Abstract
We investigated whether oral administration of Lactobacillus casei strain Shirota activates the cellular immune system and ameliorates influenza virus (IFV) titer in the nasal site in upper respiratory IFV infection by using aged mice. Natural killer activity of splenocytes and lung cells of aged mice fed an L. casei strain Shirota diet (L.casei strain Shirota group) was significantly (P < 0.01 and P < 0.05) increased compared to those fed a control diet (control group). The increases were 1.5- and 2.5-fold, respectively. In aged mice fed an XL.casei strain Shirota diet, potent induction of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), which play a very important role in excluding IFV, was evident in nasal lymphocytes. IFN-γ and TNF-α production increased 12- and 3.5-fold, respectively. In this model of upper respiratory IFV infection, the titer of IFV in the nasal washings of aged mice fed an L.casei strain Shirota diet was significantly (P < 0.05) lower than that in aged mice fed a control diet (101.6 ± 0.6 and 102.2 ± 0.5, respectively). These findings suggest that oral administration of L.casei strain Shirota activates not only systemic cellular immunity but also local cellular immunity and that it ameliorates IFV infection.
Influenza is an acute viral respiratory infection that results in high morbidity and significant mortality (2, 10). In particular, influenza infection causes deaths in older adults (5). It is presumed that declining host immune responses, particularly cellular immunity, account for the increased susceptibility to influenza virus (IFV) infection of the aged. Several studies have shown diminished natural killer (NK) cell and cytotoxic T-lymphocyte activity in aged mice compared to those in the young mice (3, 14). An increased susceptibility to IFV infection associated with the impaired immune function of T helper 1 (Th1) cells has also been reported in the senescence-accelerated mouse (7).
Lactic acid bacteria and their products are reported to have beneficial effects on host homeostasis, including activation of the immune system (8, 9). Lactobacillus casei strain Shirota, a lactic acid bacteria, was originally isolated from the human intestine and has been used commercially for a long time to produce fermented milk. Various aspects of the effects of L. casei strain Shirota have been studied intensively.L. casei strain Shirota exhibits marked activity against transplantable and 3-methylcholanthrene-induced tumors (13, 20) and anti-infectious activity against various pathogens such as Listeria monocytogenes and herpes simplex virus (17, 25). We have previously reported that intranasal administration of L. casei strain Shirota enhanced cellular immunity in the respiratory tract and protected against IFV infection in mice (12).
The purpose of the present study was to investigate whether oral administration of L. casei strain Shirota activates not only the systemic immune system but also the local immune system and whether it ameliorates IFV infection in the upper respiratory tract. Special attention was focused on the possibility of inhibiting IFV infection through oral administration of L. casei strain Shirota.
MATERIALS AND METHODS
Mice.
BALB/c female mice, 15 months old, were obtained from Japan SLC, Inc. (Hamamatsu-shi, Japan) and used for the experiments.
L. casei strain Shirota.
L. casei strain Shirota was originally isolated from human feces at the Yakult Central Institute for Microbiological Research (Tokyo, Japan). L. casei strain Shirota cells were cultured for 24 h 2at 37°C in MRS broth (Difco Laboratories, Detroit, Mich.), collected by centrifugation, and washed several times with sterile distilled water. L. casei strain Shirota cells were killed by heating for 30 min at 100°C and lyophilized. A 0.05% (wt/wt) concentration of L. casei strain Shirota was added to an MM-3 diet (Funabashi Farms, Funabashi-shi, Japan) (L. casei strain Shirota diet). The control diet was the MM-3 diet without L. casei strain Shirota.
Virus.
Influenza A/PR/8/34 (H1N1) (PR8) virus was grown in the allantoic sacs of 11-day-old chicken embryos for 2 days at 34°C according to the method of Yasui et al. (27). The allantoic fluid was removed and stored at −80°C. The titer of the virus in the allantoic fluid was expressed as the 50% egg infective dose (EID50) (26). Serial 10-fold dilutions of the allantoic fluid were injected into embryonated eggs, and the presence of virus in the allantoic fluid of each egg was determined on the basis of hemagglutinating capacity 2 days after injection. The titer of the virus was 109.2 EID50/ml.
Preparation of splenocytes and lung cells.
After mice were given the control or L. casei strain Shirota diet for 4 months, they were anesthetized with diethyl ether and killed by exsanguination. The spleen was removed and a single-cell suspension was prepared by pressing the tissue gently. After the removal of debris, erythrocytes were depleted by hypotonic lysis. The cells were washed with RPMI 1640 medium (Sigma) supplemented with 100 U of penicillin/ml and 100 μg of streptomycin/ml and then resuspended in medium supplemented with 10% heat-inactivated fetal calf serum (FCS). The lungs were removed, minced finely, and incubated for 90 min with 150 U of collagenase (Yakult Honsya Co., Tokyo, Japan) in 15 ml of medium. To dissociate tissue into single cells, collagenase-treated lungs were gently tapped in the plastic dish. After debris removal, erythrocytes were depleted by hypotonic lysis. The cells were washed with medium and then resuspended in medium supplemented with 10% FCS. The cells were counted by trypan blue exclusion and microscopy and then resuspended at the appropriate concentrations.
Assay for NK cell-mediated cytotoxicity.
Appropriate numbers of cells from spleens and lungs were added to 2 × 104 51Cr-labeled YAC-1 cells in 96-half-area-well culture plates (Corning, Corning, N.Y.) in a total volume of 0.1 ml of medium containing 10% FCS. The plates were gently centrifuged for 5 min at 50 × g and then incubated for 4 h at 37°C in 5% CO2. After incubation, the plates were centrifuged for 5 min at 50 × g, and a 50-μl sample was removed from each well for gamma scintillation counting. The percentage of specific release of 51Cr was calculated by using the following formula: % 51Cr release = [fr]cpm experimental − cpm spontaneous [fd]cpm total −cpm spontaneous [/fr] × 100
Nasal lymphocyte culture.
Nasal lymphocytes were prepared according to the method of Asanuma et al. (1) with modification. Briefly, the head of each mouse was removed, and the lower jaw was cut off. Nasal-associated lymphoid tissue (NALT) was separated from the rest of the nasal tissue by peeling away the palate. NALT is localized bilaterally on the posterior side of the palate. After obtaining the palate, the tip and the lower half of the palate were cut off. The NALT fragment was teased gently with forceps and the nasal lymphocytes were released in medium and passed through a nylon mesh. A single-cell suspension was prepared after the depletion of erythrocytes. Then, 2.5 × 105 cells were cultured in the presence of concanavalin A (ConA) (Sigma) at a concentration of 2 μg/ml in 0.1 ml of medium with 5% FCS in a 96-half-area-well culture plate. Supernatants were collected on day 3 for the determination of cytokine concentrations and then stored at−80°C for further analysis.
ELISA for determination of cytokine concentrations.
Gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-4 (IL-4) concentrations were determined by sandwich enzyme-linked immunosorbent assay (ELISA) with the DuoSet mouse IFN-γ, TNF-α, and IL-4 ELISA kits (Genzyme), respectively, according to the recommended protocols.
Infection.
After mice were given the control or L. casei strain Shirota diet for 4 months, they were anesthetized by intraperitoneal injection of sodium amobarbital (0.25 μg per mouse) and then infected by dropping 1 μl of fluid containing 103.5 EID50 of PR8 into each nostril (2 μl per mouse). Three days after inoculation, the viral titer in nasal washings was measured by using the method of Tamura et al. (23). Thereafter, the mice were anesthetized with diethyl ether and killed by exsanguination. The head of each mouse was removed, and the lower jaw was cut off. A needle attached to a syringe was inserted into the posterior opening of the nasopharynx, and a total of 1 ml of phosphate-buffered saline containing 0.1% bovine serum albumin was injected. This procedure was repeated three times, and the outflow was collected each time as nasal washing. The nasal washing was centrifuged for 5 min at 13,000 × g to remove cellular debris and used for virus titration. The viral titer in each experimental group was expressed as the mean ± standard deviation of the viral titer of each washing specimen from all mice in each group.
Statistical analyses.
Differences in NK cell activity in splenocytes and lung cells, cytokine concentrations produced by nasal lymphocytes, and viral titer in the nasal washings between the L. casei strain Shirota and control groups were examined by means of Student’s t test. Probability values of less than 5% were considered significant.
RESULTS
Effect of oral administration of L. casei strain Shirota on NK cell activity in splenocytes.
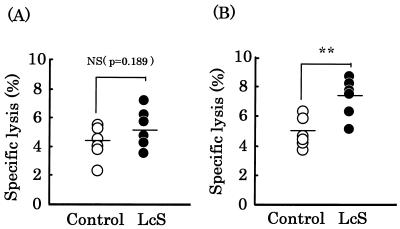
We investigated the effect of oral administration of L. casei strain Shirota on NK cell activity in splenocytes (Fig. 1). NK cell activity in control and L. casei strain Shirota groups at an effector-to-target cell (E/T) ratio of 25:1 was 4.3 ± 1.2 and 5.3 ± 1.4, respectively (Fig. 1A), with no significant difference between the groups (P = 0.189). However, NK cell activity in the control and L. casei strain Shirota groups at an E/T ratio of 50:1 was 4.9 ± 1.0 and 7.4± 1.3, respectively (Fig. 1B), with a significant (P< 0.01) difference between the groups. NK cell activity in the L. casei strain Shirota group was 1.5 times that in the control group. Similar results were obtained in three independent experiments conducted in the same manner.
FIG. 1.
Effect of oral administration of L. casei strain Shirota on NK cell activity of splenocytes. Splenocytes from mice fed either the control diet (○) or the L. casei strain Shirota diet (•) for 4 months were examined using a short-term 51Cr release assay. The E/T ratio was 25:1 (A) or 50:1 (B). Each bar represents the mean value for 6 mice. **, statistically significant difference from the control value by Student’s t test with a P value of <0.01; NS, no significant difference from the control value.
Effect of oral administration of L. casei strain Shirota on pulmonary NK cell activity.
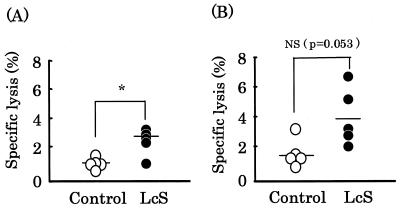
We investigated the effect of oral administration of L.casei strain Shirota on pulmonary NK cell activity (Fig. 2). NK cell activity in the control and L. casei strain Shirota groups at an E/T ratio of 12.5:1 was 1.7 ± 1.0 and 4.0 ± 2.0, respectively (Fig. 2B), with no significant difference between the groups (P = 0.053). However, NK cell activity in the control and L.casei strain Shirota groups at an E/T ratio of 6.25:1 was 1.0 ± 0.4 and 2.5 ± 0.9, respectively (Fig. 2A), with a significant (P < 0.05) difference between the groups. NK cell activity in the L. casei strain Shirota group was 2.5 times that in the control group. Similar results were obtained in two independent experiments conducted in the same manner.
FIG. 2.
Effect of oral administration of L. casei strain Shirota on pulmonary NK cell activity. Lung cells from mice fed either the control diet (○) or the L. casei strain Shirota diet (•) for 4 months were examined using a short-term 51Cr release assay. The E/T ratio was 6.25:1 (A) or 12.5:1 (B). Each bar represents the mean value for 5 mice. *, statistically significant difference from the control value by Student’s t test at a P value of <0.05; NS, no significant difference from the control value.
Effect of oral administration of L. casei strain Shirota on production of various cytokines by nasal lymphocytes.
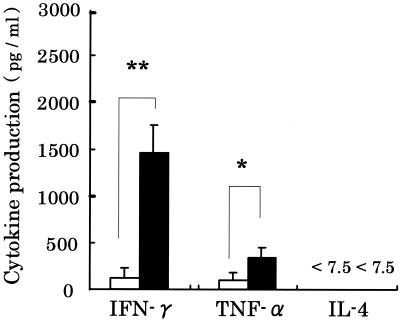
We measured production of various cytokines by nasal lymphocytes stimulated with ConA, a T-cell mitogen, to confirm the activation of T cells by oral administration of L.casei strain Shirota (Fig. 3). The concentration of IFN-γ in the L. casei strain Shirota group was significantly (P < 0.01) different from that in the control group. The IFN-γ concentration in the L. casei strain Shirota group was about 12 times greater than that in the control group (Fig. 3). The concentration of TNF-α in the L. casei strain Shirota group was also significantly (P < 0.05) different from that in the control group. The TNF-α concentration in the L. casei strain Shirota group was about 3.5 times greater than that in the control group (Fig. 3). IL-4 was not detectable in either group. Similar results were obtained in two independent experiments conducted in the same manner.
FIG. 3.
Effect of oral administration of L. casei strain Shirota on production of IFN-γ, TNF-α, and IL-4 by nasal lymphocytes. Nasal lymphocytes from aged mice fed either the control diet (n = 8) (white bar) or the L. casei strain Shirota diet (n = 8) (black bar) were cultured in the presence of ConA. Supernatants were collected 72 h after the initiation of culture, and the concentrations of various cytokines were determined. Each bar represents the mean ± standard deviation (error bar) of triplicate samples. * and **, statistically significant difference from the control by Student’s t test at a P value of <0.01 and <0.05, respectively.
Effect of oral administration of L. casei strain Shirota on viral titer in nasal washings of mice inoculated with IFV.
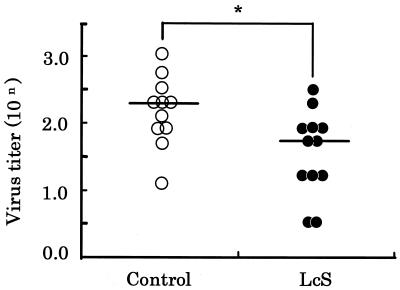
As shown in Fig. 4, the viral titers in the control and L. casei strain Shirota groups were 102.2 ± 0.5 and 101.6 ± 0.6, respectively. The reduction in virus titer in the L. casei strain Shirota group was about one-third. The viral titer in the L. casei strain Shirota group was significantly (P < 0.05) lower than that in the control group.
FIG. 4.
Effect of oral administration of L. casei strain Shirota on viral titer in nasal washings. Aged mice were fed either the control diet (○) or the L. casei strain Shirota diet (•) for 4 months. One day thereafter, the aged mice were intranasally infected with PR8, and 3 days later the viral titers in the nasal washings from the infected mice were measured. Each bar represents the mean value for 11 or 12 mice. *, statistically significant difference from the control value by Student’s t test at a P value of <0.05.
DISCUSSION
It is suggested that both humoral and cellular immunity are important for protection against IFV infection (3, 7, 27). Two strains of lactic acid bacteria which modulate the different immune systems, each in its own way have been studied (28). One strain was Bifidobacterium breve YIT 4064, which enhances humoral immunity. It has been reported that oral administration of this strain augmented production of antigen-specific immunoglobulin G in the serum and protected against IFV infection in mice (27). The other strain was L. casei strain Shirota, which enhances cellular immunity. We have previously reported that intranasal administration of this strain enhanced cellular immunity in the respiratory tract and protected against IFV infection in mice (12). In this study, we found that oral administration of L.casei strain Shirota activated not only the systemic immune system but also the local immune system and that it ameliorated IFV infection in aged mice.
It was found that L. casei strain Shirota administered orally augmented NK cell activity in splenocytes and enhanced systemic cellular immunity in aged mice. Takagi et al. (21) also reported augmentation of NK cell activity in splenocytes by means of oral administration of L.casei strain Shirota in tumor-bearing mice. In studies with humans, Nagao et al. (16) reported that oral administration of L. casei strain Shirota enhanced the NK cell activity of peripheral blood mononuclear cells in low-NK individuals. These results suggest that L.casei strain Shirota normalizes declining cellular immunity such as in aged or tumor-bearing mice. Furthermore, it was obvious that L. casei strain Shirota administered orally augmented pulmonary NK cell activity in aged mice. This is the first report that oral administration of L. casei strain Shirota affected the local immune system. Influenza is a respiratory tract disease, and it is important for the enhancement of local cellular immunity in the respiratory site for protection against influenza infection (19). In this study, we also found that L. casei strain Shirota administered orally enhanced IFN-γ and TNF-α production of nasal lymphocytes. This is very interesting because nasal lymphocytes are activated by oral administration of L. casei strain Shirota. Tamura et al. (23) and Asanuma et al. (1) reported that NALT had a very important role for protection against airborne viruses and other infectious agents. Cytokines are important in the development of various immune cells. Therefore, changes in cytokine profiles will affect the immune response and IFV infection. IFN-γ produced by Th1 and NK cells exerts direct antiviral effects and regulates several aspects of the immune response, including stimulation of macrophages and NK cell activity (4). TNF-α is produced by different immune cells, including monocytes; NK, B, and T cells; and neutrophils (29). In vitro treatment with TNF-α resulted in a decreased production of infectious virus, an inhibition of viral protein synthesis, and a reduction in the cytopathic effect of the virus (24).
In this study, we observed a significant decrease in the titer of IFV in the respiratory tract of mice fed L. casei strain Shirota. This appears to be a very important phenomenon. We have previously shown that the titer of virus in nasal washings of mice inoculated with L. casei strain Shirota intranasally before infection was significantly lower than that in mice not inoculated with L. casei strain Shirota. Furthermore, we have shown that the survival rate of mice in the L. casei strain Shirota-treated group was significantly higher than that of mice in the nontreated group by using a murine model of IFV infection in which virus moves from the upper respiratory tract to the lower respiratory tract (12). There are two possible mechanisms by which oral administration of L. casei strain Shirota ameliorates IFV infection. One possibility is the augmentation of respiratory NK cell activity, and the other is potent induction of IFN-γ and TNF-α at the nasal site. Further, it was reported that IFV infection depressed IL-2 production (6) and significantly reduced NK cell activity (18). Although the mechanism by which IFV induces immunosuppression is not completely understood, there is evidence to suggest that an induced immunosuppressed state plays a role in the pathogenesis of the disease (15). It can be speculated that oral administration of L. casei strain Shirota maintained cellular immunity after IFV infection. Regarding this point, further study is needed.
The mechanism underlying the enhancement of local cellular immunity upon oral administration of L. casei strain Shirota is not clear, but there has been a study to determine the route by which L. casei strain Shirota is recognized by host immune systems (22). L. casei strain Shirota administered orally was taken up by M cells in the Peyer’s patches and was scarcely taken up by epithelial cells on the lamina propria of the intestine. Therefore, it was presumed that L. casei strain Shirota was taken up by M cells in the Peyer’s patches and stimulated macrophages, T cells, NK cells, and other cells, and these cells migrated to the mesenteric lymph nodes (MLN). In fact, the production of IFN-γ by MLN cells of aged mice fed L. casei strain Shirota for 1 month was significantly increased (unpublished data). We think that these lymphocytes, such as Th1 cells and NK cells in MLN, would migrate via the thoracic duct and bloodstream to the spleen, lungs, and NALT.
Recently it has been reported that one of the lactobacilli,Lactobacillus rhamnosus GG (ATCC 53103) reduced respiratory infections and their severity in children (11). Since L. casei strain Shirota is used to produce fermented milk and is safe, it may be useful for elderly individuals with low immune function to prevent respiratory infections.
Acknowledgments
We thank S. Tamura, H. Asanuma, and T. Kurata (National Institute of Health, Tokyo, Japan) for help with the study and M. Watanuki for helpful discussion.
REFERENCES
- 1.Asanuma, H., A. H. Thompson, T. Iwasaki, Y. Sato, Y. Inaba, C. Aizawa, T. Kurata, and S. Tamura. 1997. Isolation and characterization of mouse nasal-associated lymphoid tissue. J. Immunol. Methods 202:123–131. [DOI] [PubMed] [Google Scholar]
- 2.Barker, W. H., and J. P. Mullooly. 1981. Underestimation of the role of pneumonia and influenza in causing excess mortality. Am. J. Public Health 71:643–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender, B. S., M. P. Johnson, and P. A. Small. 1991. Influenza in senescent mice: impaired cytotoxic T-lymphocyte activity is correlated with prolonged infection. Immunology 72:514–519. [PMC free article] [PubMed] [Google Scholar]
- 4.Boeham, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-γ. Annu. Rev. Immunol. 15:749–795. [DOI] [PubMed] [Google Scholar]
- 5.Brody, J. A., and D. B. Brock. 1985. Handbook of the biology of aging, p.3–26. In C. E. Finch and E. L. Schneider (ed.), Epidemiologic and statistical characteristics of this United States elderly population. Van Nostrand Reinhold, New York, N.Y.
- 6.Del Gobbo, V., N. Villani, S. Marini, E. Balestra, and R. Calio. 1990. Suppressor cells induced by influenza virus inhibit interleukin-2 production in mice. Immunology 69:454–459. [PMC free article] [PubMed] [Google Scholar]
- 7.Dong, L., I. Mori, M. J. Hossain, and Y. Kimura. 2000. The senescence-accelerated mouse shows aging-related defects in cellular but not humoral immunity against influenza virus infection. J. Infect. Dis. 182:391–396. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes, C. F., and K. M. Shahani. 1990. Anticarcinogenic and immunological properties of dietary lactobacilli. J. Food Prot. 53:704–710. [DOI] [PubMed] [Google Scholar]
- 9.Gilliland, S. E. 1989. Acidophilus milk products: a review of potential benefits to consumers. J. Dairy Sci. 72:2483–2494. [DOI] [PubMed] [Google Scholar]
- 10.Glezen, W. P., A. A. Payne, and D. N. Snyder. 1982. Mortality and influenza. J. Infect. Dis. 146:313–321. [DOI] [PubMed] [Google Scholar]
- 11.Hatakka, K., E. Savilahti, A. Pö nkä, J. H. Meurman, T. Poussa, L. Näse, M. Saxelin, and R. Korpela. 2001. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomized trial. BMJ 322:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hori, T., J. Kiyoshima, K. Shida, H. Yasui. 2001. Effect of intranasal administration of Lactobacillus casei Shirota on influenza virus infection of upper respiratory tract in mice. Clin. Diagn. Lab. Immunol. 8:593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuzaki, T. 1998. Immunomodulation by treatment with Lactobacillus casei strain Shirota. Int. J. Food Microbiol. 41:133–140. [DOI] [PubMed] [Google Scholar]
- 14.Meydani, S. N., L. M. Stocking, A. C. Shapiro, M. Meydani, and J. B. Blumberg. 1988. Fish oil and tocopherol-induced changes in ex vivo synthesis of spleen and lung leukotriene B4 (LTB4) in mice. Ann. N. Y. Acad. Sci. 524:395–397. [Google Scholar]
- 15.Mitchell, D. M., A. J. McMichael, and J. R. Lamb. 1985. The immunology of influenza. Br. Med. Bull. 41:80–85. [DOI] [PubMed] [Google Scholar]
- 16.Nagao, F., M. Nakayama, T. Muto, K. Okumura. 2000. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci. Biotechnol. Biochem. 64:2706–2708. [DOI] [PubMed] [Google Scholar]
- 17.Nomoto, K., S. Miake, S. Hashimoto, T. Yokokura, M. Mutai, Y. Yoshikai, and K. Nomoto. 1985. Augmentation of host resistance to Listeria monocytogenes infection by Lactobacillus casei. J. Clin. Lab. Immunol. 17:91–97. [PubMed] [Google Scholar]
- 18.Santoro, M. G., C. Favalli, A. Mastino, B. M. Jaffe, M. Esteban, and E. Garaci. 1988. Antiviral activity of a synthetic analog of prostaglandin A in mice infected with influenza A virus. Arch. Virol. 99:89–100. [DOI] [PubMed] [Google Scholar]
- 19.Stein-Streilein, J., and J. Guffee. 1986. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J. Immunol. 136:1435–1441. [PubMed] [Google Scholar]
- 20.Takagi, A., T. Matsuzaki, M. Sato, K. Nomoto, M. Morotomi, and T. Yokokura. 1999. Inhibitory effect of oral administration of Lactobacillus casei on 3-methylchoranthrene-induced carcinogenesis in mice. Med. Microbiol. Immunol. 188:111–116. [DOI] [PubMed] [Google Scholar]
- 21.Takagi, A., T. Matsuzaki, M. Sato, K. Nomoto, M. Morotomi, and T. Yokokura. 2001. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis 22:599–605. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi, M., S. Iwata, N. Yamazaki, and H. Fujiwara. 1991. Phagocytosis of the lactic acid bacteria by M cells in the rabbit Peyer’s patches. J. Clin. Electron Microsc. 24:5–6. [Google Scholar]
- 23.Tamura, S., K. Miyata, K. Matsuo, H. Asanuma, H. Takahashi, K. Nakajima, Y. Suzuki, C. Aizawa, and T. Kurata. 1996. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant combined recombinant nucleoprotein. J. Immunol. 156:3892–3900. [PubMed] [Google Scholar]
- 24.Van Campen, H. 1994. Influenza A virus replication is inhibited by tumor necrosis factor-α in vitro. Arch. Virol. 136:439–446. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe, T., and H. Saito. 1986. Protection of mice against herpes simplex virus infection by a Lactobacillus casei preparation (LC9018) in combination with inactivated viral antigen. Microbiol. Immunol. 30:111–122. [DOI] [PubMed] [Google Scholar]
- 26.Webster, R. G., and B. A. Askonas. 1980. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur. J. Immunol. 10:396–401. [DOI] [PubMed] [Google Scholar]
- 27.Yasui, H., J. Kiyoshima, T. Hori, and K. Shida. 1999. Protection against influenza virus infection of mice fed Bifidobacterium breve YIT4064. Clin. Diagn. Lab. Immunol. 6:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasui, H., K. Shida, T. Matsuzaki, and T. Yokokura. 1999. Immunomodulatory function of lactic acid bacteria. Antonie Leeuwenhoek 76:383–389. [PubMed] [Google Scholar]
- 29.Zhang, M., and K. J. Tracy. 1998. Tumor necrosis factor, p.517–548. In A. D. Thompson (ed.), The cytokine handbook, 3rd ed. Academic Press, San Diego, Calif.