of glucose uptake. J Biol Chem. 1961 Feb;236:269–272. [PubMed] [Google Scholar]
- Pain V. M., Manchester K. L. The influence of electrical stimulation in vitro on protein synthesis and other metabolic parameters of rat extensor digitorum longus muscle. Biochem J. 1970 Jun;118(2):209–220. doi: 10.1042/bj1180209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkett M. O., Perlman R. L. Stimulation of sugar transport in rat diaphragm by 8-bromoguanosine 3', 5'-monophosphate. Biochim Biophys Acta. 1975 Aug 13;399(2):473–477. doi: 10.1016/0304-4165(75)90277-9. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., SMITH G. H. Regulation of glucose uptake by muscle. 2. The effects of insulin, anaerobiosis and cell poisons on the penetration of isolated rat diaphragm by sugars. Biochem J. 1958 Nov;70(3):501–508. doi: 10.1042/bj0700501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Senft G., Schultz G., Munske K., Hoffmann M. Influence of insulin on cyclic 3',5'-AMP phosphodiesterase activity in liver, skeletal muscle, adipose tissue, and kidney. Diabetologia. 1968 Dec;4(6):322–329. doi: 10.1007/BF01211766. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarui S., Saito Y., Fujimoto M., Okabayashi T. Effects of insulin on diaphragm muscle independent of the variation of tissue levels of cyclic AMP and cyclic GMP. Arch Biochem Biophys. 1976 May;174(1):192–198. doi: 10.1016/0003-9861(76)90338-6. [DOI] [PubMed] [Google Scholar]
- Taylor W. M., Halperin M. L. Stimulation of glucose transport in rat adipocytes by insulin, adenosine, nicotinic acid and hydrogen peroxide. Role of adenosine 3':5'-cyclic monophosphate. Biochem J. 1979 Feb 15;178(2):381–389. doi: 10.1042/bj1780381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres H. N., Flawià M. M., Hernaez L., Cuatrecasas P. Effects of insulin on the adenylyl cyclase activity of isolated fat cell membranes. J Membr Biol. 1978 Sep 29;43(1):1–18. doi: 10.1007/BF01869039. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas O., Walaas E., Gronnerod O. Hormonal regulation of cyclic-AMP-dependent protein kinase of rat diaphragm by epinephrine and insulin. Eur J Biochem. 1973 Dec 17;40(2):465–477. doi: 10.1111/j.1432-1033.1973.tb03215.x. [DOI] [PubMed] [Google Scholar]
- Walaas O., Walaas E., Lystad E., Alertsen A. R., Horn R. S., Fossum S. A stimulatory effect of insulin on phosphorylation of a peptide in sarcolemma-enriched membrane preparation from rat skeletal muscle. FEBS Lett. 1977 Aug 15;80(2):417–422. doi: 10.1016/0014-5793(77)80489-4. [DOI] [PubMed] [Google Scholar]
- Walaas O., Walaas E., Lystad E., Alertsen A. R., Horn R. S. The effect of insulin and guanosine nucleotides on protein phosphorylations by sarcolemma membranes from skeletal muscle. Mol Cell Endocrinol. 1979 Oct;16(1):45–55. doi: 10.1016/0303-7207(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Cushman S. W., Salans L. B. Mechanism of insulin action on glucose transport in the isolated rat adipose cell. Enhancement of the number of functional transport systems. J Biol Chem. 1978 Nov 25;253(22):8002–8005. [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- Woo Y. T., Manery J. F. Cyclic AMP phosphodiesterase activity at the external surface of intact skeletal muscles and stimulation of the enzyme by insulin. Arch Biochem Biophys. 1973 Feb;154(2):510–519. doi: 10.1016/0003-9861(73)90003-9. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Gould M. K. Effect of prolonged anaerobiosis on 125I-insulin binding to rat soleus muscle: permissive effect of ATP. Am J Physiol. 1978 Dec;235(6):E606–E613. doi: 10.1152/ajpendo.1978.235.6.E606. [DOI] [PubMed] [Google Scholar]


