Abstract
Apo2 Ligand or Tumour Necrosis Factor (TNF)-Related Apoptosis-Inducing Ligand (Apo2L/TRAIL) is a member of the TNF gene superfamily that selectively induces apoptosis in tumor cells of diverse origins through engagement of death receptors. We have recently demonstrated that Type I interferons (IFN-α and β) induce apoptosis in multiple myeloma (MM) cell lines and in plasma cells from MM patients. Moreover, Apo2L selectively induces apoptosis of patient MM tumor cells while sparing non-malignant cells. Apo2L induction is one of the earliest events following IFN administration in these cells. IFNs activate Caspases and the mitochondrial-dependent apoptotic pathway mediated by Apo2L production. Cell death induced by IFNs and Apo2L can be blocked by a dominant-negative Apo2L receptor, DR5, and is regulated by members of the Bcl-2 family of proteins. This review is focused on the apoptotic signaling pathways regulated by Apo2L and Bcl-2-family proteins and summarizes what is known about their clinical role.
Keywords: Multiple myeloma, Apoptosis, Apo2L/TRAIL, Bcl-2 family proteins, Interferon
APOPTOSIS ACTIVATION IN MM
Among hematologic malignancies, multiple myeloma (MM) constitutes 10% of the cancers and ranks as the second most frequently occurring hematologic cancer in the United States, after non-Hodgkin lymphoma [1]. MM is a malignancy characterized by a very slow proliferation of malignant plasma cells leading to their accumulation within the bone marrow. This suggests that resistance to apoptosis, a genetically regulated cell death process, may play a critical role both in the pathogenesis and resistance to treatment of MM [2]. Moreover, inducers of apoptosis may not merely have a lethal effect but also support their immortalization in the bone marrow.
Apoptosis execution may proceed through the intrinsic and extrinsic apoptotic pathways. Radiation and chemotherapeutic agents act primarily through the intrinsic pathway, in which mitochondria play the central role [3]. Once cells are committed to die, apoptogenic factors, such as cytochrome c (cyt c), are released from mitochondria to stimulate the association of the adapter Apaf-1 with Caspase-9 in the apoptosome complex, to initiate activation of a cascade of cysteine protease (caspases), which culminates in activation of effector caspases-3, -6 and -7 and proteolytic targeting of key intracellular proteins (Fig. 1, [4]).
FIGURE 1.
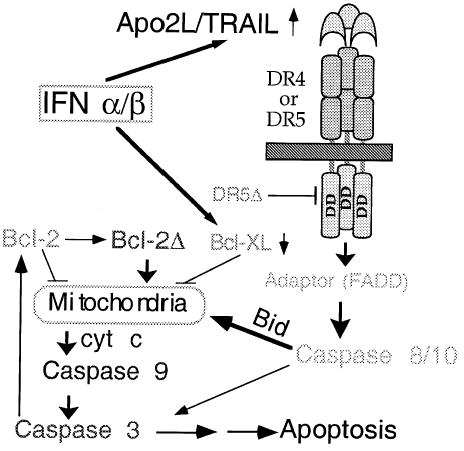
Model for activation of apoptosis in MM by IFNs. Following transcriptional induction by IFNs, Apo2L engages its receptor DR5 (or DR4) and, through an adaptor intermediate (FADD), recruits caspase 8 to the cell membrane. A similar pathway is activated by the trimeric Apo2L/TRAIL prepared for clinical studies [17]. Following caspase 8 activation by proteolysis, Bid is cleaved and translocates to mitochondria, causing release of low levels of cytochrome c into the cytosol, leading to caspase 9 and 3 activation. This results in attack of the anti-apoptotic protein Bcl-2 on the mitochondrial membranes, producing a truncated Bcl-2Δ protein, which causes release of more cyt c, caspase activation, and apoptosis. Bcl-xL transcriptional down-regulation is an additional mechanism by which IFNs may decrease levels of anti-apoptotic proteins shifting the balance towards a pro-apoptotic state (modified from Ref. [4], with permission).
The signaling pathway initiated by tumor necrosis factor (TNF) and the related Fas ligand (Apo-1) through their cognate receptors constitutes the extrinsic apoptotic pathway [5]. These cytokines are expressed as membrane-bound ligands that can be cleaved to a soluble form, which then engage their respective receptors, followed by recruitment of Caspase 8 or -10 through the Fas-associated death domain protein (FADD) [6] adapter molecule. Once activated, Caspase 8 can proteolytically cleave Bid, with the truncated Bid targeting mitochondria and inducing cyt c release, thus leading to further amplification of the caspase cascade [7,8]. The link between the extrinsic and intrinsic pathways of apoptosis through engagement of mitochondria by truncated Bid further supports the critical role of mitochondria in apoptosis.
Recently, we reported that genotoxic stress, such as ionizing radiation (IR), and IFN-induced apoptosis in MM are characterized by two distinct stages of cyt c release and a positive feedback loop linking caspase activation to cyt c release and mitochondrial dysfunction [4,9]. Irradiation also generates reactive oxygen species (ROS) in MM, associated with depletion of reduced glutathione (GSH) and collapse of mitochondrial membrane potential (Δψm). Radiation, exogenous ROS, and caspase 3 all induce Δψm drop and cyt c release from mitochondria, which could be prevented by molecular (dominant negative caspase-9) and pharmacologic (zVAD-fmk) caspase inhibitors and overexpression of Bcl-2. Exogenous ROS also induces mitochondrial permeability transition (PT) pore opening and cyt c release in isolated mitochondria, which could be blocked by inhibition of PT with cyclosporin A. These results indicate that the late ROS production is associated with decreased Δψm, PT, and GSH, events associated with caspase activation and cyt c release in MM [9].
The Bcl-2 family of proteins play a pivotal role in regulating cyt c release and apoptosis [10]. This expanding family consists of both anti-apoptotic molecules, such as Bcl-2, Bcl-XL and Mcl-1, as well as pro-apoptotic ones. The pro-apoptotic members may be classified as Bcl-2 homology domain (BH) 3-only (Bid, Bik) or multi-domain (Bax, Bak), depending on whether they contain one or three BH3 (BH1-3) domains. Bcl-2, which contains an additional BH4 domain as well as a transmembrane (TM) domain which anchors it primarily to the outer mitochondrial membrane, can block the release of cyt c from mitochondria [3] and prevent the activation of Caspase 3 in many cells, including MM [11,12]. In contrast, Bax and Bid can promote cyt c release from mitochondria, and thus activate the caspase cascade. The interactions between these pro- and anti-apoptotic molecules seem to be the determining factor for cell survival. Not surprisingly, most death modulators function by acting through Bcl-2 family proteins to regulate cyt c release.
APO2L/TRAIL, A PROMISING TUMOR SPECIFIC LIGAND FOR TREATMENT OF MM
Apo2L/TRAIL (TNFSF10) was originally cloned by virtue of its sequence homology to Fas Ligand (FasL, TNFSF6) and the TNF superfamily [13,14]. Various studies show that Apo2L potently induces apoptosis in a broad range of cancer cell lines, but not in many normal cells [15–17]. This apparent protection of normal cells from the cytotoxic effect of Apo2L is believed to be based on a unique set of decoy receptors (DcR); these cells either have DcR1 (TNFRSF 10C) and/or DcR2 (TNFRSF 10D), which have a truncated cytoplasmic death domain, rendering them unable to signal and competing instead for receptor binding to Apo2L [5].
Exogenous Apo2L induces apoptosis in cell lines derived from many cancers, including leukemia and MM. Our recent study, examining the apoptotic pathways in MM following treatment with IFNs, found that Type I but not II IFNs induce typical apoptosis through activation of Apo2L in U266 (an IL-6 dependent MM cell line resistant to IR and Fas), NCI-H929, but not IM-9 [4]. IFNs may impact on the expression of a number of genes associated with apoptosis but also with cell cycle progression [18]. In the MM cell lines PCM6, NOP-2, U266 and RPMI8226, IFN-α-induced apoptosis was further enhanced in the presence of IL-6, via activation of caspase 3 [19]. When treated with IFN α and β, U266 cells showed multiple characteristics of apoptosis, blocked by the pancaspase inhibitor z-VAD-fmk or Bcl-2 expression [4]. Apo2L mRNA was induced dramatically by IFN-β, with no significant changes in expression levels of the Apo2L receptors DR4 and DR5 (TNFRSF10A-B), or of Caspase-8 and Fas (TNFRSF6). We have shown that the promoter region of Apo2L contains 2 IFN-stimulated regulatory elements (ISREs) and IFNs can significantly induce the promoter activity of this gene [20]. Apo2L protein was induced as early as 6 h following treatment with IFN-α and β, with a more pronounced expression observed at later times. There was only a slight change in Apo2L levels following IFN-γ treatment early on, with no additional change at a later time. The induction of Apo2L is of functional significance since U266 cells were sensitive to Apo2L, but not Fas agonistic antibodies. Moreover, a dominant negative Apo2L receptor, DR5Δ, was able to substantially block IFN and Apo2L-induced molecular and cellular changes associated with apoptosis. A cross-talk between these two pathways has been proposed [21].
Apoptosis was also induced by IFN-α and β in plasma cells freshly isolated from the bone marrow of 10 MM patients. Only patient samples containing malignant (CD38+/CD45−/dim) plasma cells, but not the non-plasma lymphocytes used as control, underwent apoptosis upon treatment with IFNs. This was further confirmed by triple staining for CD38, CD45 and Annexin V. Almost all CD38+/CD45−/dim cells became Annexin V positive when treated with IFNs and Apo2L. In contrast, the majority of non-plasma cells were insensitive to Apo2L and IFNs, as also indicated by other reports [22,23]. Apo2L induces apoptosis in plasma cells derived from many MM patients, one of which had a persistent tumor following extensive chemotherapy (13 cycles), suggesting that Apo2L may be useful for treating resistant or relapsing myeloma. Apo2L, therefore, seems effective at inducing apoptosis in MM patient samples regardless of their resistance or sensitivity to chemotherapeutic drugs [4,23], or prior exposure to chemotherapy [4,24]. In mouse models, Apo2L has been shown to provide remarkable efficacy against tumor xenografts of many cancers, including MM. Moreover, combinations of Apo2L and certain DNA damaging drugs or radiotherapy [8] may exert synergistic anti-tumor xenograft activity [23].
Some discrepancy between the effectiveness of Apo2L/TRAIL in published reports is largely based on the use of various recombinant forms: an amino-terminal polyhistidine [13], Gal-4 leucine zipper (LZ [16], and Flag-epitope tagged, with the tags allowing trimerization or crosslinking to enhance activity against certain cell lines [4,8]. Currently, the variant preferred for clinical application contains amino acids 114–281 of human Apo2L/TRAIL without any added exogenous sequences [17] and is, therefore, the least likely to be immunogenic in human patients and it has been optimized for generation of a trimeric molecule [15,25]. All normal human cell types tested are refractory to the non-tagged, optimized version of Apo2L/TRAIL [25]. Some normal cell types are resistant to this optimized ligand version, while being quite sensitive to apoptosis-induction by the non-optimized or antibody-crosslinked variants.
In addition to being tumor specific, Apo2L-based therapy is also attractive since it is not dependent on p53 and, therefore, it should be effective in p53-deficient tumors. Mutations in the p53 tumor suppressor gene occur with a frequency of 12.5% in lymphoid malignancies. Interestingly, an additive apoptosis effect of Apo2L/TRAIL and Adeno-p53 was observed in the induction of apoptosis in MM cell lines expressing nonfunctional p53 and low levels of bcl-2 [26]. Those MM cells resistant to Ad-p53 had high levels of the DR4, DR5, and DcR1 receptors, with the Ad-p53 treatment having no effect on the expression of DcR1.
Apo2L potently induces apoptosis of MM cells from patients and the majority of MM cell lines, including cells sensitive or resistant to dexamethasone (Dex), doxorubicin (Dox), melphalan and mitoxantrone. Apo2L also overcomes the survival effect of IL-6, a multifunctional cytokine affecting growth and survival of normal B cell lineage and MM cells. The status of the Apo2L receptors could not predict Apo2L sensitivity of MM cells. Dox, an agent commonly used to treat MM, up-regulated the expression of the Apo2L receptor DR5 and synergistically enhanced the effect of Apo2L not only against MM cells sensitive to, but also against those resistant to, Dox- or Dex- induced apoptosis. Nuclear factor (NF)-kappaB inhibitors, such as SN50 or the proteasome inhibitor PS-341, currently in Phase II clinical trials, also enhanced the proapoptotic activity of Apo2L against Apo2L-senstive MM cells, whereas SN50 reversed the Apo2L resistance of ARH- 77 and IM-9 MM cells. Importantly, normal B lymphocytes were not sensitized to Apo2L by either Dox, SN50 or PS-341 [23]. Moreover, Apo2L effectively kills MM cells in vitro irrespective of refractoriness to Dex and chemotherapy. Forced expression of procaspase-8 or FLIP antisense oligonucleotides also sensitized Apo2L-resistant cells to Apo2L. Moreover, the cell permeable NF-kappaB inhibitor SN50, which sensitizes Apo2L-resistant cells to Apo2L, also inhibited cIAP2 protein expression. Finally, CHX, BIM and SN50 facilitated the cleavage and activation of procaspase-8 in Apo2L-resistant cells, confirming that inhibition of Apo2L-induced apoptosis occurs at this level and that these agents sensitize MM cells by relieving this block [27].
Apo2L also induces synergistic cytotoxicity and apoptosis in adriamycin-resistant 8226/Dox40 human MM cells when combined with subtoxic concentrations of adriamycin, indicating its potential use in drug-resistant MM [28].
ROLE OF BCL-2 FAMILY PROTEIN EXPRESSION IN MM
We found that IFN-induced apoptosis in MM was dependent on modulation of the Bcl-2 family of proteins in three MM cell lines and in plasma cells isolated from 10 MM patients. Apo2L induction was at least partially necessary for proteolytic cleavage dependent activation of apoptotic activities of Bcl-2 and Bid (Fig. 1). Interestingly, during IR-induced apoptosis of IM-9 cells Bcl-2 and not Bid was the proteolytic target of Caspases, resulting in its conversion in situ from an anti-apoptotic to a pro-apoptotic molecule to promote cyt c release and apoptosis [12]. This effect was specific since IFNs and Apo2L, but not Fas mAb, induced Bid and Bcl-2 cleavage in U266 and patient-derived plasma cells. Bid cleavage products appeared at a time preceding the initial release of cyt c from mitochondria into the cytosol and the activation of Caspase-8. In contrast, Bcl-2 was cleaved at 48 h after IFN treatment. These results indicate that Bcl-2 family proteins are modulated in IFN-induced apoptosis, with Bid and Bcl-2 cleavage corresponding to the early and late stages of cyt c release.
In addition, we observed also that IFN-α induces down-regulation of Bcl-xL, which is expressed at high levels in these cells (Chen and Almasan, unpublished). Other reports indicate that the effect of several therapeutic agents could be mediated by down regulation of Bcl-2 family protein expression (Table I). Expression of these proteins may be regulated also by myeloma survival factors: interleukin-6, INF-α, and insulin-like growth factor 1 [29]. IL-6, a major survival factor for MM tumor cells, induces signaling through the STAT proteins. One STAT family member, Stat3, was reported to be constitutively activated in bone marrow mononuclear cells from patients with MM and in the IL-6-dependent human MM cell line U266. Bcl-xL expression can be inhibited by blocking IL-6 receptor signaling from Janus kinases to the Stat3 protein, demonstrating that Stat3 signaling is essential for the survival of MM tumor cells [30]. INF-α has an IL-6-like effect and potently extends the survival of human MM cells through upregulation of Mcl-1. This regulation is not dependent on an IL-6 autocrine loop but depends on STAT-3 activation [31]. Other inhibitors, whose down-regulation may contribute to apoptosis of MM are the transcription factor NF-kappaB (NF-kappaB) [32,33] the cellular inhibitor of apoptosis protein-2 and FLICE inhibitory protein [27]. Thus NF-kappa B is downregulated following dexa-methaone, thalidomide and proteasome inhibitor (PS-341) treatments (Table I).
TABLE I.
Apoptosis inducers in MM and their mechanism of action
| Apoptosis mechanism | Treatment agent | References |
|---|---|---|
| Intrinsic | Arsenic trioxide | [43–46] |
| Cyt c release | Radiation, IFNs, Apo2L | [4,12,47] |
| Bcl-2 (down) | Hexamethylene bisacetamide, its cleavage | [48] [44] [12] |
| Bcl-2 (up) | doxorubicin, etoposide, and hydrogen peroxide | [49] |
| Bcl-XL, Mcl-1 | IL6 and Stat-3 mediated down-regulation | [50–55] |
| Caspase (active) | Radiation, Act D, anti-sense | [9,12,20,27,55–60] |
| Extrinsic | Fas in MM patients, immune privilege, CD40 | [61–67] |
| Apo2L (Up) | Interferon, radiation | [4,8,20] |
| DR4, DR5 (up) | Doxorubicin, p53 | [26] |
| Bid (active) | Apo2L, IFNs | [4,8] |
| IAPs. P13 K, NFKb | Thalidomide, dexamethasone, inhibitors (SN50) | [32,33,66] |
Bcl-2, frequently expressed in follicular lymphomas bearing the t(14;18) chromosomal translocation, it is also widely expressed in many other B- and T-cell lymphomas without bcl-2 rearrangement. Many lymphoid and myeloid cell lines also express bcl-2 protein with no correlation being shown with differentiation stage [34]. Bcl-2 was shown to be expressed in all eight human MM cell lines examined and in normal lymph node and bone marrow plasma cells, even though DNA rearrangements of the bcl-2 locus were evident in only one of these myeloma cell lines [35]. Bcl-2 overexpression is associated with resistance to Dex and paclitaxel, but not gemcitabine or melphalan, in MM cells [36].
Another study found no correlation between bcl-2 expression and clinico-biological parameters, response to therapy or overall survival in MM patients [37]. Globally, the number of bcl-2 (+) plasma cells and the intensity of protein expression in neoplastic gammopathies were significantly higher than in reactive plasmacytosis and bcl-2 levels tended to increase with disease stage. Bcl-2 was suggested to possibly be relevant to the pathogenesis of malignant gammopathies, prolonging the survival of plasma cells by preventing apoptosis and increasing the chance of acquiring additional gene defects. Bcl-2 expression could also contribute to the resistance to chemotherapy observed in MM disease.
An earlier study has suggested that apoptosis-induced by Apo2L and TNF-α in human MM cells is not blocked by BCL-2 [38]. However, Bcl-2 expression levels in these cells have not been determined. Another study based on antisense strategy has suggested that Mc1-1, rather than Bcl-2 or Bcl-x(L), is an essential survival protein for myeloma cells [39]. Although Bcl-2 ASO treatment alone had no effect, it sensitized MM cell lines to Dex, whereas Bcl-x(L) ASO in combination with Dex still had no effect.
IFNs and most recently, Apo2L, have been suggested to play an important role in immune surveillance [40,41]. One mechanism by which IFNs may achieve their role in immune surveillance could be through regulation of other cytokines, with Apo2L possibly being an important mediator for these effects. These findings support a rationale to explore Apo2L, as well as the therapies that modulate its expression, in the management of MM, as a novel alternative for this currently incurable disease. This might be examined especially where α-interferon may be effective, such as advanced MM. It remains to be determined whether IFNs can induce Apo2L during MM therapy or whether Apo2L can induce MM cell death in vivo. Given the lack of cytotoxicity of Apo2L towards most other types of blood cells, as well as other cell types in mice [16], and non-human primates [15], it would be of interest to examine whether Apo2L could be utilized for treating MM patients. The molecular mechanisms of cell sensitivity to IFNs, Apo2L and Fas, and their application to clinical treatment of MM warrant further investigation. Further insights may be provided by therapeutic targets identified through microarray technologies (Oancea and Almasan, unpublished) [21,42].
Footnotes
Supported in part by research grants from the National Cancer Institute CA81504 and CA82858 (A.A).
References
- 1.Hussein MA, Juturi JV, Lieberman I. “Multiple myeloma: present and future”. Curr Opin Oncol. 2002;14:31–35. doi: 10.1097/00001622-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Puthier D, Pellat-Deceunynck C, Barille S, Robillard N, Rapp MJ, Juge-Morineau N, Harousseau JL, Bataille R, Amiot M. “Differential expression of Bcl-2 in human plasma cell disorders according to proliferation status and malignancy”. Leukemia. 1999;13:289–294. doi: 10.1038/sj.leu.2401302. [DOI] [PubMed] [Google Scholar]
- 3.Wang X. “The expanding role of mitochondria in apoptosis”. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 4.Chen Q, Gong B, Mahmoud-Ahmed A, Zhou A, Hsi ED, Hussein M, Almasan A. “Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon- induced apoptosis in multiple myeloma”. Blood. 2001;98:2183–2192. doi: 10.1182/blood.v98.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashkenazi A. “Targeting death and decoy receptors of the tumour-necrosis factor superfamily”. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 6.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. “Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5”. Immunity. 2000;12:611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 7.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. “Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors”. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 8.Gong B, Almasan A. “Apo2 ligand/TNF-related apoptosis-inducing ligand and death receptor 5 mediate the apoptotic signaling induced by ionizing radiation in leukemic cells”. Cancer Res. 2000;60:5754–5760. [PubMed] [Google Scholar]
- 9.Chen Q, Chai Y-C, Mazumder S, Jiang C, Macklis RM, Chisolm GM, Almasan A. “The late increase in intracellular free radical oxygen species during apoptosis is dependent on cytochrome c release caused by caspase-mediated feedback amplification”. Cell Death Diff. 2002;10(3):1–12. doi: 10.1038/sj.cdd.4401148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cory S, Adams JM. “The bcl2 family: regulators of the cellular life-or-death switch”. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 11.Chinnaiyan A, Orth K, O’Rourke K, Duan H, Poirier G, Dixit V. “Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases”. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Gong B, Almasan A. “Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis”. Cell Death Differ. 2000;7:227–233. doi: 10.1038/sj.cdd.4400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. “Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family”. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 14.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. “Identification and characterization of a new member of the TNF family that induces apoptosis”. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 15.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. “Safety and antitumor activity of recombinant soluble Apo2 ligand”. J Clin Investig. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. “Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo”. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 17.LeBanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Ashkenazi A. “Tumor cell resistance to death receptor induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax”. Nat Med. 2002;8:274–278. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 18.Sangfelt O, Erickson S, Castro J, Heiden T, Einhorn S, Grander D. “Induction of apoptosis and inhibition of cell growth are independent responses to interferon-alpha in hematopoietic cell lines”. Cell Growth Differ. 1997;8:343–352. [PubMed] [Google Scholar]
- 19.Minami R, Muta K, lseung C, Abe Y, Nishimura J, Nawata H. “Interleukin-6 sensitizes multiple myeloma cell lines for apoptosis induced by interferon-alpha”. Exp Hematol. 2000;28:244–255. doi: 10.1016/s0301-472x(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 20.Gong B, Almasan A. “Genomic organization and transcriptional regulation of the human Apo2L/TRAIL gene”. Biochem Biophys Res Commun. 2000;278:747–752. doi: 10.1006/bbrc.2000.3872. [DOI] [PubMed] [Google Scholar]
- 21.Kumar-Sinha C, Varambally S, eekumar A, Chinnaiyan AM. “Molecular cross-talk between the TRAIL and interferon signaling pathways”. J Biol Chem. 2002;277:575–585. doi: 10.1074/jbc.M107795200. [DOI] [PubMed] [Google Scholar]
- 22.Gazitt Y. “TRAIL is a potent inducer of apoptosis in myeloma cells derived from multiple myeloma patients and is not cytotoxic to hematopoietic stem cells”. Leukemia. 1999;13:1817–1824. doi: 10.1038/sj.leu.2401501. [DOI] [PubMed] [Google Scholar]
- 23.Mitsiades CS, Treon SP, Mitsiades N, Shima Y, Richardson P, Schlossman R, Hideshima T, Anderson KC. “TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications”. Blood. 2001;98:795–804. doi: 10.1182/blood.v98.3.795. [DOI] [PubMed] [Google Scholar]
- 24.Lincz LF, Yeh TX, Spencer A. “TRAIL-induced eradication of primary tumour cells from multiple myeloma patient bone marrows is not related to TRAIL receptor expression or prior chemotherapy”. Leukemia. 2001;15:1650–1657. doi: 10.1038/sj.leu.2402251. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D, Ashkenazi A. “Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions”. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, El-Deiry WS, Gazitt Y. “Additive effect of Apo2L/TRAIL and Adeno-p53 in the induction of apoptosis in myeloma cell lines”. Exp Hematol. 2001;29:962–970. doi: 10.1016/s0301-472x(01)00677-4. [DOI] [PubMed] [Google Scholar]
- 27.Mitsiades N, Mitsiades CS, Poulaki V, Anderson KC, Treon SP. “Intracellular regulation of tumor necrosis factor-related apoptosis- inducing ligand-induced apoptosis in human multiple myeloma cells”. Blood. 2002;99:2162–2171. doi: 10.1182/blood.v99.6.2162. [DOI] [PubMed] [Google Scholar]
- 28.Jazirehi AR, Ng CP, Gan XH, Schiller G, Bonavida B. “Adriamycin sensitizes the adriamycin-resistant 8226/Dox40 human multiple myeloma cells to Apo2L/tumor necrosis factor-related apoptosis- inducing ligand-mediated (TRAIL) apoptosis”. Clin Cancer Res. 2001;7:3874–3883. [PubMed] [Google Scholar]
- 29.Jourdan M, De Vos J, Mechti N, Klein B. “Regulation of Bcl-2-family proteins in myeloma cells by three myeloma survival factors: interleukin-6, interferon-alpha and insulin-like growth factor 1”. Cell Death Differ. 2000;7:1244–1252. doi: 10.1038/sj.cdd.4400758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, Dalton WS, Jove R. “Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells”. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 31.Puthier D, Thabard W, Rapp M, Etrillard M, Harousseau J, Bataille R, Amiot M. “Interferon alpha extends the survival of human myeloma cells through an upregulation of the Mcl-1 anti-apoptotic molecule”. Br J Haematol. 2001;112:358–363. doi: 10.1046/j.1365-2141.2001.02575.x. [DOI] [PubMed] [Google Scholar]
- 32.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP, Anderson KC. “Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications”. Blood. 2002;99:4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 33.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi N, Treon SP, Anderson KC. “Biologic sequelae of nuclear factor-kappaB blockade in multiple myeloma: therapeutic applications”. Blood. 2002;99:4079–4086. doi: 10.1182/blood.v99.11.4079. [DOI] [PubMed] [Google Scholar]
- 34.Akagi T, Kondo E, Yoshino T. “Expression of Bcl-2 protein and Bcl-2 mRNA in normal and neoplastic lymphoid tissues”. Leuk Lymphoma. 1994;13:81–87. doi: 10.3109/10428199409051655. [DOI] [PubMed] [Google Scholar]
- 35.Pettersson M, Jernberg-Wiklund H, Larsson LG, Sundstrom C, Givol I, Tsujimoto Y, Nilsson K. “Expression of the bcl-2 gene in human multiple myeloma cell lines and normal plasma cells”. Blood. 1992;79:495–502. [PubMed] [Google Scholar]
- 36.Gazitt Y, Fey V, Thomas C, Alvarez R. “Bcl-2 overexpression is associated with resistance to dexamethasone, but not melphalan, in multiple myeloma cells”. Int J Oncol. 1998;13:397–405. doi: 10.3892/ijo.13.2.397. [DOI] [PubMed] [Google Scholar]
- 37.Miguel-Garcia A, Orero T, Matutes E, Carbonell F, Miguel-Sosa A, Linares M, Tarin F, Herrera M, Garcia-Talavera J, Carbonell-Ramon F. “bcl-2 expression in plasma cells from neoplastic gammopathies and reactive plasmacytosis: a comparative study”. Haematologica. 1998;83:298–304. [PubMed] [Google Scholar]
- 38.Gazitt Y, Shaughnessy P, Montgomery W. “Apoptosis-induced by TRAIL AND TNF-alpha in human multiple myeloma cells is not blocked by BCL-2”. Cytokine. 1999;11:1010–1019. doi: 10.1006/cyto.1999.0536. [DOI] [PubMed] [Google Scholar]
- 39.Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL, Bataille R, Amiot M. “Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells”. Blood. 2002;100:194–199. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- 40.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. “Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development”. J Exp Med. 2002;195:161–169. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. “Increased susceptibility to tumor initiation and metastasis in TNF- related apoptosis-inducing ligand-deficient mice”. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 42.Chauhan D, Auclair D, Robinson EK, Hideshima T, Li G, Podar K, Gupta D, Richardson P, Schlossman RL, Krett N, Chen LB, Munshi NC, Anderson KC. “Identification of genes regulated by dexamethasone in multiple myeloma cells using oligonucleotide arrays”. Oncogene. 2002;21:1346–1358. doi: 10.1038/sj.onc.1205205. [DOI] [PubMed] [Google Scholar]
- 43.Ishitsuka K, Hanada S, Uozumi K, Utsunomiya A, Arima T. “Arsenic trioxide and the growth of human t-cell leukemia virus type i infected t-cell lines”. Leuk Lymphoma. 2000;37:649–655. doi: 10.3109/10428190009058521. [DOI] [PubMed] [Google Scholar]
- 44.Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, Kim BK, Lee YY. “Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis”. Cancer Res. 2000;60:3065–3071. [PubMed] [Google Scholar]
- 45.Hussein MA. “Arsenic trioxide: a new immunomodulatory agent in the management of multiple myeloma”. Med Oncol. 2001;18:239–242. doi: 10.1385/MO:18:4:239. [DOI] [PubMed] [Google Scholar]
- 46.Alemany M, Levin J. “The effects of arsenic-trioxide (As2O3) on human megakaryocytic leukemia cell lines. With a comparison of its effects on other cell lineages”. Leuk Lymphoma. 2000;38:153–163. doi: 10.3109/10428190009060329. [DOI] [PubMed] [Google Scholar]
- 47.Chauhan D, Pandey P, Ogata A, Teoh G, Krett N, Halgren R, Rosen S, Kufe D, Kharbanda S, Anderson K. “Cytochrome c-dependent and -independent induction of apoptosis in multiple myeloma cells”. J Biol Chem. 1997;272:29995–29997. doi: 10.1074/jbc.272.48.29995. [DOI] [PubMed] [Google Scholar]
- 48.Siegel DS, Zhang X, Feinman R, Teitz T, Zelenetz A, Richon VM, Rifkind RA, Marks PA, Michaeli J. “Hexamethylene bisacetamide induces programmed cell death (apoptosis) and down-regulates BCL-2 expression in human myeloma cells”. Proc Natl Acad Sci USA. 1998;95:162–166. doi: 10.1073/pnas.95.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu Y, Xu FH, Liu J, Vescio R, Berenson J, Fady C, Lichtenstein A. “Upregulated expression of BCL-2 in multiple myeloma cells induced by exposure to doxorubicin, etoposide, and hydrogen peroxide”. Blood. 1996;88:1805–1812. [PubMed] [Google Scholar]
- 50.Kawamura C, Kizaki M, Ikeda Y. “Bone morphogenetic protein (BMP)-2 induces apoptosis in human myeloma cells”. Leuk Lymphoma. 2002;43:635–639. doi: 10.1080/10428190290012182. [DOI] [PubMed] [Google Scholar]
- 51.Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S, Reed JC, Lichtenstein A. “BCL-X expression in multiple myeloma: possible indicator of chemoresistance”. Cancer Res. 1998;58:256–262. [PubMed] [Google Scholar]
- 52.Juturi J, Bukowski RA, Bocock K, Bloom T, Finke J, Hussein MA. “High, intermittent dose of all-trans retinoic acid in combination with alpha-interferon for advanced multiple myeloma”. Haematologica. 2001;86:776–777. [PubMed] [Google Scholar]
- 53.Puthier D, Derenne S, Barille S, Moreau P, Harousseau JL, Bataille R, Amiot M. “Mcl-1 and Bcl-xL are co-regulated by IL-6 in human myeloma cells”. Br J Haematol. 1999;107:392–395. doi: 10.1046/j.1365-2141.1999.01705.x. [DOI] [PubMed] [Google Scholar]
- 54.Schwarze MM, Hawley RG. “Prevention of myeloma cell apoptosis by ectopic bcl-2 expression or interleukin 6-mediated up-regulation of bcl-xL”. Cancer Res. 1995;55:2262–2265. [PubMed] [Google Scholar]
- 55.Zhang B, Gojo I, Fenton RG. “Myeloid cell factor-1 is a critical survival factor for multiple myeloma”. Blood. 2002;99:1885–1893. doi: 10.1182/blood.v99.6.1885. [DOI] [PubMed] [Google Scholar]
- 56.Gong B, Almasan A. “Differential upregulation of p53-responsive genes by genotoxic stress in hematopoietic cells containing wild-type and mutant p53”. Gene Expr. 1999;8:197–206. [PMC free article] [PubMed] [Google Scholar]
- 57.Almasan A. “Cellular commitment to radiation-induced apoptosis”. Radiat Res. 2000;153:347–350. doi: 10.1667/0033-7587(2000)153[0347:cctria]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 58.Mazumder S, Gong B, Almasan A, Cyclin E. “induction by genotoxic stress leads to apoptosis of hematopoietic cells”. Oncogene. 2000;19:2828–2835. doi: 10.1038/sj.onc.1203623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazumder S, Chen Q, Gong B, Drazba JA, Buchsbaum JC, Almasan A. “Proteolyfic cleavage of cyclin E leads to inactivation of associated kinase activity and amplification of apoptosis in hematopoietic cells”. Mol Cell Biol. 2002;22:2398–2409. doi: 10.1128/MCB.22.7.2398-2409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Secchiero P, Gonelli A, Celeghini C, Mirandola P, Guidotti L, Visani G, Capitani S, Zauli G. “Activation of the nitric oxide synthase pathway represents a key component of tumor necrosis factor-related apoptosis-inducing ligand- mediated cytotoxicity on hematologic malignancies”. Blood. 2001;98:2220–2228. doi: 10.1182/blood.v98.7.2220. [DOI] [PubMed] [Google Scholar]
- 61.Kanda Y, Ara C, Chizuka A, Yamamoto R, Hamaki T, Suguro M, Matsuyama T, Takezako N, Miwa A, Tohma J, Shirakawa K, Yatomi T, Nakamura N, Hirai H, Togawa A. “Lack of correlation between clinical characteristics and serum soluble Fas ligand levels in patient with multiple myeloma”. Leuk Lymphoma. 2001;40:351–356. doi: 10.3109/10428190109057934. [DOI] [PubMed] [Google Scholar]
- 62.Tong AW, Seamour B, Chen J, Su D, Ordonez G, Frase L, Netto G, Stone MJ. “CD40 ligand-induced apoptosis is Fas-independent in human multiple myeloma cells”. Leuk Lymphoma. 2000;36:543–558. doi: 10.3109/10428190009148403. [DOI] [PubMed] [Google Scholar]
- 63.Greil R, Egle A, Villunger A. “On the role and significance of Fas (Apo1/CD95) ligand (FasL) expression in immune privileged tissues and cancer cells using multiple myeloma as a model”. Leuk Lymphoma. 1998;31:477–490. doi: 10.3109/10428199809057607. [DOI] [PubMed] [Google Scholar]
- 64.Hata H, Matsuzaki H, Takeya M, Takatsuki K. “Fas/Apo-1 (CD95)-mediated and CD95-independent apoptosis of malignant plasma cells”. Leuk Lymphoma. 1996;24:35–42. doi: 10.3109/10428199609045712. [DOI] [PubMed] [Google Scholar]
- 65.Shima Y, Nishimoto N, Yoshizaki K, Kishimoto T. “Fas antigen/APO-1 (CD95) expression on myeloma cells”. Leuk Lymphoma. 1996;23:521–531. doi: 10.3109/10428199609054860. [DOI] [PubMed] [Google Scholar]
- 66.Shiao RT, Miglietta L, Khera SY, Wolfson A, Freter CE. “Dexamethasone and suramin inhibit cell proliferation and interleukin-6- mediated immunoglobulin secretion in human lymphoid and multiple myeloma cell lines”. Leuk Lymphoma. 1995;17:485–494. doi: 10.3109/10428199509056862. [DOI] [PubMed] [Google Scholar]
- 67.Robertson MJ, Manley TJ, Pichert G, Cameron C, Cochran KJ, Levine H, Ritz J. “Functional consequences of APO-1/Fas (CD95) antigen expression by normal and neoplastic hematopoietic cells”. Leuk Lymphoma. 1995;17:51–61. doi: 10.3109/10428199509051703. [DOI] [PubMed] [Google Scholar]


