“I have, on many occasions, examined normal blood and normal tissues using methods that ensure that such organisms are not overlooked, and I have never, in a single instance, found bacteria. I therefore conclude that bacteria do not occur in the blood or tissues of healthy animals or humans” (R. Koch, 1878 [12]).
“In Coaquet (Peru) was the origin of an infectious disease which covered them with warts, made them suffer and exhausted them. These warts, like bubons all over the body could be of the size of an egg and finish by spliting. Blood and other substances then came out” (Pedro Pizarro, 1571 [authors’ translation of the first description of bartonellosis]).
The genus Bartonella contains numerous recently described species, many of which are new and emerging human pathogens. Until 1990, only two diseases were recognized to be caused by Bartonella species: Carrión’s disease, due to Bartonella bacilliformis, and trench fever, due to B. quintana (47). More recently, B. quintana has also been associated with endocarditis and bacteremia in homeless people (29, 91) and with bacillary angiomatosis (BA), which was first described in 1983 (51, 52, 82, 93) and which is an AIDS-related disease. Another important cause of BA, however, is B. henselae (81). This organism is closely related to B. quintana and was first identified in 1990 by PCR amplification of the gene for bacterial 16S rRNA (82) and characterized as a new species in 1992 (77). B. henselae is now known to cause a number of other clinical syndromes in immunocompetent and immunocompromised patients. These include cat scratch disease (CSD), peliosis hepatis (74), relapsing bacteremia with fever, and endocarditis (39, 42). Other Bartonella species have recently been implicated as human pathogens. B. clarridgeiae is possibly another agent of CSD (56), B. elizabethae (24, 75) and B. vinsonii subsp. berkhoffii may cause endocarditis (85), B. vinsonii subsp. arupensis has been found in a patient with fever and a valvulopathy (97), and B. grahamii may cause uveitis (49).
People usually become infected with Bartonella species incidentally, as the organisms are normally found in the reservoir hosts of Bartonella species, which include animals such as cats and dogs that live in close contact with people. The Bartonella species are unique because of this specific association with mammalian reservoir hosts, in which they cause chronic bacteremia with no or few symptoms. This is contrary to the existing premise that cultures of blood from healthy individuals should be sterile. Cats can be infected and become bacteremic with Bartonella species such as B. clarridgeiae and B. henselae. Wild rats are the reservoirs of B. grahamii, B. taylorii, and B. doshiae and of the newly described species B. tribocorum (41), while dogs can be infected with B. vinsonii subsp. berkhoffii (9). Recently, B. alsatica has been isolated from healthy rabbits trapped in France (40) and B. weissii has been isolated from cattle and cats (10, 17). Arthropod vectors, including ticks, fleas, and lice, have been proposed for almost all the Bartonella species; and transmission of the organisms to people may also occur by scratches or bites from reservoir hosts, in particular, cats.
In this minireview we describe the presently recognized Bartonella species, their reservoirs and vectors, and the diseases that they cause. We also speculate on the possible natural history of the diseases caused by the Bartonella species.
BACTERIOLOGY
Members of the genus Bartonella are short, pleomorphic, gram-negative rods that are fastidious aerobic and oxidase-negative organisms within the α2 subgroup of the class Proteobacteria. They have a close evolutionary homology with members of the genera Brucella, Agrobacterium, and Rhizobium. The Bartonella species grow on axenic medium at 37°C with 5% carbon dioxide but can also be grown in broth with fetal bovine serum and in tissue culture (60). Growth in axenic medium is hemin dependent (97), and agar should be enriched with rabbit and horse blood, which gives better growth than sheep blood. All Bartonella species grow slowly on blood agar, with primary isolates typically appearing after 12 to 14 days but sometimes requiring 45 days to be visible (70). In subcultures, colonies usually appear after only 3 to 5 days. B. bacilliformis grows best in vitro at 28°C, has polar peritrichous flagella, and is highly motile. B. clarridgeiae is also flagellated. The susceptibilities of Bartonella species to antibiotics has been evaluated (67, 68, 90); and the bacteria have been found to be very susceptible to beta-lactams (except oxacillin and cephalothin), aminoglycosides, macrolides (but not clindamycin), tetracyclines, and rifampin. There was considerable variability in the susceptibilities of isolates to the fluoroquinolones. Only the aminoglycosides (gentamicin, tobramycin, and amikacin) were found to be bactericidal.
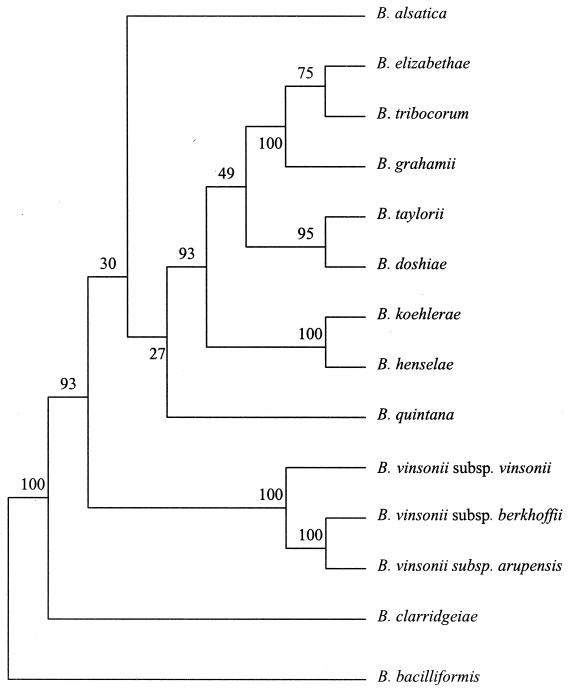
The genus Bartonella contains 16 species, most of which have been reclassified from the genus Rochalimeae (B. quintana, B. henselae, B. elizabethae, and B. vinsonii) (11) and from the genus Grahamella (B. talpae, B. peromysci, B. grahamii, B. taylorii, and B. doshiae) (6) (Fig. 1). B. bacilliformis was first reported in 1909, and before recent taxonomic changes, it was the only member of the genus (Table 1). The Bartonella species are all closely related, having over 98% homology in the sequences of their 16S rRNA genes.
FIG. 1.
Parsimony tree for Bartonella species derived from internal transcribed spacer sequences. The support of each branch, as indicated by 100 bootstrap samples, is indicated by the value at the node (adapted from reference 43).
TABLE 1.
Epidemiology of Bartonella species
| Bartonella sp. | Yr of discovery (reference) | Yr of first cultivation (reference) | Reservoir (reference) | Vector (reference) | Current geographic distribution (reference) |
|---|---|---|---|---|---|
| B. bacilliformis | 1909 (63) | 1919 (63) | Humans (27, 63) | Phlebotomines (L. verrucarum) (2) | Peru (63), Ecuador and Columbia (2), Bolivia and Chile (33), Oand Guatemala (33) |
| B. quintana | 1914 (59) | 1961 (69) | Humans (69) | Human body lice (Pediculus humanis corporis) (69) | Worldwide (86) |
| B. talpae | 1905 (6) | Moles | United Kingdom (6) | ||
| B. peromysci | 1942 (6) | Mice (Peromyscus spp.) (6) | United States (6) | ||
| B. henselae | 1950 (25) | 1990 (89) | Cats | Fleas (Ctenocephalides felis) | Worldwide |
| B. clarridgeiae | 1995 (22) | 1995 (56) | Cats | Fleas (Ctenocephalides felis) | Cosmopolite (7, 38) |
| B. koehlerae | 1999 (30) | 1999 (30) | Cats (supposed reservoir) (30) | Fleas (supposed vectors) (30) | California (30) |
| B. vinsonii subsp. vinsonii | 1946 (3) | 1996 (3) | Voles (Microtus pennsylvanicus) (3) | Canada (38) | |
| B. vinsonii subsp. berkhoffii | 1995 (9) | 1995 (9) | Dogs (9, 57) | Fleas and ticks | Cosmopolite |
| B. vinsonii subsp. arupensis | 1999 (96) | 1999 (96) | Cattle (96) | ||
| B. elizabethae | 1986 (24) | 1993 (24) | Rats (5) | Fleas | |
| B. grahamii | 1995 (6) | 1995 (6) | Rats (Clethrionomys glareolus) (6) | United Kingdom (6) | |
| B. taylori | 1995 (6) | 1995 (6) | Rats (Apodemus spp.) (6) | United Kingdom (6) | |
| B. doshiae | 1995 (6) | 1995 (6) | Rats (Microtus agrestis) (6) | United Kingdom (6) | |
| B. tribocorum | 1998 (41) | 1998 (41) | Rats (Rattus rattus) (41) | ||
| B. alsatica | 1999 (40) | 1999 (40) | Rabbits (40) | Fleas or ticks (40) | France (40) |
| B. weissii | 1999 (17) | 1999 (17) | Deer, elk, beef, cattle (17) | United States, France (17) | |
| B. birtlesii | 2000 (4) | 2000 (4) | Rats (Apodemus spp.) (4) | France (4) |
NATURAL HISTORY OF BARTONELLA INFECTIONS
As for many vector-borne disease agents, it seems that the Bartonella species also have a natural cycle. The cycle contains a reservoir host in which the Bartonella species cause a chronic intraerythrocytic bacteremia and a vector that transmits the bacteria from the reservoir hosts to new susceptible hosts. These could be the natural reservoir hosts, new competent reservoir hosts, or incidental hosts. There is usually a specific association between the natural host, the vector, and the Bartonella species which determines the spectrum of hosts (natural or incidental) possible and the geographic distribution of the organisms.
Natural infection in the host.
Natural Bartonella infections begin with the inoculation of the bacteria, and this is usually associated with the feeding of the arthropod vector. Differences in the clinical presentations of individuals with primary infections may be due to several factors. The size of the inoculum may vary, and this could explain the differences in the severities of the clinical signs that might occur. Variations in strain virulence may also, however, contribute to differences in the intensity of illness (72). Host responses, modulated by immune responses to Bartonella infections, can vary and can induce variations in the intensities of clinical signs during initial infections.
With all other known bacteria, prolonged bacteremia is associated with signs of septicemia in the host. Bartonella bacteremias in the natural hosts, however, can be asymptomatic. This is contrary to our present understanding of bacteremia and goes against the idea originated by Koch that bacteria do not occur in the blood of healthy animals or humans (12). Bartonella may be the single bacterial genus capable of producing asymptomatic bacteremia in mammals and, thus, may be an exception to Koch’s postulate. Using confocal microscopy, we have shown that B. henselae occurs within naturally infected asymptomatic cat erythrocytes (Fig. 2) (83) and that B. quintana occurs in human erythrocytes (unpublished data). As the Bartonella species are intraerythrocytic and, hence, might be less exposed to the immune system, their hosts may become adapted to the chronic bacteremia.
FIG. 2.
Presence of B. henselae (arrow)within naturally infected cat erythrocytes, as seen by confocal microscopy.
Recently, the kinetics of the colonization of B. triborum in rat erythrocytes has been reported (87). The organism multiplies until there are an average of eight Bartonella species per cell and thereafter remains in the cell for the life of the erythrocyte. It was suggested that this nonhemolytic intracellular colonization of erythrocytes is a bacterial persistence strategy that preserves the Bartonella species for potential transmission by arthropods. The host, then, could contaminate blood-feeding arthropods such as ticks, fleas (50), sand flies, or lice (14, 76), which could then subsequently infect a new host. The role of antibodies in the control of the multiplication of bacteria living in erythrocytes has been demonstrated in mice experimentally infected with B. grahamii (53), but in humans high antibody titers are associated with bacteremia (D. Raoult, unpublished data). Only B. bacilliformis has been reported to cause hemolysis (62).
Transmission between natural hosts.
Evidence has accumulated that Bartonella species may be inoculated by arthropod vectors, through the bites and scratches of reservoir hosts, and perhaps, by needles and syringes in drug addicts (23). Arthropod vectors have been widely studied; and fleas have been shown to be infected with B. henselae (50), body lice have been shown to be infected with B. quintana (14), and ticks (61) have been shown to be infected with B. henselae, B. quintana, B. washoensis, and B. vinsonii subsp. berkhoffii (18).
Incidental infections caused by animal species.
People are the incidental hosts of numerous Bartonella species. Infections can present in two clinical forms, depending on the immune status of the host. When the incidental host is immunocompetent, the infection is usually controlled locally by the immune system. The clinical manifestations of Bartonella infections are then local or regional. CSD results from B. henselae infection, and in immunocompetent people and immunocompetent mice, the disease usually presents as regional lymphadenopathy. Occasionally, visceral organ involvement has been described (31), but bacteremia has been reported only very rarely in immunocompetent hosts (89). B. henselae infections in immunocompromised hosts, however, result in bacteremia and other systemic conditions including BA and bacillary peliosis. Bartonella bacteremias in incidental hosts are manifested as systemic signs, and in people with existing heart valve abnormalities, the bacteremias may result in endocarditis, as reported with many other bacteria (15). Endocarditis due to B. henselae (75), B. elizabethae (24), B. vinsonii subsp. berkhoffii (85), and B. vinsonii subsp. arupensis (96) has been reported in patients with existing valve lesions.
Mammalian host specificity
There is apparently a species-specific association between Bartonella species and their animal hosts or vectors (32, 44, 58). All Bartonella species appear to be associated mainly with a mammalian host. After the primary infection, which might or might not be symptomatic, an asymptomatic chronic bacteremia occurs in the natural mammalian host. The host is then a competent reservoir from which an arthropod vector can become infected and Bartonella species can be transmitted to other susceptible hosts. Analysis of the sequences of the 16S rRNA, gltA, and groEL genes of the Bartonella species shows that they are clustered into phylogenetically related groups (Fig. 1) (44). Each of the Bartonella species in each cluster has a particular mammal as a reservoir host. One cluster contains B. bacilliformis, the second contains B. quintana, and the third contains Bartonella species isolated from rodents of the New World (e.g., United States and Peru); the Bartonella species isolated from felines are found in an additional two clusters. The close relationships between the Bartonella species with the same mammalian reservoir support the hypothesis of a species-specific association (44). The geographic distributions of the different Bartonella species vary considerably, with B. henselae and B. quintana occurring worldwide and B. clarridgeiae, B. elizabethae, B. weissii, and B. vinsonii subsp. berkhoffii occurring in Europe and the United States. B. vinsonii subsp. vinsonii and B. koehlerae and have been found exclusively in the Americas, while B. grahamii, B. taylorii, B. doshiae, B. tribocorum, B. birtlesii, and B. alsatica have been found only in Europe. B. bacilliformis occurs only in a restricted area of South America (Peru, Colombia, and Ecuador). The geographic distributions of the Bartonella species may reflect the geographic distributions of their hosts or of their vectors. For example, in the case of B. bacilliformis, because of favorable climatic conditions, Lutzomyia verrucarum, the phlebotomine vector of the bacterium, occurs only in localized regions of South America (62). Continent-specific Bartonella species are mainly associated with rodents, and phylogenetic analyses show that there are marked genetic differences between Bartonella species associated with indigenous New World rodents and those associated with Old World rodents (6, 44, 65). The phylogenetic relationships between the Bartonella species isolated from Rattus species suggest that these rodents carried the organisms from the Old World to the New World during times of conquest, the intercontinental migration of populations, and commercial exchange. In rural areas, contact between Rattus rattus and rodents from the New World can occur. Where clusters of these rodents are found, the same species of Bartonella can be isolated from both New World and Old World rodents. Of course, the present classification could be contradicted in the future. The ubiquitous species B. quintana and B. henselae have hosts (people and cats, respectively) and vectors (body lice and cat fleas, respectively) with worldwide distributions.
BARTONELLOSES IN PEOPLE
People are the hosts and reservoirs of B. bacilliformis and B. quintana; other mammals are the reservoirs for the other Bartonella species. There is usually a highly specific association between a Bartonella species and the mammalian species which is its reservoir. Sometimes, however, the organisms from animals may incidentally infect people, causing systemic diseases in those who are immunocompromised or who have preexisting heart valve abnormalities. In immunocompetent people, Bartonella species usually cause only localized disease.
Carrión’s disease.
Carrión’s disease is caused by B. bacilliformis. Briefly, the epidemiological cycle of Carrión’s disease begins when infected sand flies (L. verrucarum) transmit B. bacilliformis to susceptible people during feeding. The Bartonella organisms localize in the capillary endothelial cells, and this primary infection is asymptomatic in most cases (95). In some patients Oroya fever occurs when organisms enter erythrocytes and hemolysis occurs due to erythrophagocytosis by histiocytes and macrophages. In this acute stage of infection, the Bartonella species may be observed in erythrocytes. The level of erythrocyte parasitization can reach 100%, resulting in severe anemia and, occasionally, death. Death could also occur because of opportunistic infections (specifically, salmonellosis) following infection-induced immunosuppression. The fatality rate without treatment can be 40% (63). A chronic asymptomatic bacteremia which may last for up to 15 months (33) usually follows, and B. bacilliformis may be transmitted to sand flies that feed on patients during this time (Fig. 3). Seroepidemiological studies in areas of endemicity have shown that more than half the people infected with B. bacilliformis are asymptomatic. The majority of infected people are children or young adults (63). In the chronic stage of infection, people develop cutaneous eruptions, the verrugae of Carrión’s disease. These individuals can, presumably, also serve as reservoirs for the bacteria. The disease has a very limited geographic distribution, with most cases having been reported in arid areas at 500 to 3,000 m above sea level in the Peruvian Andes between southwestern Colombia and central Peru. The disease has, however, also been reported to occur in Bolivia, Chile, and Guatemala and at high elevations in Colombia and Ecuador (33). Most of the suspected and confirmed cases of bartonellosis in Ecuador have been reported in an arid coastal province (63). The disease was apparently unknown in Colombia until an outbreak occurred in 1936, peaked between 1938 and 1940, and subsided after 1941 (2). Large epidemics of febrile anemia characterize the history of bartonellosis in Peru. For a long time it was reported that only people born in areas of endemicity developed the verruga peruana (Peruvian warts) of the chronic stage of the disease and that only foreigners developed the acute febrile form of the disease (63). A prospective study in the national hospital Cayetano Heredia in Peru, however, has shown that 58.8% of the 145 patients with Oroya fever were in fact born in areas of endemicity (63). People are still the only known reservoirs of B. bacilliformis, and they serve as sources of infection for sand flies, which are the vectors of the disease. The treatment of acute infection is based on tetracyclines and chloramphenicol. Interestingly, they are poorly effective in preventing or treating verruga peruana. This chronic stage is treated with streptomycin or rifampin (63).
FIG. 3.
Putative natural history of B. bacilliformis infection.
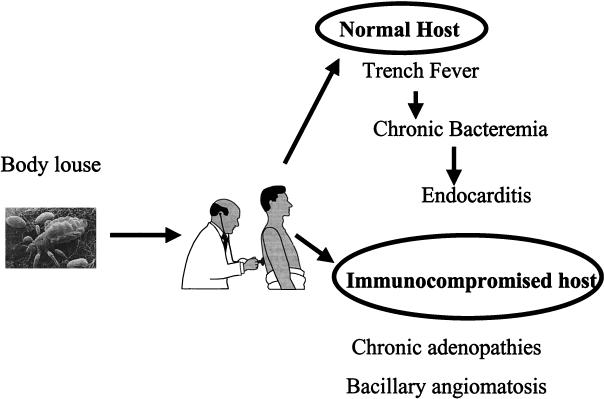
B. quintana infections.
Trench fever or quintan fever is a recurrent fever caused by B. quintana, which is transmitted by human body lice. The lice cause pruritis and broken skin, through which B. quintana, present at high concentrations in the feces of infected lice, may enter the body. People are the only proven animal hosts for B. quintana and are probably the natural reservoirs of the organism. Although B. quintana is usually present in the blood of patients during the febrile stages of trench fever, infections may persist long after the disappearance of all clinical signs; this persistent bacteremia may facilitate the spread of the bacteria by lice. It has been shown that lice tend to migrate away from febrile hosts, and they may then spread the infection to people in close contact with the ill individual (59). The disease has an acute onset, with severe headache and pretibial pain. The acute signs usually resolve spontaneously, but in some patients they may recur after about 5 days. In some patients there may be six or more recurrences of the disease (59). In other patients relapses may occur many years after the initial illness or the patients may be bacteremic but have no clinical signs (14). The prolonged bacteremias that occur in patients with B. quintana infections may be associated with the development of endocarditis and bacillary angiomatosis (Fig. 4). Trench fever occurred in millions of troops in World War I, but with the introduction of louse control measures by armed forces, the disease was thought to no longer be a threat (69). Recently, however, the disease has reemerged, and an outbreak of bacteremia due to B. quintana (91) was reported in Seattle, Washington, in 1994. Infections were characterized by relapsing fever, and the major risk factors for acquiring these B. quintana infections included poor living conditions and chronic alcoholism. These are also the risk factors found in human immunodeficiency virus (HIV)-infected patients who develop BA (91) and endocarditis (75). Subsequent studies have shown that the seroprevalences of antibodies against B. quintana are high in homeless people in both the United States and Europe. In a 1997 study at the emergency departments of the university hospitals of Marseille, France (13), the blood of 14% of homeless people whose blood was sampled was found to be positive by culture, and half of these people had chronic bacteremia without fever (14). Serology showed that 30% had specific antibodies to B. quintana, and the DNA of the organism was detected in lice from three homeless patients (14). Trench fever responds favorably to tetracycline (69). However, chronic bacteremia is not controlled by doxycycline (D. Raoult, unpublished data).
FIG. 4.
Putative natural history of B. quintana infection.
Endocarditis.
B. quintana, B. henselae, B. elizabethae, and two B. vinsonii subspecies, B. vinsonii subsp. berkhoffii and B. vinsonii subsp. arupensis, have been associated with endocarditis in patients with existing valvulopathies (34). In the largest series of Bartonella endocarditis cases reported, 13 of 22 patients had previously been diagnosed with a valvulopathy. B. quintana was the etiologic agent in five patients, and B. henselae was the etiologic agent in four patients. Patients with Bartonella endocarditis produce antibodies that react with Chlamydia pneumoniae, Chlamydia psittaci, and Chlamydia trachomatis (66). Cases of endocarditis caused by B. vinsonii subsp. berkhoffii (85) and B. vinsonii subsp. arupensis (96) have also been described, and in the latter study the organism was isolated from the blood of a rancher with preexisting cardiac valve disease. All together, these data suggest that previous valve lesions predispose an individual to endocarditis with nonhuman Bartonella species, with B. quintana endocarditis being more frequently diagnosed in patients without previous heart diseases. On the basis of retrospective analysis, prescription of an aminoglycoside appears to be critical in the outcome of endocarditis (D. Raoult, unpublished data).
CSD.
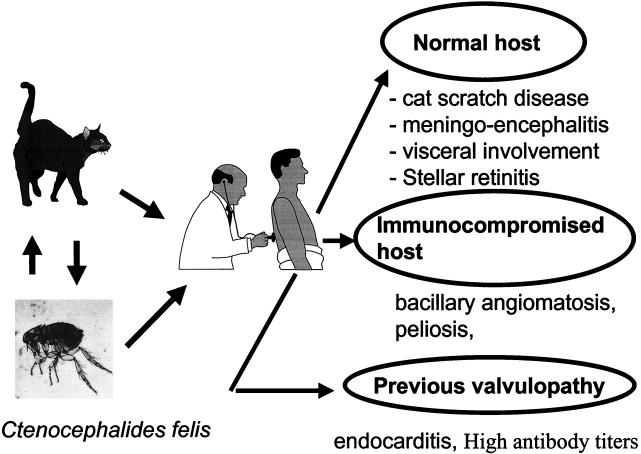
CSD is usually a self-limiting regional lymphadenitis. At the inoculation site there is usually an erythematous papule, and later, the lymph nodes draining the site become enlarged and tender. They usually regress in size over a period of weeks or months, but the lymphadenitis may become suppurative in 10% of patients. Complications such as rash, hepatosplenomegaly, lytic bone lesions, and deep lymphadenitis can occur in 5% of patients, most often in children. In immunocompetent hosts, there is usually no bacteremia (16, 100). Serological studies have shown that the vast majority of cases of CSD are due to B. henselae (78). The first isolation of B. henselae from a patient with CSD lymphadenitis was made by Dolan et al. (26) in 1993. The organism was also isolated from the blood of an asymptomatic cat, indicating that domestic cats are reservoirs of B. henselae (79). CSD has been reported worldwide and seems to be the most common Bartonella infection in people today. Between 1 and 2% of people with CSD will suffer neuroretinitis, which includes disk edema and exudates of the macula (71). In the United States, Jackson et al. (45) studied epidemiological databases and estimated that approximately 24,000 cases of CSD occur each year, with a calculated incidence of 9.3/10,000 ambulatory patients per year. In various studies, the seroprevalence of antibodies to B. henselae in people has ranged from 3.6 to 6% (7). Although CSD may occur in people of any age, most patients are under 18 years of age (16), perhaps because children are more likely to have close and rough contact with cats. The incidence of CSD is seasonal, with most cases occurring in August to October in northern temperate areas (16). The prevalence of the disease also varies with the geographic location (45). Jameson et al. (46) reported that the prevalence of antibodies to B. henselae was higher in areas with warm humid climates, where there was a higher prevalence and intensity of cat flea infestations. Cats may infect humans either directly through scratches and bites or indirectly via the cat flea (Ctenocephalides felis), which is the arthropod vector (Fig. 5). Both the Houston and Marseille serotypes of B. henselae can cause CSD (28, 64). B. clarridgeiae may also cause some cases of CSD (56) (Table 2). No antibiotics have proved effective in the treatment of CSD. Together with the fact that few isolates were recovered by culture of pus from patients with CSD, this may be caused by the fact that clinical symptoms are related to the immune reaction rather than bacterial multiplication.
FIG. 5.
Putative natural history of B. henselae infection.
TABLE 2.
Conditions caused by Bartonella species in people
| Bartonella sp. | Condition |
|---|---|
| B. grahamii | Uveitis |
| B. elizabethae | Endocarditis |
| B. vinsonii subsp. berkhoffii | Endocarditis |
| B. vinsonii subsp. arupensis | Fever in a patient with valvulopathy |
| B. henselae | CSD, BA, peliosis hepatis, endocarditis, bacterema, neuroretinitis |
| B. clarridgeiae | CSD (based on serology only) |
| B. bacilliformis | Carrion’s disease (acute Oroya fever and chronic verruga peruana) |
| B. quintana | BA, endocarditis, trench fever, chronic bacteremia |
BA, peliosis hepatis, and immunodeficiency.
BA and peliosis hepatis are vascular proliferative diseases that occur particularly in immunocompromised patients with Bartonella infections, mainly those infected with HIV (74) (94). In 1983, subcutaneous lesions named BA were observed in HIV-infected patients (93), and B. henselae DNA was later amplified from the lesions (82). At the same time, B. henselae was recovered from febrile patients with AIDS but without skin lesions (89). Subsequently, B. quintana has also been described as a causative agent of BA (51) (70). Among a series of 49 patients who were infected with Bartonella species identified by molecular biology-based techniques and who had clinical lesions consistent with BA (52), 53% were infected with B. henselae and 47% were infected with B. quintana. There are clinical and epidemiological differences between patients with BA due to B. henselae and B. quintana. Subcutaneous and lytic bone lesions are strongly associated with B. quintana infections, whereas peliosis hepatis was associated exclusively with B. henselae infections (52). Patients with B. henselae infections were more likely to be exposed to cats and their fleas, while those infected with B. quintana were more likely to be homeless and exposed to lice. In a study of 37 patients with BA in the literature (35), the presence of lymphadenopathy was significantly associated with B. henselae infections but not with B. quintana infections. Neurological disorders were significantly associated with B. quintana infections. Epidemiological risk factor analysis revealed that cat exposure was specified by 25 of the 37 patients and cat contact was recorded by 11 (84.6%) of the 13 B. henselae-infected patients. Bartonella infections of the central nervous system have also been proposed as a cause of meningitis and neuropsychiatric deterioration in HIV-infected patients with antibodies to Bartonella species in their cerebrospinal fluid and serum (88). HIV-infected patients with BA or peliosis hepatis can present with concomitant Bartonella bacteremia, and bacteremia can also occur in immunocompromised patients in the absence of focal BA (92). BA responds dramatically to macrolide antibiotics (51, 52).
BARTONELLA SPECIES IN ANIMALS
Rodents.
Numerous rats and mice are known to have intraerythrocytic Bartonella bacteremias. B. talpae was first observed in 1905 in the erythrocytes of moles and other rodents (6), and in 1995, three new Bartonella species were isolated from the blood of small woodland mammals in the United Kingdom (6). B. grahamii was isolated from the blood of Clethrionomys glareolus rats, B. taylori was isolated from the blood of Apodemus rat species, and B. doshiae was isolated from the blood of Microtus agrestis rats. In a recent survey, Bartonella species were found in the blood of intradomiciliary animals and Phyllotis mice in the Huayllacallàn Valley in Peru (5). Erythrocyte-associated bacteria were observed in blood smears from only one mouse, but two Bartonella species were isolated from Rattus norvegicus. One was indistinguishable from B. elizabethae, and the second was a distinct new Bartonella species. Ellis et al. (32) analyzed whole-blood specimens from 325 R. norvegicus rats and 92 R. rattus rats trapped in Portugal and at 13 different sites in the United States. Bartonella species were isolated from the blood of 63 R. norvegicus rats and 11 R. rattus rats. The overall prevalence of Bartonella bacteremias in both species was 18%, with the prevalence of Bartonella species in R. norvegicus rats being significantly high in California (45%); Los Angeles, California (56%); and Portugal (100%). The prevalence of Bartonella bacteremias in R. rattus rats did not differ from the overall prevalence except in California, where the prevalence was 60%. Two isolates were identical to B. elizabethae, and all 63 Bartonella species isolated from R. norvegicus rats were closely related and clustered with B. elizabethae and B. grahamii. B. tribocorum was obtained from the blood of wild R. norvegicus rats in eastern France (41), and it was observed that intraerythrocytic Bartonella bacteremias are lifelong in experimentally infected rats (87). It is not known how the Bartonella species carried by rodents can be transmitted to people, but 61% of Xenopsylla cheopis collected from rats have been found to be infected with Bartonella species, including B. elizabethae (7). Also, the pathogenicities of most rodent Bartonella species are not known, although one patient with a B. elizabethae infection and another patient with a B. grahamii infection have been described (49).
Felines.
Domestic cats are most commonly infected with B. henselae, although they may also be infected with B. clarridgeiae, B. koehlerae, and B. weissii (7). The prevalence of blood culture-positive cats is high worldwide and is up to 41% in California (50). Transmission electron microscopy (54) and confocal microscopy (Fig. 2) (83) have shown that B. henselae occurs within the erythrocytes of bacteremic cats, and such infections can persist for up to a year (54). The cat flea (C. felis) can transmit B. henselae between cats and is probably the main vector of the organism (21).
Other felines can be infected with bartonellas, with B. henselae having been isolated from a cheetah (Acinonyx jubatus) (48) and a strain named B. henselae Humboldt having been isolated from four mountain lions in California (99). Antibodies to B. henselae have been found in 30% of captive wild felids (98) and in free-ranging Florida panthers (Puma concolor coryi), mountain lions, and cougars (84).
There are conflicting reports on the clinical signs that might be seen in cats experimentally infected with B. henselae. Three studies have reported that cats show clinical signs including swelling at the site of inoculation, fever, lethargy, anorexia, myalgia, behavioral and/or neurological changes, and lymphadenopathy (36, 55, 72). Reproductive failures and delayed conceptions have been reported in female cats inoculated with B. henselae. Infections were not spread by sexual contact, and kittens from pregnant queens were free of infection (37). In other studies, cats infected with B. henselae have shown no clinical signs (1, 80); therefore, there might be variations in the pathogenicities of B. henselae strains for cats.
Canids.
B. vinsonii subsp. berkhoffii was first isolated from a female domestic dog with endocarditis (9). Subsequently, the organism has been found in another dog with endocarditis (57) and it has been implicated in granulomatous lymphadenitis, granulomatous rhinitis, peliosis hepatis, myocardial inflammation, cardiac arrhythmias, syncope, and sudden death in dogs (8). The organism may also be found in healthy dogs, with one dog having had a persistent infection for 16 months (57). In this dog, B. vinsonii subsp . berkhoffii was isolated in 8 of 10 blood cultures performed over the 16-month period and the dog had antibodies at titers of ≥1:64 against B. vinsonii subsp . berkhoffii but not against B. clarridgeiae or B. henselae. A seroepidemiological study has identified tick exposure as a risk factor for the presence of B. vinsonii antibodies in dogs (73). Recent studies in California have implicated coyotes as the wildlife reservoir of B. vinsonii subsp. berkhoffii, with 76% of animals being seropositive and bacteremia being present in 28% of animals (19, 20).
Rabbits.
Of 9 of 30 (30%) wild rabbits (Oryctolagus cuniculus) from eastern France that were culture positive for B. alsatica (40), 2 that were sent for postmortem examination had no gross abnormalities and also appeared normal by histopathology.
Ungulates.
In recent studies in the United States, the prevalences of Bartonella bacteremias have been determined to be 15% in elk (Cervus elaphus), 90% in mule deer, 0% in 84 bighorn sheep (Ovis canadensis), and about 50% in domestic cattle (Bos taurus) (10). Analyses of partial sequences of the citrate synthase genes of the isolates showed they were all closely related to one another and also to B. weissii.
CONCLUSION
The available data on the Bartonella species have expanded rapidly in recent years as this group of organisms has been found to be responsible for a growing spectrum of emerging and reemerging diseases. We now have new insights into the natural history of the Bartonella species and can see that these bacteria have adapted to their mammalian reservoir hosts in unique ways. They cause chronic intraerythrocytic infections, with up to half of the reservoir host populations being bacteremic at any one time. This bacteremia is the source of the vector infection. The Bartonella bacteremias, however, result in few (and, if present, very subtle) clinical signs in their specific reservoir hosts, and this contradicts Koch’s observation that the blood of healthy humans or animals is free of bacteria.
Acknowledgments
We acknowledge J. M. Rolain for photography with confocal microscopy.
REFERENCES
- 1.Abbott, R. C., B. B. Chomel, R. W. Kasten, K. A. Floyd-Hawkins, Y. Kikuchi, J. E. Koehler, and N. C. Pedersen. 1997. Experimental and natural infection with Bartonella henselae in domestic cats. Comp. Immunol. Microbiol. Infect. Dis. 20:41–51. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, B. 1995. A review of bartonellosis in Ecuador and Colombia. Am. J. Trop. Med. Hyg. 52:354–359. [DOI] [PubMed] [Google Scholar]
- 3.Baker, J. A. 1946. A rickettsial infection in Canadian voles. J. Exp. Med. 84:37–51. [PubMed] [Google Scholar]
- 4.Bermond, D., R. Heller, F. Barrat, G. Delacour, C. Dehio, A. Alliot, H. Monteil, B. Chomel, H. Boulouis, and Y. Piémont. 2000. Bartonella birtlesii sp. nov., isolated from small mammals (Apodemus spp.). Int. J. Syst. Evol. Microbiol. 50:1973–1979. [DOI] [PubMed] [Google Scholar]
- 5.Birtles, R. J., J. Canales, P. Ventosilla, E. Alvarez, H. Guerra, A. Llanos-Cuenta, D. Raoult, N. Doshi, and T. G. Harrison. 1999. Survey of Bartonella species infecting intradomicillary animals in the Huayllacallan Valley, Ancash, Peru, a region endemic for human bartonellosis. Am. J. Trop. Med. Hyg. 60:799–805. [DOI] [PubMed] [Google Scholar]
- 6.Birtles, R. J., T. G. Harrison, N. A. Saunders, and D. H. Molyneux. 1995. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb.nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int. J. Syst. Bacteriol. 45:1–8. [DOI] [PubMed] [Google Scholar]
- 7.Breitschwerdt, E. B., and D. Kordick. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 13:428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breitschwerdt, E. B., C. E. Atkins, T. T. Brown, D. L. Kordick, and P. S. Snyder. 1999. Bartonella vinsonii subsp. berkhoffii and related members of the alpha subdivision of the Proteobacteria in dogs with cardiac arrhythmias, endocarditis, or myocarditis. J. Clin. Microbiol. 37:3618–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breitschwerdt, E. B., D. L. Kordick, D. E. Malarkey, B. Keene, T. L. Hadfield, and K. Wilson. 1995. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J. Clin. Microbiol. 33:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitschwerdt, E. B., S. Sontakke, A. Cannedy, S. I. Hancock, and J. Bradley. 2001. Infection with Bartonella weissii and detection of nanobacterium antigens in a North Carolina beef herd. J. Clin. Microbiol. 39:879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner, D. J., S. O’Connor, H. H. Winkler, and A. G. Steigerwalt. 1993. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int. J. Syst. Bacteriol. 43:777–786. [DOI] [PubMed] [Google Scholar]
- 12.Brock, T. D. 1999. Robert Koch, a life in medicine and bacteriology, p.1–364. ASM Press, Washington, D.C.
- 13.Brouqui, P., P. Houpikian, H. Tissot-Dupont, P. Toubiana, Y. Obadia, V. Lafay, and D. Raoult. 1996. Survey of the seroprevalence of Bartonella quintana in homeless people. Clin. Infect. Dis. 23:756–759. [DOI] [PubMed] [Google Scholar]
- 14.Brouqui, P., B. La Scola, V. Roux, and D. Raoult. 1999. Chronic Bartonella quintana bacteremia in homeless patients. N. Engl. J. Med. 340:184–189. [DOI] [PubMed] [Google Scholar]
- 15.Brouqui, P., and D. Raoult. 2001. Endocarditis due to rare and fastidious bacteria. Clin. Microbiol. Rev. 14:177–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carithers, H. A. 1985. Cat-scratch disease: an overview based on a study of 1200 patients. Am. J. Dis. Child. 139:1124–1133. [DOI] [PubMed] [Google Scholar]
- 17.Chang, C. C., B. Chomel, R. Kasten, R. Heller, K. M. Kocan, H. Ueno, K. Yamamoto, V. Bleich, B. Pierce, B. Gonzales, P. Swift, W. Boyce, S. Jang, H. J. Boulouis, and Y. Piémont. 2000. Bartonella spp. isolated from wild and domestic ruminants in North America. Emerg. Infect. Dis. 6:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang, C. C., B. B. Chomel, R. W. Kasten, V. Romano, and N. Tietze. 2001. Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J. Clin. Microbiol. 39:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang, C. C., R. W. Kasten, B. B. Chomel, D. C. Simpson, C. M. Hew, D. L. Kordick, R. Heller, Y. Piemont, and E. B. Breitschwerdt. 2000. Coyotes (Canis latrans) as the reservoir for a human pathogenic Bartonella sp.: molecular epidemiology of Bartonella vinsonii subsp. berkhoffii infection in coyotes from central coastal California. J. Clin. Microbiol. 38:4193–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang, C. C., K. Yamamoto, B. B. Chomel, R. W. Kasten, D. C. Simpson, C. R. Smith, and V. L. Kramer. 1999. Seroepidemiology of Bartonella vinsonii subsp. berkhoffii infection in California coyotes, 1994-1998. Emerg. Infect. Dis. 5:711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chomel, B. B., R. W. Kasten, K. Floyd-Hawkins, B. Chi, K. Yamamoto, J. Roberts-Wilson, A. Nikos Gurfield, R. C. Abbott, N. C. Pedersen, and J. E. Koehler. 1996. Experimental transmission of Bartonella henselae by the cat flea. J. Clin. Microbiol. 34:1952–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarridge, J. E., T. J. Raich, D. Pirwani, B. Simon, L. Tsai, M. C. Rodriguez-Barradas, R. Regnery, A. Zollo, D. C. Jones, and C. Rambo. 1995. Strategy to detect and identify Bartonella species in routine clinical laboratory yields Bartonella henselae from human immunodeficiency virus-positive patient and unique Bartonella strain from his cat. J. Clin. Microbiol. 33:2107–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comer, J. A., C. Flynn, R. L. Regnery, D. Vlahov, and J. E. Childs. 1996. Antibodies to Bartonella spp. in inner-city Baltimore intravenous drug users. Arch. Intern. Med. 156:2491–2495. [PubMed] [Google Scholar]
- 24.Daly, J. S., M. G. Worthington, D. J. Brenner, W. C. Moss, D. G. Hollis, R. S. Weyant, A. G. Steigerwalt, R. E. Weaver, M. I. Daneshvar, and S. P. O’Connor. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debré, R., M. Lamy, M. L. Jammet, L. Costil, and P. Mozziconacci. 1950. La maladie des griffes du chat. Soc. Med. Hop. Paris 66:76–79. [PubMed] [Google Scholar]
- 26.Dolan, M. J., M. T. Wong, R. L. Regnery, J. H. Jorgensen, M. Garcia, J. Peters, and D. Drehner. 1993. Syndrome of Rochalimaea henselae adenitis suggesting cat scratch disease. Ann. Intern. Med. 118:331–336. [DOI] [PubMed] [Google Scholar]
- 27.Dooley, J. R. 1976. Bartonellosis, p.190–193. In H. Chapman, H. Binford, and D. Connor (ed.), Pathology of tropical and extraordinary diseases. Armed Forces Institute of Pathology, Washington, D.C.
- 28.Drancourt, M., R. J. Birtles, G. Chaumentin, F. Vandenesch, J. Etienne, and D. Raoult. 1996. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet 347:441–443. [DOI] [PubMed] [Google Scholar]
- 29.Drancourt, M., J. L. Mainardi, P. Brouqui, F. Vandenesch, A. Carta, F. Lehnert, J. Etienne, E. Vigier, F. Goldstein, J. Acar, and D. Raoult. 1995. Bartonella (Rochalimaea) quintana endocarditis in homeless patients: report of three cases. N. Engl. J. Med. 332:419–423. [DOI] [PubMed] [Google Scholar]
- 30.Droz, S., B. Chi, E. Horn, A. G. Steigerwalt, A. M. Whitney, and D. J. Brenner. 1999. Bartonella koehlerae sp. nov., isolated from cats. J. Clin. Microbiol. 37:1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn, M. W., F. E. Berkowitz, J. J. Miller, and J. A. Snitzer. 1997. Hepatosplenic cat-scratch disease and abdominal pain. Pediatr. Infect. Dis. J. 16:269–272. [DOI] [PubMed] [Google Scholar]
- 32.Ellis, B. A., R. L. Regnery, L. Beati, F. Bacellar, M. Rood, G. G. Glass, E. Marston, T. G. Ksiazek, D. Jones, and J. E. Childs. 1999. Rats of the genus Rattus are reservoir hosts for pathogenic Bartonella species: an Old World origin for a new world disease? J. Infect. Dis. 180:220–224. [DOI] [PubMed] [Google Scholar]
- 33.Ellis, B. A., L. D. Rotz, J. A. D. Leake, F. Samalvides, J. Bernable, G. Ventura, C. Padilla, P. Villaseca, L. Beati, R. Regnery, J. E. Childs, J. G. Olson, and C. P. Carrillo. 1999. An outbreak of acute bartonellosis (Oroya fever) in the Urubamba region of Peru, 1998. Am. J. Trop. Med. 61:344–349. [DOI] [PubMed] [Google Scholar]
- 34.Fournier, P. E., H. Lelievre, S. J. Eykyn, J. L. Mainardi, T. J. Marrie, F. Brunel, C. Roure, J. Nash, D. Clave, E. James, C. Benoit-Lemercier, L. Deforges, H. Tissot-Dupont, and D. Raoult. 2001. Epidemiological and clinical features of Bartonella endocarditis: a case control study. Medicine 80:245–251. [DOI] [PubMed] [Google Scholar]
- 35.Gasquet, S., M. Maurin, P. Brouqui, H. Lepidi, and D. Raoult. 1998. Bacillary angiomatosis in immunocompromised patients: a clinicopathological and microbiological study of seven cases and review of literature. AIDS 12:1793–1803. [DOI] [PubMed] [Google Scholar]
- 36.Guptill, L., L. Slater, C. Wu, L. T. Glickman, J. T. Crippen, and H. HogenEsch. 1999. Immune response of neonatal specific pathogen-free cats to experimental infection with Bartonella henselae. Vet. Immunol. Immunopathol. 71:233–243. [DOI] [PubMed] [Google Scholar]
- 37.Guptill, L., L. N. Slater, C. C. Wu, T. L. Lin, L.T. Glickman, D. F. Welch, J. Tobolski, and H. HogenEsch. 1998. Evidence of reproductive failure and lack of perinatal transmission of Bartonella henselae in experimentally infected cats. Vet. Immunol. Immunopathol. 65:177–189. [DOI] [PubMed] [Google Scholar]
- 38.Gurfield, A. N., H. J. Boulouis, B. B. Chomel, R. Heller, R. W. Kasten, K. Yamamoto, and Y. Piemont. 1997. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselae strains in domestic cats. J. Clin. Microbiol. 35:2120–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadfield, T. L., R. Warren, M. Kass, E. Brun, and C. Levy. 1993. Endocarditis caused by Rochalimaea henselae. Hum. Pathol. 24:1140–1141. [DOI] [PubMed] [Google Scholar]
- 40.Heller, R., M. Kubina, P. Mariet, P. Riegel, G. Delacour, C. Dehio, F. Lamarque, R. Kasten, H. J. Boulouis, H. Monteil, B. Chomel, and Y. Piemont. 1999. Bartonella alsatica sp. nov., a new Bartonella species isolated from the blood of wild rabbits. Int. J. Syst. Bacteriol. 49:283–288. [DOI] [PubMed] [Google Scholar]
- 41.Heller, R., P. Riegel, Y. Hansmann, G. Delacour, D. Bermond, C. Dehio, F. Lamarque, H. Monteil, B. Chomel, and Y. Piémont. 1998. Bartonella tribocorum sp. nov., a new Bartonella species isolated from the blood of wild rats. Int. J. Syst. Bacteriol. 48:1333–1339. [DOI] [PubMed] [Google Scholar]
- 42.Holmes, A. H., T. C. Greenough, G. J. Balady, R. L. Regnery, B. E. Anderson, J. C. Oikeane, J. D. Fonger, and E. L. McCrone. 1995. Bartonella henselae endocarditis in an immunocompetent adult. Clin. Infect. Dis. 21:1004–1007. [DOI] [PubMed] [Google Scholar]
- 43.Houpikian, P., and D. Raoult. 2001. 16S/23S rRNA intergenic spacer regions for phylogenetic analysis, identification, and subtyping of Bartonella species. J. Clin. Microbiol. 39:2768–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houpikian, P., and D. Raoult. 2001. Molecular phylogeny of the genus Bartonella: what is the current knowledge? FEMS Microbiol. Lett. 200:1–7. [DOI] [PubMed] [Google Scholar]
- 45.Jackson, L. A., B. A. Perkins, and J. D. Wenger. 1993. Cat scratch disease in the United States: an analysis of three national databases. Am. J. Public Health 83:1707–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jameson, P., C. Greene, R. Regnery, M. Dryden, A. Marks, J. Brown, J. Cooper, B. Glaus, and R. Greene. 1995. Prevalence of Bartonella henselae antibodies in pet cats throughout regions of North America. J. Infect. 172:1145–1149. [DOI] [PubMed] [Google Scholar]
- 47.Karem, K. L., C. D. Paddock, and R. L. Regnery. 2000. Bartonella henselae, B. quintana, and B. bacilliformis: historical pathogens of emerging significance. Microbes Infect. 2:1193–1205. [DOI] [PubMed] [Google Scholar]
- 48.Kelly, P. J., J. J. A. Rooney, E. L. Marston, D. C. Jones, and R. L. Regnery. 1998.Bartonella henselae isolated from cats in Zimbabwe. Lancet 351:1706. [DOI] [PubMed] [Google Scholar]
- 49.Kerkhoff, F., A. M. Bergmans, A. van der Zee, and A. Rothova. 1999. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J. Clin. Microbiol. 37:4034–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koehler, J. E., C. A. Glaser, and J. W. Tappero. 1994. Rochalimaea henselae infection: a new zoonosis with the domestic cat as a reservoir. JAMA 271:531–535. [DOI] [PubMed] [Google Scholar]
- 51.Koehler, J. E., F. D. Quinn, T. G. Berger, P. E. Leboit, and J. W. Tappero. 1992. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N. Engl. J. Med. 327:1625–1631. [DOI] [PubMed] [Google Scholar]
- 52.Koehler, J. E., M. A. Sanchez, C. S. Garrido, M. J. Whitfeld, F. M. Chen, T. G. Berger, M. C. Rodriguez-Barradas, P. E. Leboit, and J. W. Tappero. 1997. Molecular epidemiology of Bartonella infections in patients with bacillary angiomatosis-peliosis. N. Engl. J. Med. 337:1876–1883. [DOI] [PubMed] [Google Scholar]
- 53.Koesling, J., T. Aebischer, C. Falch, R. Schülein, and C. Dehio. 2001. Cutting edge: antibody-mediated cessation of hemotropic infection by the intraerythrocytic mouse pathogen Bartonella grahamii. J. Immunol. 167:11–14. [DOI] [PubMed] [Google Scholar]
- 54.Kordick, D. L., and E. B. Breitschwerdt. 1995. Intraerythrocytic presence of Bartonella henselae. J. Clin. Microbiol. 33:1655–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kordick, D. L., and E. B. Breitschwerdt. 1997. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am. J. Vet. Res. 58:492–497. [PubMed] [Google Scholar]
- 56.Kordick, D. L., E. J. Hilyard, T. L. Hadfield, K. H. Wilson, A. G. Steigerwalt, D. J. Brenner, and E. B. Breitschwerdt. 1997. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease). J. Clin. Microbiol. 35:1813–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kordick, D. L., B. Swaminathan, C. E. Greene, K. H. Wilson, A. M. Whitney, S. O’Connor, D. G. Hollis, G. M. Matar, A. G. Steigerwalt, G. B. Malcolm, P. S. Hayes, T. L. Hadfield, E. B. Breitschwerdt, and D. J. Brenner. 1996. Bartonella vinsonii subsp. berkhofii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int. J. Syst. Bacteriol. 46:704–709. [DOI] [PubMed] [Google Scholar]
- 58.Kosoy, M., E. Saito, D. Green, E. Marston, D. Jones, and J. Childs. 2000. Experimental evidence of host specificity of Bartonella infection in rodents. Comp. Immunol. Microbiol. Infect. Dis. 23:221–238. [DOI] [PubMed] [Google Scholar]
- 59.Kostrzewski, J. 1949. The epidemiology of trench fever. Bul.Acad. Polonaise Sci. Lett. Classe Med. 7:233–263. [PubMed] [Google Scholar]
- 60.La Scola, B., and D. Raoult. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J. Clin. Microbiol. 37:1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lucey, D., M. J. Dolan, C. W. Moss, M. Garcia, D. G. Hollis, and S. Weigner. 1992. Relapsing illness due to Rochalimaea henselae in immunocompetent hosts: implication for therapy and new epidemiological associations. Clin. Infect. Dis. 14:683–688. [DOI] [PubMed] [Google Scholar]
- 62.Maguina, C., and E. Gotuzzo. 2000. Bartonellosis—new and old. Infect. Dis. Clin. N. Am. 14:1–22. [DOI] [PubMed] [Google Scholar]
- 63.Maguina Vargas, C. 1998. Bartonellosis o enfermedad de carrion. Nuevos aspectos de una vieja enfermedad, p.7–195. AFA Editores Importadores, Lima, Peru.
- 64.Mainardi, J. L., C. Figliolini, F. W. Goldstein, P. Blanche, M. Baret-Rigoulet, N. Galezowski, P. E. Fournier, and D. Raoult. 1998. Cat scratch disease due to Bartonella henselae serotype Marseille (Swiss cat) in a seronegative patient. J. Clin. Microbiol. 36:3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marston, E. L., J. W. Sumner, and R. L. Regnery. 1999. Evaluation of intraspecies genetic variation within the 60-kDa heat-shock protein gene (groEL) of Bartonella species. Int. J. Syst. Bacteriol. 49:1015–1023. [DOI] [PubMed] [Google Scholar]
- 66.Maurin, M., F. Eb, J. Etienne, and D. Raoult. 1997. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J. Clin. Microbiol. 35:2283–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maurin, M., S. Gasquet, C. Ducco, and D. Raoult. 1995. MICs of 28 antibiotic compounds for 14 Bartonella (formerly Rochalimaea) isolates. Antimicrob. Agents Chemother. 39:2387–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maurin, M., and D. Raoult. 1993. Antimicrobial susceptibility of Rochalimaea quintana, Rochalimaea vinsonii and the newly recognized Rochalimaea henselae. J. Antimicrob. Chemother. 32:587–594. [DOI] [PubMed] [Google Scholar]
- 69.Maurin, M., and D. Raoult. 1996. Bartonella (Rochalimaea) quintana infections. Clin. Microbiol. Rev. 9:273–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maurin, M., V. Roux, A. Stein, F. Ferrier, R. Viraben, and D. Raoult. 1994. Isolation and characterization by immunofluorescence, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blot, restriction fragment length polymorphism-PCR, 16S rRNA gene sequencing, and pulsed-field gel electrophoresis of Rochalimaea quintana from a French patient with bacillary angiomatosis. J. Clin. Microbiol. 32:1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monahan, S. 2000. Neuroretinitis: a clinical syndrome of cat-scratch disease. Clin. Eye Vision Care 12:155–159. [DOI] [PubMed] [Google Scholar]
- 72.O’Reilly, K. L., R. W. Bauer, R. L. Freeland, L. D. Foil, K. J. Hughes, K. R. Rohde, A. F. Roy, R. W. Stout, and P. C. Triche. 1999. Acute clinical disease in cats following infection with a pathogenic strain of Bartonella henselae (LSU 16). Infect. Immun. 67:3066–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pappalardo, B. L., M. T. Correa, C. C. York, C. Y. Peat, and E. B. Breitschwerdt. 1997. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am. J. Vet. Res. 58:467–471. [PubMed] [Google Scholar]
- 74.Perkocha, L. A., S. M. Geaghan, B. T. S. Yen, S. L. Nishimura, S. P. Chan, R. Garcia-Kennedy, G. Honda, A. C. Stoloff, H. Z. Klein, R. L. Goldman, S. Van Meter, L. Ferrel, and P. E. Leboit. 1990. Clinical and pathological features of bacillary peliosis hepatis in association with human immunodeficiency virus infection. N. Engl. J. Med. 323:1581–1586. [DOI] [PubMed] [Google Scholar]
- 75.Raoult, D., P. E. Fournier, M. Drancourt, T. J. Marrie, J. Etienne, J. Cosserat, P. Cacoub, Y. Poinsignon, P. Leclercq, and A. M. Sefton. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann. Intern. Med. 125:646–652. [DOI] [PubMed] [Google Scholar]
- 76.Raoult, D., and V. Roux. 1999. The body louse as a vector of reemerging human diseases. Clin. Infect. Dis. 29:888–911. [DOI] [PubMed] [Google Scholar]
- 77.Regnery, R. L., B. E. Anderson, J. E. Clarridge, M. C. Rodriguez-Barradas, D. C. Jones, and J. H. Carr. 1992. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J. Clin. Microbiol. 30:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Regnery, R. L., M. Martin, and J. G. Olson. 1992. Naturally occuring Rochalimaea henselae infection in domestic cat. Lancet 340:557–558. [DOI] [PubMed] [Google Scholar]
- 79.Regnery, R. L., T. G. Olson, B. A. Perkins, and W. Bibb. 1992. Serological response to Rochalimaea henselae antigen in suspected cat-scratch disease. Lancet 339:1443–1445. [DOI] [PubMed] [Google Scholar]
- 80.Regnery, R. L., J. A. Rooney, A. M. Johnson, S. L. Nesby, P. Manzewitsch, K. Beaver, and J. G. Olson. 1996. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am. J. Vet. Res. 57:1714–1719. [PubMed] [Google Scholar]
- 81.Relman, D. A., S. Falkow, P. E. Leboit, L. A. Perkocha, K. W. Min, D. F. Welch, and L. N. Slater. 1991. The organism causing bacillary angiomatosis, peliosis hepatis, and fever and bacteremia in immunocompromised patients. N. Engl. J. Med. 324:1514. [DOI] [PubMed] [Google Scholar]
- 82.Relman, D. A., J. S. Loutit, T. M. Schmidt, S. Falkow, and L. S. Tompkins. 1990. The agent of bacillary angiomatosis: an approach to the identification of uncultured pathogens. N. Engl. J. Med. 323:1573–1580. [DOI] [PubMed] [Google Scholar]
- 83.Rolain, J. M., B. La Scola, Z. Liang, B. Davoust, and D. Raoult. 2001. Immunofluorescent detection of intraerythrocytic Bartonella henselae in naturally infected cats. J. Clin. Microbiol. 39:2978–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rotstein, D. S., S. K. Taylor, J. Bradley, and E. B. rieitschwerdt. 2000. Prevalence of Bartonella henselae antibody in Florida panthers. J. Wildl. Dis. 36:157–160. [DOI] [PubMed] [Google Scholar]
- 85.Roux, V., S. J. Eykyn, S. Wyllie, and D. Raoult. 1999. First report of Bartonella vinsonii subspecies berkhoffii as an agent of afebrile blood culture-negative endocarditis in man. J. Clin. Microbiol. 38:1698–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roux, V., and D. Raoult. 1999. Body lice as tools for diagnosis and surveillance of reemerging diseases. J. Clin. Microbiol. 37:596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schülein, R., A. Seubert, C. Gilles, C. Lanz, Y. Hansmann, Y. Piemont, and C. Dehio. 2001. Invasion and persistent intracellular colonization of erythrocytes: a unique parasitic strategy of the emerging pathogen Bartonella. J. Exp. Med 193:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwartzman, W. A., M. Patnaik, F. J. Angulo, B. R. Visscher, E. N. Miller, and J. B. Peter. 1995. Bartonella (Rochalimaea) antibodies, dementia, and cat ownership among men infected with human immunodeficiency virus. Clin. Infect. Dis. 21:954–959. [DOI] [PubMed] [Google Scholar]
- 89.Slater, L. N., D. F. Welch, D. Hensel, and D. W. Coody. 1990. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N. Engl. J. Med. 323:1587–1593. [DOI] [PubMed] [Google Scholar]
- 90.Sobraques, M., M. Maurin, R. Birtles, and D. Raoult. 1999. In vitro susceptibilities of four Bartonella bacilliformis strains to 30 antibiotic compounds. Antimicrob. Agents Chemother. 43:2090–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spach, D. H., A. S. Kanter, M. J. Dougherty, A. M. Larson, M. B. Coyle, D. J. Brenner, B. Swaminathan, G. M. Matar, D. F. Welch, R. K. Root, and W. E. Stamm. 1995. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N. Engl. J. Med. 332:424–428. [DOI] [PubMed] [Google Scholar]
- 92.Spach, D. H., and J. E. Koehler. 1998. Bartonella-associated infections. Emerg. Infect. Dis. 12:137–155. [DOI] [PubMed] [Google Scholar]
- 93.Stoler, M. H., T. A. Bonfiglio, R. T. Steigbigel, and M. Pereira. 1983. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am. J. Clin. Pathol. 80:714–718. [DOI] [PubMed] [Google Scholar]
- 94.Tappero, J. W., J. C. Mohle-Boetani, J. E. Koehler, B. Swaminathan, T. G. Berger, P. E. Leboit, L. L. Smith, J. D. Wenger, R. W. Pinner, C. A. Kemper, and A. L. Reingold. 1993. The epidemiology of bacillary angiomatosis and bacillary peliosis. JAMA 269:770–775. [PubMed] [Google Scholar]
- 95.Walker, D. H., H. Guerra, and C. Maguina. 1999. Bartonelloses, p.492–497. In R. L. Guerrant, D. H. Walker, and P. F. Weller (ed.), Tropical infectious diseases: principles, pathogens & practice. Churchill Livingstone, Philadelphia, Pa.
- 96.Welch, D., K. Carrol, E. Hofmeister, D. Persing, D. Robison, A. Steigerwalt, and D. Brenner. 1999. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J. Clin. Microbiol. 37:2598–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wong, M. T., D. C. Thornton, R. C. Kennedy, and M. J. Dolan. 1995. A chemically defined liquid medium that supports primary isolation of Rochalimae (Bartonella) henselae from blood and tissue specimens. J. Clin. Microbiol. 33:742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamamoto, K., B. Chomel, L. Lowenstine, L. Phillips, J. Blackwell, R. Kasten, and N. Pedersen. 1997. Prevalence of Bartonella henselae antibodies in captive wild felids, california, and association with ectoparasite infestation. Epidémiol. Santé Anim., p.31–32. (In French.)
- 99.Yamamoto, K., B. B. Chomel, R. W. Kasten, C. C. Chang, T. Tseggai, P. R. Decker, M. Mackowiak, K. A. Floyd-Hawkins, and N. C. Pedersen. 1998. Homologous protection but lack of heterologous-protection by various species and types of Bartonella in specific pathogen-free cats. Vet. Immunol. Immunopathol. 65:191–204. [DOI] [PubMed] [Google Scholar]
- 100.Zangwill, K. M., D. H. Hamilton, B. A. Perkins, R. L. Regnery, B. D. Plikaytis, J. L. Hadler, M. L. Cartter, and J. D. Wenger. 1993. Cat scratch disease in Connecticut— epidemiology, risk factors, and evaluation of a new diagnostic test. N. Engl. J. Med. 329:8–13. [DOI] [PubMed] [Google Scholar]