Abstract
Channel catfish (Ictalurus punctatus) that survive infection with the parasitic ciliate Ichthyophthirius multifiliis acquire immunity to subsequent challenge and produce specific antibodies in serum that immobilize the parasite in vitro. Cellular surface protein antigens targeted by these antibodies are referred to as immobilization antigens (i-antigens). By using an immobilizing mouse monoclonal antibody as a ligand, the i-antigen of I. multifiliis isolate G5 was purified to homogeneity by immunoaffinity chromatography, and its immunogenicity was confirmed by inoculating rabbit and channel catfish to produce immobilizing antisera. To test the purified i-antigen as a subunit vaccine, channel catfish fingerlings were injected intraperitoneally (i.p.) with purified i-antigen at a dose of 10 μg/fish in complete Freund’s adjuvant on day 1, followed by a second i.p. injection of the same amount of i-antigen in incomplete Freund’s adjuvant on day 15. Negative control fish were immunized similarly with either bovine serum albumin (BSA) or an immobilization-irrelevant I. multifiliis protein. On day 84, the fish were challenged with live I. multifiliis G5 theronts at a dose of 15,000 cells per fish. Seventy-two percent of the fish immunized with i-antigen survived the challenge. All negative control fish died within 16 days of exposure. There was a significant difference in the median days to death between the negative control fish injected with BSA and the fish that died following vaccination with i-antigen. Fish injected with i-antigen developed high immobilizing antibody titers in serum. This is the first demonstration of a direct role for i-antigens in the elicitation of protective immunity, suggesting that these proteins by themselves serve as effective subunit vaccines against I. multifiliis.
The obligate parasitic ciliate Ichthyophthirius multifiliis is one of the most common and destructive protozoan pathogens of freshwater fish. The free-swimming, highly motile infective theront penetrates into the epithelia of the skin and gills, where it transforms into a large (500-μm) feeding trophont. After a period of growth it leaves the host and replicates within a protective cyst in the aqueous environment. Although the disease (commonly referred to as “Ich” or “white spot disease”) is usually fatal, fish that survive infection develop immunity to subsequent parasite challenge (3, 10, 13, 17, 22). Our laboratory is focused on elucidation of the mechanisms of this protective immune response.
The first observation that sera from immune fish immobilize the parasite in vitro was reported in 1974 (17), where it was postulated that this effect corresponds to protection in vivo. It was subsequently found that antibody binding to parasite cell and ciliary surface antigens causes immobilization (3, 4). The target antigens of immobilization have been purified by immunoaffinity chromatography (20) and have been characterized as a class of highly abundant, glycosyl-phosphatidyl-inositol-anchored surface membrane proteins (5). The structures of these proteins (referred to as immobilization antigens [i-antigens]) are analogous to those of the surface antigens found on the free-living ciliates Paramecium and Tetrahymena (2, 24). To date, 10 different I. multifiliis isolates have been classified into five immobilization serotypes (serotypes A to E) on the basis of in vitro immobilization (11).
Experimental evidence supports the hypothesis that immobilizing antibodies play a role in protective immunity. Channel catfish passively immunized by intraperitoneal injection of immobilizing mouse monoclonal antibodies (MAbs) are protected against subsequent lethal challenge (19). Furthermore, parasites colonized in the epithelia of naive fish are induced to leave following the injection of i-antigen-specific MAbs or F(ab)2 fragments. This response requires cross-linking of surface i-antigen by bivalent antibody at subimmobilizing concentrations (7). Mouse immunoglobulin (Ig) G antibodies reach the surface epithelia of fish within 12 h of intravenous or intraperitoneal injection. Immobilizing mouse IgM antibodies or fish serum antibodies (tetrameric 750-kDa IgM-like molecules), however, are not found in the surface mucus of fish following passive transfer. Presumably, this is due to their large molecular mass, which precludes transport to the skin. Nevertheless, specific immobilizing antibodies have been detected in the skin of actively immunized fish, and these are postulated to offer protection by the same mechanisms by which passively administered mouse antibodies offer protection (26).
An important goal of I. multifiliis research is the development of an effective and practical vaccine to protect fish from infection. Fish have been successfully immunized in the laboratory by intraperitoneal injection of live theronts (1) or by surface exposure followed by treatment (4). Parasites introduced into the peritoneal cavity establish infection and grow for about 21 days before they become surrounded by granulomatous tissue and die (15). Interestingly, intraperitoneal infection elicits an immune response that effectively blocks infection by challenge by surface exposure. While live parasites elicit protection under controlled circumstances, vaccines that comprise live parasites are not practical for large-scale field use because I. multifiliis is an obligate parasite and is difficult to grow in large quantities. Also, the danger of inadvertent outbreaks exists if live parasites are used for vaccination. For these reasons we have investigated the use of purified i-antigen as a subunit vaccine. While passive immunization experiments have suggested that the i-antigens of I. multifiliis play an important role in immunity, direct evidence that these proteins by themselves stimulate protective immunity had not been established until now. Here we demonstrate that the vaccination of channel catfish with affinity-purified i-antigens elicits protective immunity against a potentially lethal parasite challenge.
MATERIALS AND METHODS
Propagation and collection of parasites.
The I. multifiliis G5 isolate used in the present study has been characterized previously (5, 19) and is maintained by serial passage on juvenile channel catfish (Ictalurus punctatus) (23). Live theronts were obtained by previously described methods (12). Five heavily infected fish with visible parasites over the entire body surface were placed in a beaker containing 3 liters of carbon-filtered water, and trophonts were dislodged from the fish skin. Parasites were collected with a 200-mesh sieve, transferred into about 100 ml of carbon-filtered water, and allowed to develop into theronts (18 to 20 h at 22°C). Theronts were passed through a 400-mesh sieve, harvested by centrifugation at 1,000 × g for 2 min, and washed once in 50 ml of carbon-filtered water. Cells were used immediately, or pellets were frozen in liquid nitrogen and stored at −70°C.
Purification of protein antigens from I. multifiliis.
Two mouse MAbs, MAbs G3-61 and G3-74, were used for the preparation of immunoaffinity columns and the subsequent purification of I. multifiliis protein antigens. These MAbs (IgG1) were generated against the I. multifiliis G3 isolate (7, 19). MAb G3-61 specifically immobilizes the I. multifiliis G3 isolate as well as the G5 isolate and was used to purify i-antigen from I. multifiliis G5 in the present study. MAb G3-74 is a nonimmobilizing antibody that was used to purify a 14-kDa immobilization-irrelevant protein from I. multifiliis G5.
Immunoaffinity chromatography was performed as described previously (20), with modifications. Briefly, 10 mg of MAb purified on a protein A agarose column (Boehringer Mannheim, Mannheim, Germany) was coupled to a 2-ml AminoLink column (Pierce, Rockford, Ill.) by the manufacturer’s protocols. Parasite membrane protein was solubilized in 1% (vol/vol) Triton X-114 (Sigma, St. Louis, Mo.) in 10 mM Tris-HCl buffer (pH 7.5) and was isolated by phase separation at 30°C as described previously (12). Five milliliters of parasite membrane protein (2 to 5 mg/ml in 10 mM Tris-HCl buffer [pH 7.5]) was applied to the affinity columns. Unbound proteins were washed off the columns with 20 ml of 10 mM Tris-HCl buffer (pH 7.5). Antigens were eluted from the affinity columns with 10 mM glycine buffer (pH 3.0) and neutralized with 1 M Tris-HCl buffer (pH 7.5), and the protein concentration was determined either by determination of the absorbance at 280 nm or by the bicinchoninic acid method (Pierce).
SDS-PAGE and Western blotting.
One-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were done by standard protocols (20). A mouse MAb against channel catfish Ig heavy chain (MAb MAF13) was provided by Norman W. Miller, Department of Microbiology, University of Mississippi Medical Center, Jackson. The conjugate of this MAb and alkaline phosphatase was prepared and used as a secondary antibody for Western blotting and enzyme-linked immunosorbent assay (ELISA).
MALDI-TOF.
To determine the molecular mass of the purified i-antigen from isolate G5, matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry was performed on a Hewlett-Packard G23051 MALDI-TOF mass spectrometer located at the Complex Carbohydrate Research Center, University of Georgia. The analytic parameters used were as follows: laser energy, 7.00 μJ; vacuum, 1.26 × 10−6 torr; polarity, positive; and matrix, sinapanic acid (3,5-dimethoxy-4-hydroxy cinnamic acid).
Experimental fish.
Channel catfish (I. punctatus) fingerlings (weight, 10 to 15 g) with no previous history of exposure to I. multifiliis were obtained from a local hatchery. Prior to immunization, the fish were treated with formalin (25 ppm) to remove any possible external parasites and were maintained in a flowthrough system. The fish were fed daily with commercial trout starter feed (Purina, St. Louis, Mo.). The water temperature was maintained at 20 to 23°C, and water quality (NH3 and NO2 levels and pH) was monitored daily with standard test kits.
Production of immobilizing antisera.
Antisera were prepared in rabbits and channel catfish. The animals were bled before immunization to test for background immobilizing activity. Two adult female specific-pathogen-free New Zealand White rabbits were each injected intradermally with 100 μg of purified i-antigen in complete Freund’s adjuvant (CFA) and were boosted 3 weeks later by intradermal injection of 100 μg of i-antigen in incomplete Freund’s adjuvant (IFA). Blood was collected by cardiocentesis 3 weeks after the second injection. Ten channel catfish weighing 15 to 20 g each were injected twice intraperitoneally with 10 μg of purified i-antigen in CFA and IFA, respectively, at a 2-week interval and were bled from the caudal vein or artery 4 weeks after the second injection. The sera from rabbit and channel catfish blood were heat inactivated at 56°C for 30 min and stored at −80°C.
Vaccination procedure.
Channel catfish were distributed into eight 38-liter aerated and conditioned aquaria at a density of 35 fish per aquarium. The vaccine trial consisted of four groups, each consisting of 70 randomly assorted fish (two aquaria per group). Fish were immunized by intraperitoneal injection of protein or live theronts by using a 1-ml syringe fitted with a 26-gauge needle. The fish in group 1 were immunized on day 1 with 10 μg of i-antigen in CFA and on day 14 with 10 μg of i-antigen in IFA. The fish in group 2 were immunized on day 1 with 10 μg of bovine serum albumin (BSA) in CFA and on day 14 with 10 μg of BSA in IFA. The fish in group 3 were immunized on day 1 with 10 μg of a 14-kDa I. multifiliis protein in CFA and on day 14 with 10 μg of a 14-kDa I. multifiliis protein in IFA. The fish in group 4 were immunized on day 1 with 8,000 live theronts without adjuvant and on day 35 with 10,000 live theronts without adjuvant.
Challenge.
A small-scale experiment was carried out to determine the proper number of theronts for challenge. Small subgroups (ranging from two to seven fish each) from each treatment group were challenged with three different doses of theronts (5,000, 10,000, or 15,000 theronts) on day 28 by a standard protocol (14). In the large-scale trial, each of the remaining fish in the four treatment groups was exposed to 15,000 theronts on day 84. The aquaria were equipped with biological filtration, and water quality and temperature were monitored daily. Mortalities in each group were recorded daily until all fish died or recovered from infection.
Collection of catfish cutaneous mucus and serum samples.
The channel catfish were anesthetized with 100 to 200 ppm of tricaine methane sulfonate (MS-222; Argent Chemical Laboratories, Redmond, Wash.), and both lateral surfaces were gently wiped with cotton swabs. Each mucus-saturated swab was soaked in 0.1 ml of ice-cold phosphate-buffered saline (PBS; 135 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4 [pH 7.5]), and then the solution was squeezed out. The PBS-mucus solution was centrifuged at 14,000 × g for 5 min at 4°C, and the supernatant was collected as the mucus sample. Mucus samples were normalized to a protein concentration of 100 μg/ml, supplemented with 1% BSA, and stored at −80°C until use. A blood sample was taken from anesthetized fish immediately after mucus sample collection. Bleeding, preparation, and heat inactivation of sera were carried out as described previously (4).
In vitro immobilization assays.
Assays were carried out to determine the immobilizing antibody level in serum as described previously (4), with minor modifications. Briefly, serum samples were prepared in 96-well microtiter plates as a series of doubling dilutions in 50% PBS. Each well contained 50 μl of diluted serum. Approximately 100 to 200 live theronts in 50 μl of carbon-filtered water were added to each well, and the plate was incubated at room temperature (RT; 20 to 22°C). Immobilization was determined by observation under a dissection microscope, and titers were expressed as the inverse of the highest dilution in which all of the theronts were immobilized after incubation for 30 min at RT. Preimmune serum samples were used as negative controls.
Detection of mucus antibodies by ELISA.
To absorb antigen to plates, individual wells of ELISA plates (Falcon 3911Microtest III; Becton Dickinson, Franklin Lakes, N.J.) were filled with 50 μl of purified i-antigen (20 μg/ml) in 25 mM sodium acetate buffer (pH 7.5), and the plates were incubated overnight at 4°C. Control wells were coated with 2% (wt/vol) BSA. Nonspecific protein binding was blocked by overnight incubation at 4°C with 100 μl of 5% (wt/vol) BSA in Tris-buffered saline with Tween 20 (TBST; 20 mM Tris-HCl, 50 mM NaCl, 0.05% [vol/vol] Tween 20 [pH 7.5]). Mucus samples (50 μl per well) were added to the plates, and the plates were incubated for 1 h at RT. Following three washes with TBST, mouse anti-catfish Ig MAb (MAb MAF13) coupled to alkaline phosphatase (1:1,000 dilution in 2% [wt/vol] BSA in TBST) was added (50 μl/well), and the plate was incubated for 1 h at RT. After three washes with TBST, p-nitrophenylphosphate substrate was added, and the plates were incubated for 1 h in the dark at RT. The reaction was stopped with 4 N NaOH, and the plates were read at 405 nm with an ELISA reader.
Statistical analyses.
Differences in the median days to death (MDDs) among groups were calculated by one-way analysis of variance (ANOVA). Differences in the proportion of survivors among groups were calculated by the z test. All calculations were done with SigmaStat statistical software (Jandel Scientific Software, Chicago, Ill.).
RESULTS
Immunization with purified immobilization antigen.
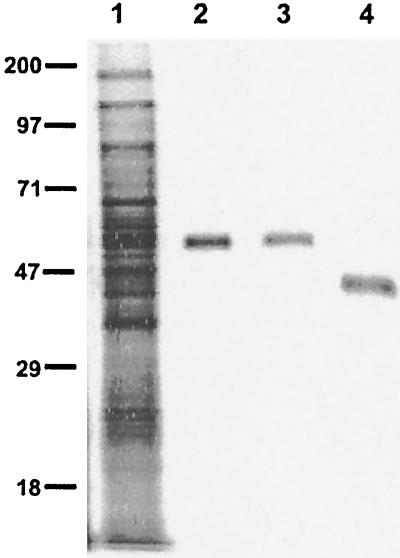
The i-antigen from I. multifiliis isolate G5 was purified to homogeneity by immunoaffinity chromatography with mouse MAb G3-61. This MAb was originally produced against I. multifiliis isolate G3 but was previously shown to immobilize both G3 and G5 isolates and confer passive protection against the G5 isolate (19). The i-antigen purified from I. multifiliis G5 comprises a single polypeptide chain. Its approximate molecular mass is 55 kDa under reducing conditions and 46 kDa under nonreducing conditions, as determined by SDS-PAGE (Fig. 1). Its more exact size by MALDI-TOF mass spectrometry analysis is 44.3 kDa. To confirm its functional identity, the affinity-purified protein was used to immunize two rabbits whose sera were then incubated with live parasites. The rabbit antisera immobilized the free-swimming theronts at a titer of 1,280 and recognized both reduced and nonreduced i-antigen on Western blots at a dilution of 1:10,000. When the G5 i-antigen was used to immunize channel catfish, a similar result was obtained, with a notable exception. Fish antisera immobilized live theronts at a titer of 640 and recognized only nonreduced i-antigen protein on Western blots at a dilution of 1:2,000. These results indicate that affinity-purified G5 i-antigen is highly immunogenic for both rabbits and channel catfish. Catfish, however, produce antibodies that react only with conformational antigenic epitopes.
FIG. 1.
Purified i-antigen of I. multifiliis G5 isolate. The G5 i-antigen was purified by immunoaffinity chromatography with immobilizing MAb G3-61 coupled to an AminoLink matrix. The purified i-antigen was resolved by SDS-PAGE on a 10% polyacrylamide gel and stained with silver nitrate. Lane 1, total membrane proteins of I. multifiliis G5 isolate (10 μg); lanes 2 and 3, reduced purified G5 i-antigen (0.5 μg); lane 4, nonreduced purified G5 i-antigen (0.5 μg). Protein standards (molecular masses [in kilodaltons]) are indicated to the left. The molecular mass of the reduced G5 i-antigen is ≈55 kDa, and that of the nonreduced G5 i-antigen is ≈46 kDa.
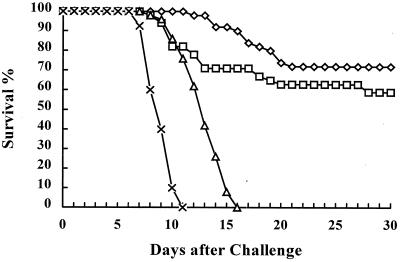
The 55-kDa i-antigen of I. multifiliis G5 was administered to channel catfish as a subunit vaccine with CFA and IFA to test if the purified protein could elicit protective immunity against homologous Ichthyophthirius infection. Preliminary experiments have shown that the purified protein without adjuvant is not an effective immunogen (X. Wang, unpublished results). Forty to 50 fish per group were tested in a large-scale vaccination trial by exposure of each fish to 15,000 theronts on day 84 after the initial injection. As shown in Fig. 2, 72% of the fish (n = 50) immunized with 10 μg of affinity-purified 55-kDa i-antigen in CFA and IFA survived lethal challenge with the homologous parasite. Negative control fish immunized with an immobilization-irrelevant 14-kDa I. multifiliis protein or BSA died by day 16 following exposure. I. multifiliis infection was visible on all fish in the negative control groups at 6 days after the challenge. Statistical analysis by the z test showed that there was no significant difference between the survival of the group into which i-antigen was injected and the group vaccinated with live theronts (P = 0.250). There was a significant difference, however, between the survival of groups vaccinated with i-antigen or live theronts and negative control groups injected with BSA or the 14-kDa protein (P < 0.001). The MDDs for fish that died in groups immunized with i-antigen or live parasites were significantly greater (P < 0.001; Kruskal-Wallis ANOVA) than the MDD for the control fish injected with BSA (Table 1).
FIG. 2.
Vaccine challenge experiment. Each fish in each group was challenged with 15,000 live I. multifiliis theronts at day 84 after the first injection. Seventy-two percent of the fish (n = 50) immunized with 10 μg of affinity-purified 55-kDa i-antigen (◊) in CFA and IFA and 59.2% of the fish (n = 49) immunized with live I. multifiliis theronts (□) survived the challenge. Negative control fish injected with an immobilization-irrelevant 14-kDa I. multifiliis protein (▵) or BSA (×) in CFA and IFA died by day 16 following challenge.
TABLE 1.
Survival of immunized channel catfish following challenge
| Vaccinea | No. of fish challengedb/no. of fish that survived | % Survival | MDDc | 25% MDDd | 75% MDDe |
|---|---|---|---|---|---|
| i-antigen | 50/36 | 72.0 | 17 | 14 | 20 |
| Live theronts | 49/29 | 59.2 | 12 | 10 | 18 |
| 14-kDa protein | 50/0 | 0 | 13 | 12 | 15 |
| BSA | 40/0 | 0 | 9 | 8 | 10 |
Channel catfish were injected with protein antigens or live theronts as described in Materials and Methods.
Each fish was exposed to 15,000 theronts by surface exposure.
MDDs were calculated by Kruskal-Wallis one way ANOVA on ranks. There is a significant difference in the MDDs among the treatment groups (P < 0.01).
Lower quartile.
Upper quartile.
Detection of i-antigen-specific serum and mucus antibody.
The amount of serum and mucus antibody detected by the immobilization assay and ELISA, respectively, correlated positively with the level of protection. Channel catfish immunized with i-antigen or live parasites had high immobilizing serum antibody titers that ranged from 160 to 640 beginning 2 weeks after the first injection, while fish injected with BSA or the 14-kDa protein had no detectable immobilizing antibody response (Table 2). Furthermore, at 9 months after challenge, the titer of immobilizing antibody in the sera of fish that survived challenge was 640. All fish were resistant to rechallenge with 15,000 theronts per fish at that time (data not shown).
TABLE 2.
Serum and mucus antibody responses over time
| Immunogen | Serum antibody titera at wk:
|
Mucus antibody OD405nb at wk:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 7 | 9 | 11 | 2 | 7 | 9 | 11 | |
| Live theronts | 160 | 240 | 480 | 480 | 0.011 | 0.008 | 0.039 | 0.085 |
| i-antigen | 120 | 640 | 640 | 480 | 0.018 | 0.004 | 0.027 | 0.049 |
| 14-kDa protein | 0 | 20 | 0 | 0 | 0.032 | 0.007 | 0.017 | 0.004 |
| BSA | 0 | 0 | 0 | 0 | 0.011 | 0.007 | 0.012 | 0.011 |
Determined by in vitro immobilization assay. The value is the median titer (n = 6).
Determined by ELISA. The value is the median value of the optical density at 405n (OD405) (n = 6).
Mucus antibodies against the i-antigen were not present at a sufficiently high concentration to immobilize parasites but were detectable by ELISA. As shown in Table 2, fish immunized with either purified G5 i-antigen or live theronts developed specific mucus antibodies against the i-antigen of isolate G5 at week 11.
Antibodies from I. multifiliis-immune fish and immobilizing mouse MAb recognize conformation-dependent epitopes.
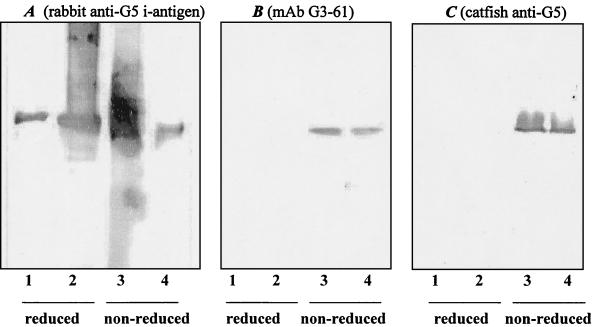
When analyzed by SDS-PAGE (Fig. 1), the i-antigen protein migrates at different mobilities under reducing or nonreducing conditions (with or without β-mercaptoethanol, respectively). Under reducing conditions the protein has a molecular mass of 55 kDa. Under nonreducing conditions its molecular mass is 46 kDa. This is likely due to the intramolecular sulfhydryl bonds that exist between repeated cysteine residues (8). As shown by Western blot analyses (Fig. 3), immobilizing MAb G3-61 and polyclonal antisera from immune channel catfish recognize only the nonreduced form of the i-antigen. In contrast, polyclonal antisera from rabbits immunized with purified isolate G5 i-antigen recognize the polypeptide under both reducing and nonreducing conditions. The reactivity of antisera or antibodies against i-antigens on blot membranes could be eliminated by preincubation of antisera or antibodies with high concentrations of I. multifiliis cilia or live theronts (data not shown). These results indicate that immobilizing antibodies recognize conformation-dependent epitopes on the i-antigen molecule and that these epitopes elicit the predominant antibody response in fish.
FIG. 3.
Western blot analyses of G5 i-antigen. Lanes 1, reduced purified G5 i-antigen (0.7 μg); lanes 2, reduced G5 membrane protein sample (5 μg); lanes 3, nonreduced G5 membrane protein sample (5 μg); lanes 4, nonreduced purified G5 i-antigen (0.7 μg). (A) The probe was rabbit anti-G5 i-antigen serum (1:5,000); (B) the probe was MAb G3-61 (1:1,000); (C) the probe was fish anti-G5 i-antigen serum (1:500). The secondary antibodies are goat anti-rabbit IgG, rabbit anti-mouse IgG, and mouse anti-catfish Ig MAb (MAb MAF13) conjugated with alkaline phosphatase, respectively. Rabbit antiserum against purified G5 i-antigen recognizes both reduced and nonreduced G5 i-antigens. Immobilizing MAb G3-61 and fish anti-G5 i-antigen serum recognize only the nonreduced forms of the protein.
DISCUSSION
The results presented here demonstrate that vaccination of naïve channel catfish with the purified i-antigen of I. multifiliis elicits immunity, thus confirming the role of the i-antigen as a protective immunogen. Previous studies have strongly implicated the involvement of i-antigens in the immune response against this highly pathogenic protozoan parasite. I. multifiliis-immune fish produce serum antibodies against i-antigens, and mucus collected from skin (a primary site of parasite infection) contains antibodies that react with the proteins on Western blots (3, 4, 6, 9, 10, 22). There is considerable evidence supporting the existence of a separate mucosal immune system in fish (11, 26), and we postulate that specific antibodies that target i-antigens in the skin and gills are responsible for protection against I. multifiliis (11). Passive transfer studies with mouse immobilizing MAbs support a model of surface immunity mediated by antibodies (7, 20). It is not yet clear whether fish antibodies generated in response to infection are transported to surface sites from central or regional lymphoid tissue or are produced locally.
Two aspects of the fish immune response to i-antigens are apparent from this and other studies in our laboratory. First, i-antigens elicit protective immunity only when they are presented in context with live parasites or are associated with adjuvants (1). It appears that inflammation and its associated “danger signals” are necessary elements for initiation of the acquired immune response. Similar results were found in other fish species immunized with individual protein antigens (21). This is of practical significance for the further development of vaccines, which will require an adjuvant system that is easy to administer (preferably orally or by immersion), that causes minimal tissue damage, and that elicits an appropriately modulated inflammatory response.
Second, fish serum antibodies (like mouse immobilizing MAbs) do not recognize i-antigens on Western blots run under reducing conditions (20). Intramolecular sulfhydryl bonds are most likely responsible for maintaining the conformation required for binding of immobilizing antibodies. Antibodies from rabbits immunized with purified i-antigen in CFA and IFA recognize both the reduced and the nonreduced forms of the protein on Western blots. The presence of different recognition sites or alternate mechanisms of antigen processing and presentation might account for this difference from the fish’s response. The fact that channel catfish were injected intraperitoneally while rabbits were inoculated intradermally could be a contributing factor. In any case, it appears that fish generate a homogeneous antibody response with regard to the recognition of conformational epitopes.
It is clear that immobilizing antibodies prevent infection, but it is also known that immunity generated in response to natural infection with I. multifiliis protects fish against infection by challenge with parasites with heterologous i-antigen serotypes (18). This raises an interesting question with regard to whether nonimmobilizing epitopes on i-antigen molecules elicit protection against parasites with heterologous serotypes or whether other protective antigens exist. To explore further the role of i-antigens in cross-protection, we are testing groups of channel catfish injected with affinity-purified i-antigens from two distinct serotypes (serotypes A and D) by challenging them with parasites with homologous or heterologous serotypes.
The experimental vaccine used in the present study consisted of i-antigen that was affinity purified directly from the parasite. While this native protein clearly served as an effective vaccine, it would be extremely difficult to collect enough protein for the production of a vaccine for large-scale field use. A heterologous system for the production of recombinant i-antigen proteins is required. Toward this end, the gene encoding the 48-kDa i-antigen of I. multifiliis strain G1 has been cloned and expressed in the free-living ciliate Tetrahymena thermophila (16). Tetrahymena has great potential for use in vaccine development. Tetrahymena cell lines can be grown in large-volume cultures, reach a remarkably high density of >5 × 106 cells per ml in a relatively short time (generation time, 1.4 h), and tolerate wide ranges of tonicity and temperatures. Importantly, T. thermophila is nonpathogenic and is generally regarded as an environmentally safe organism (25). Thus, successful expression of an I. multifiliis gene in T. thermophila should allow the production of large amounts of antigen in a purified form at relatively low cost. In addition to its use for the production of purified antigens, transformed T. thermophila can also be used as a live vaccine applied either through a bath or by injection into the animal host. We have found that up to 106 T. thermophila cells can be injected into the peritoneal cavity of channel catfish fingerlings weighing 10 to 15 g, where they survive for several days without causing adverse effects (X. Wang and H. W. Dickerson, unpublished results). Expression of native Ichthyophthirius proteins on the surface of Tetrahymena may provide the most efficient means of exposing fish to antigens, short of infection with the live parasite itself. The use of T. thermophila as an expression system could have widespread implications for the development of fish vaccines.
Acknowledgments
This work was supported by U.S. Department of Agriculture National Research Initiative grant 97-35204-4481.
We thank Jane Noe for excellent technical support in the care and maintenance of the fish and parasite cultures. We also thank for Ron Orlando and Li Zhang of CCRC, University of Georgia, for measurement of the molecular size of the i-antigen by the MALDI-TOF method.
REFERENCES
- 1.Burkart, M. A., T. G. Clark, and H. W. Dickerson. 1990. Immunization of channel catfish, Ictalurus punctatus Rafinesque, against Ichthyophthirius multifiliis (Fouquet): killed versus live vaccines. J. Fish Dis. 13:401–410. [Google Scholar]
- 2.Caron, F., and E. Meyer. 1989. Molecular basis of surface antigen variation in Paramecia. Annu. Rev. Microbiol. 43:23–42. [DOI] [PubMed] [Google Scholar]
- 3.Clark, T. G., H. W. Dickerson, and R. C. Findly. 1988. Immune response of channel catfish to ciliary antigens of Ichthyophthirius multifiliis. Dev. Comp. Immunol. 12:581–594. [DOI] [PubMed] [Google Scholar]
- 4.Clark, T. G., H. W. Dickerson, J. B. Gratzek, and R. C. Findly. 1987. In vitro response of Ichthyophthirius multifiliis to sera from immune channel catfish. J. Fish Biol. 31:203–208. [Google Scholar]
- 5.Clark, T. G., Y. Gao, J. Gaertig, X. Wang, and G. Cheng. 2001. The i-antigens of Ichthyophthirius multifiliis are GPI-anchored proteins. J. Eukaryot. Microbiol. 48:332–337. [DOI] [PubMed] [Google Scholar]
- 6.Clark, T. G., T. Lin, and H. W. Dickerson. 1995. Surface immobilization antigens of Ichthyophthirius multifiliis: their role in protective immunity. Annu. Rev. Fish Dis. 5:113–132. [Google Scholar]
- 7.Clark, T. G., T. L. Lin, and H. W. Dickerson. 1996. Surface antigen cross-linking triggers forced exit of a protozoan parasite from its host. Proc. Natl. Acad. Sci. USA 93:6825–6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, T. G., T. L. Lin, D. A. Jackwood, J. Sherrill, Y. Lin, and H. W. Dickerson. 1999. The gene for an abundant parasite coat protein predicts tandemly repetitive metal binding domains. Gene 229:91–100. [DOI] [PubMed] [Google Scholar]
- 9.Cross, M. L., and R. A. Matthews. 1992. Ichthyophthiriasis in carp. Cyprinus carpio L.: fate of parasites in immunized fish. J. Fish Dis. 15:497–505. [Google Scholar]
- 10.Cross, M. L., and R. A. Matthews. 1993. Ichthyophthirius multifiliis Fouquet (Ciliophora): the localization of sites immunogenic to the host Cyprinus carpio (L.). Fish Shellfish Immunol. 3:13–24. [Google Scholar]
- 11.Dickerson, H., and T. Clark. 1998. Ichthyophthirius multifiliis: a model of cutaneous infection and immunity in fishes. Immunol. Rev. 166:377–384. [DOI] [PubMed] [Google Scholar]
- 12.Dickerson, H. W., T. G. Clark, and R. C. Findly. 1989. Ichthyophthirius multifiliis has membrane-associated immobilization antigens. J. Protozool. 36:159–164. [DOI] [PubMed] [Google Scholar]
- 13.Dickerson, H. W., and D. L. Dawe. 1995. Ichthyophthirius multifiliis and Cryptocaryon irritans (Phylum Ciliophora), p.181–227. In P. T. K. Woo (ed.), Fish diseases and disorders. CAB International, Wallingford, United Kingdom.
- 14.Dickerson, H. W., D. L. Dawe, J. B. Gratzek, J. Brown, and S. W. Pyle. 1981. Induction of Ichthyophthirius multifiliis Fouquet infections in channel catfish Ictalurus punctatus Rafinesque: standardization of the procedure. Dev. Biol. Stand. 49:331–336. [Google Scholar]
- 15.Dickerson, H. W., A. L. Lohr, and J. B. Gratzek. 1985. Experimental intraperitoneal infection of channel catfish, Ictalurus punctatus (Rafinesque) with Ichthyophthirius multifiliis (Fouquet). J. Fish Dis. 8:139–142. [Google Scholar]
- 16.Gaertig, J., Y. Gao, T. Tishgarten, T. G. Clark, and H. W. Dickerson. 1999. Surface display of a parasite antigen in the ciliate Tetrahymena thermophila. Nat. Biotechnol. 17:462–465. [DOI] [PubMed] [Google Scholar]
- 17.Hines, R. S., and D. T. Spira. 1974. Ichthyophthiriasis in the mirror carp Cyprinus carpio (L.). V. Acquired immunity. J. Fish Biol. 6:373–378. [Google Scholar]
- 18.Leff, A. A., T. Yoshinaga, and H. W. Dickerson. 1994. Cross immunity in channel catfish against two immobilization serotypes of Ichthyophthirius multifiliis. J. Fish Dis. 17:429–432. [Google Scholar]
- 19.Lin, T. L., T. G. Clark, and H. Dickerson. 1996. Passive immunization of channel catfish (Ictalurus punctatus) against the ciliated protozoan parasite Ichthyophthirius multifiliis by use of murine monoclonal antibodies. Infect. Immun. 64:4085–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, T. L., and H. W. Dickerson. 1992. Purification and partial characterization of immobilization antigens from Ichthyophthirius multifiliis. J. Protozool. 39:457–463. [DOI] [PubMed] [Google Scholar]
- 21.Manning, M. J., and M. S. Mughal. 1985. Factors affecting the immune responses of immature fish, p.27–40. In A. E. Ellis (ed.), Fish and shellfish pathology. Academic Press, London, United Kingdom.
- 22.McCallum, H. I. 1986. Acquired resistance of black mollies Poecilia latipinna to infection by Ichthyophthirius multifiliis. Parasitology 93:251–261. [DOI] [PubMed] [Google Scholar]
- 23.Noe, J. G., and H. W. Dickerson. 1995. Sustained growth of Ichthyophthirius multifiliis at low temperature in the laboratory. J. Parasitol. 81:1022–1024. [PubMed] [Google Scholar]
- 24.Smith, D. L., M. S. Berkowitz, D. Potoczak, M. Krause, C. Raab, F. Quinn, and F. P. Doerder. 1992. Characterization of the T, L, I, S, M and P cell surface (immobilization) antigens of Tetrahymena thermophila: molecular weights, isoforms, and cross-reactivity of antisera. J. Protozool. 39:420–428. [DOI] [PubMed] [Google Scholar]
- 25.Wheatley, D. N., L. Rasmussen, and A. Tiedtke. 1994. Tetrahymena: a model for growth, cell cycle and nutritional studies, with biotechnological potential. Bioessays 16:367–372. [DOI] [PubMed] [Google Scholar]
- 26.Xu, C. 1995. Ph.D. thesis. University of Georgia.