Abstract
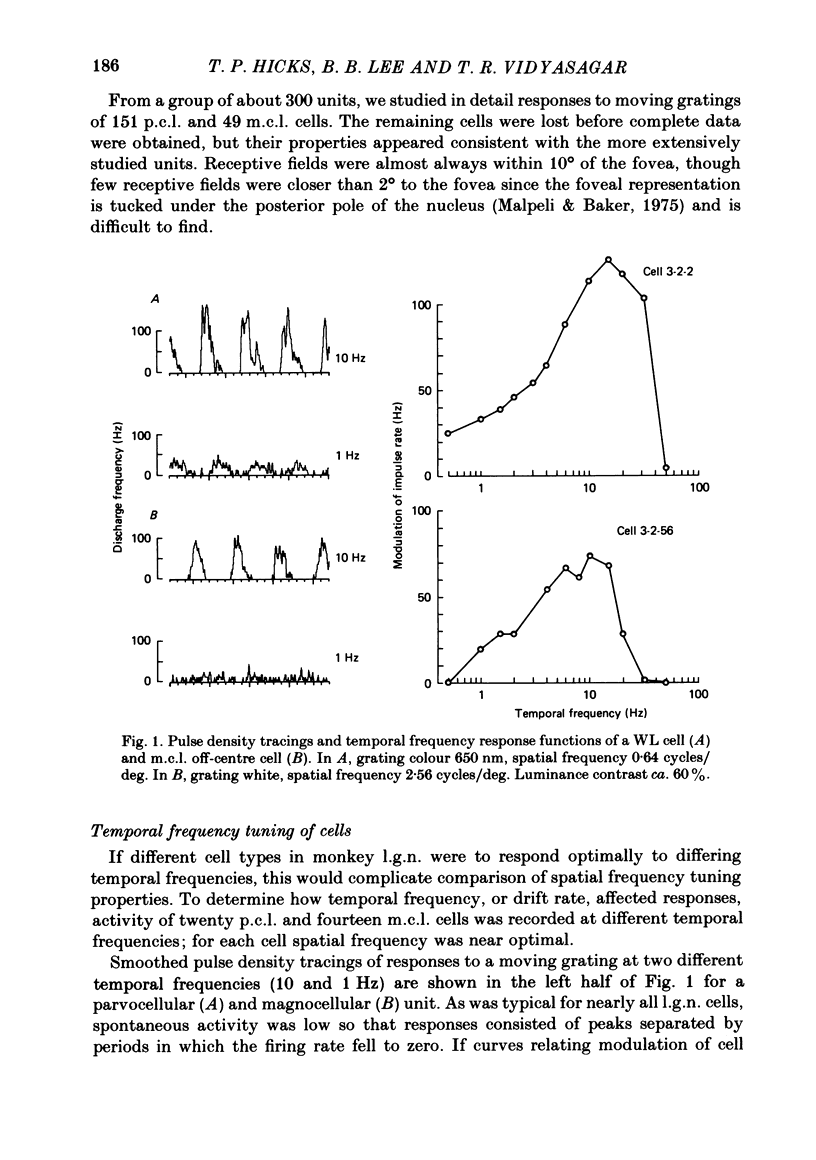
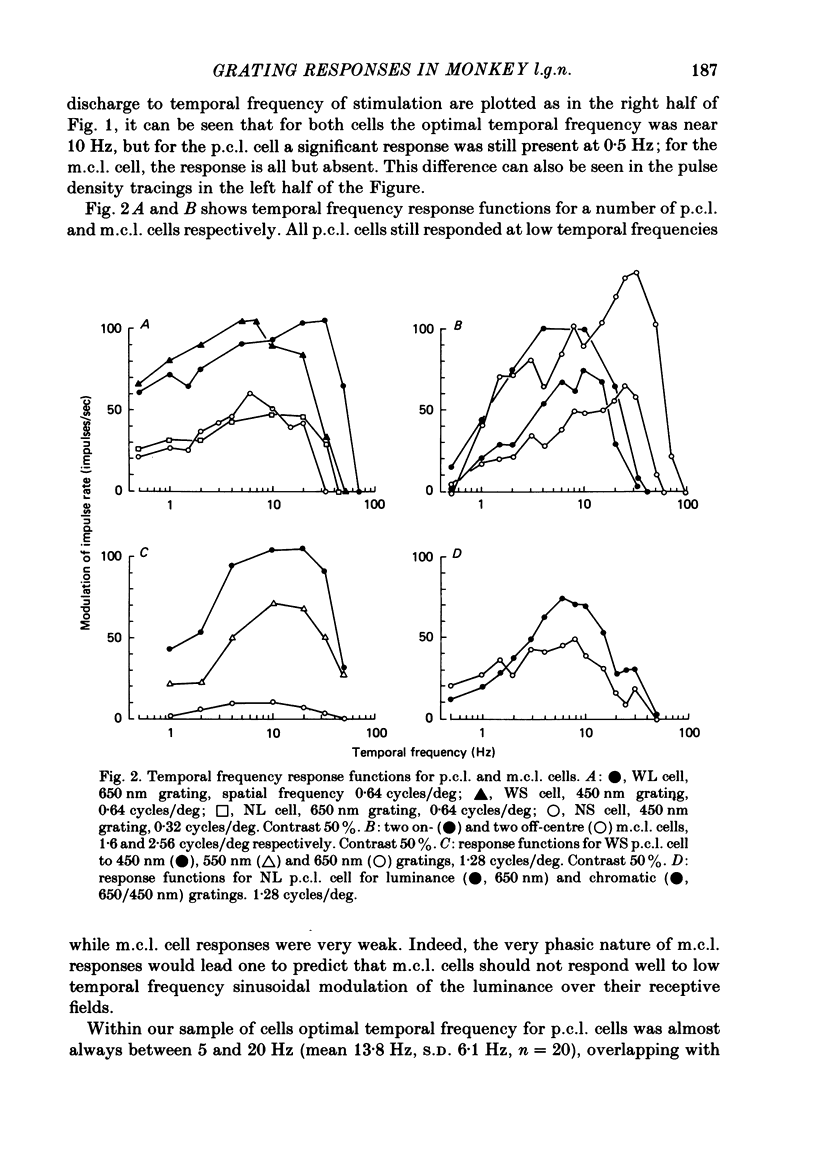
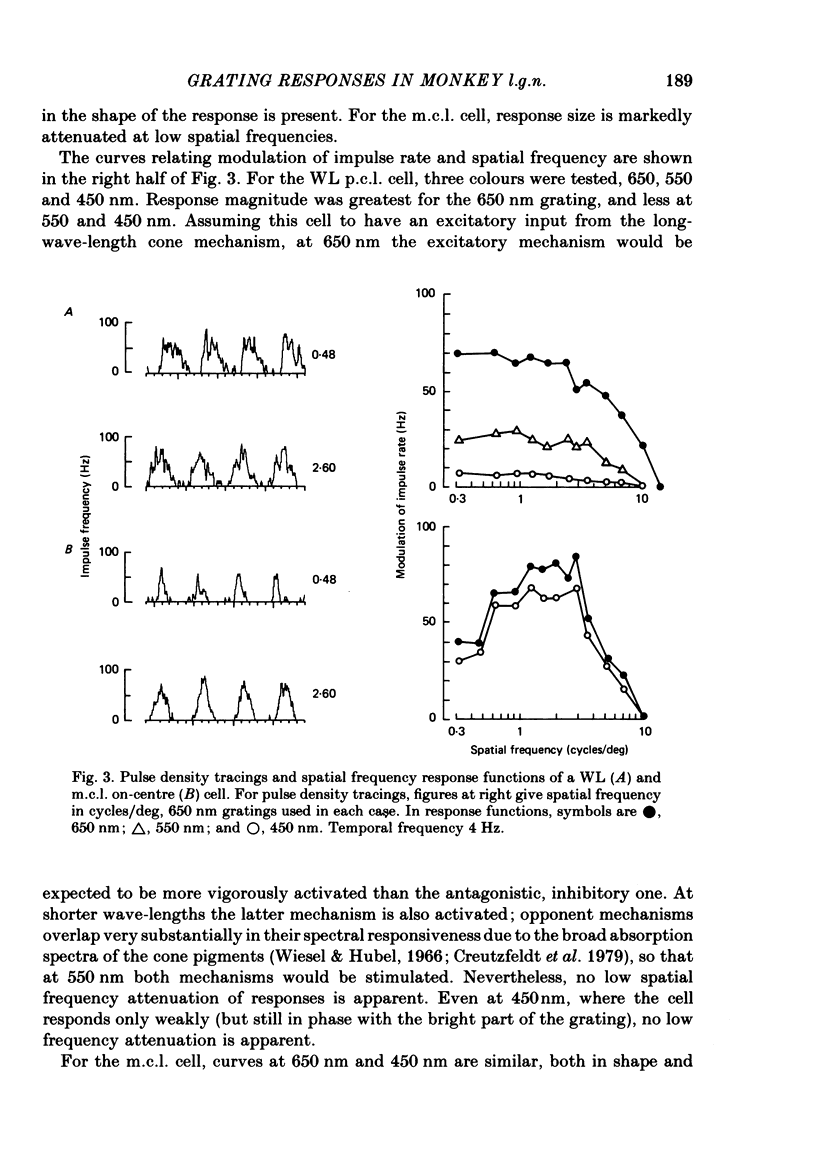
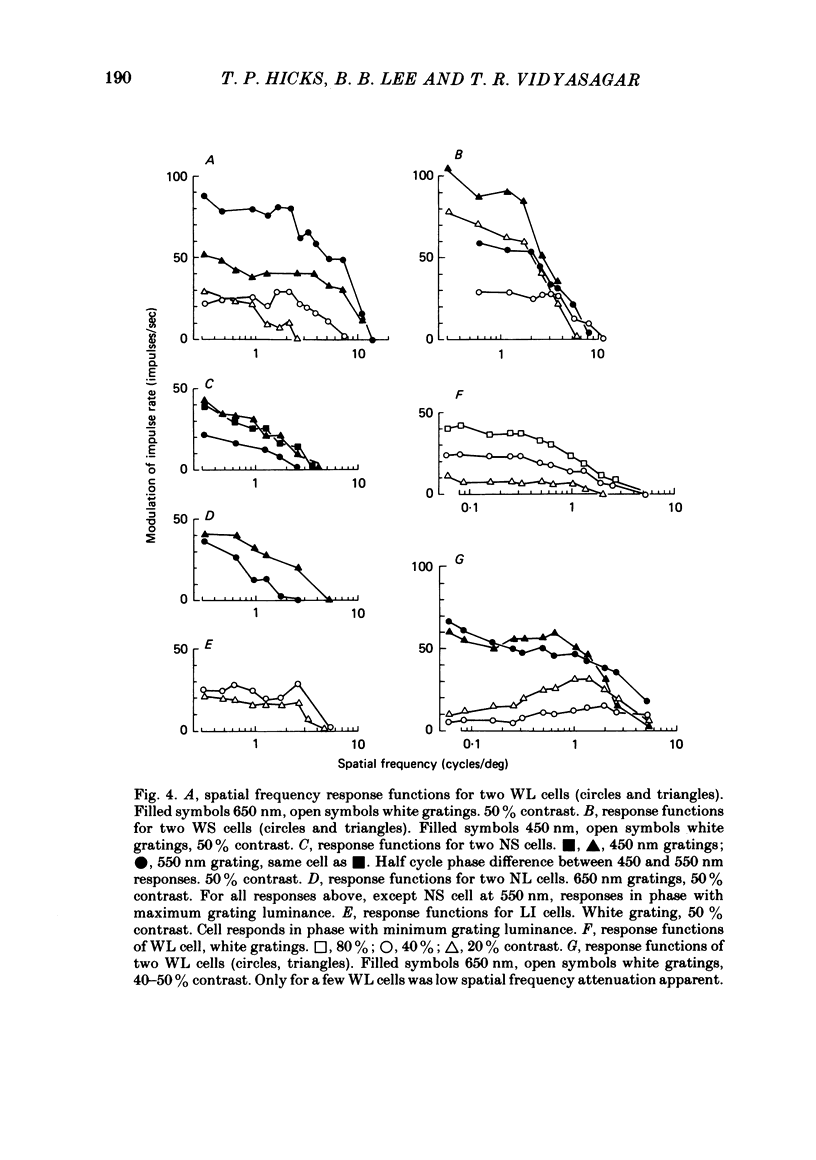
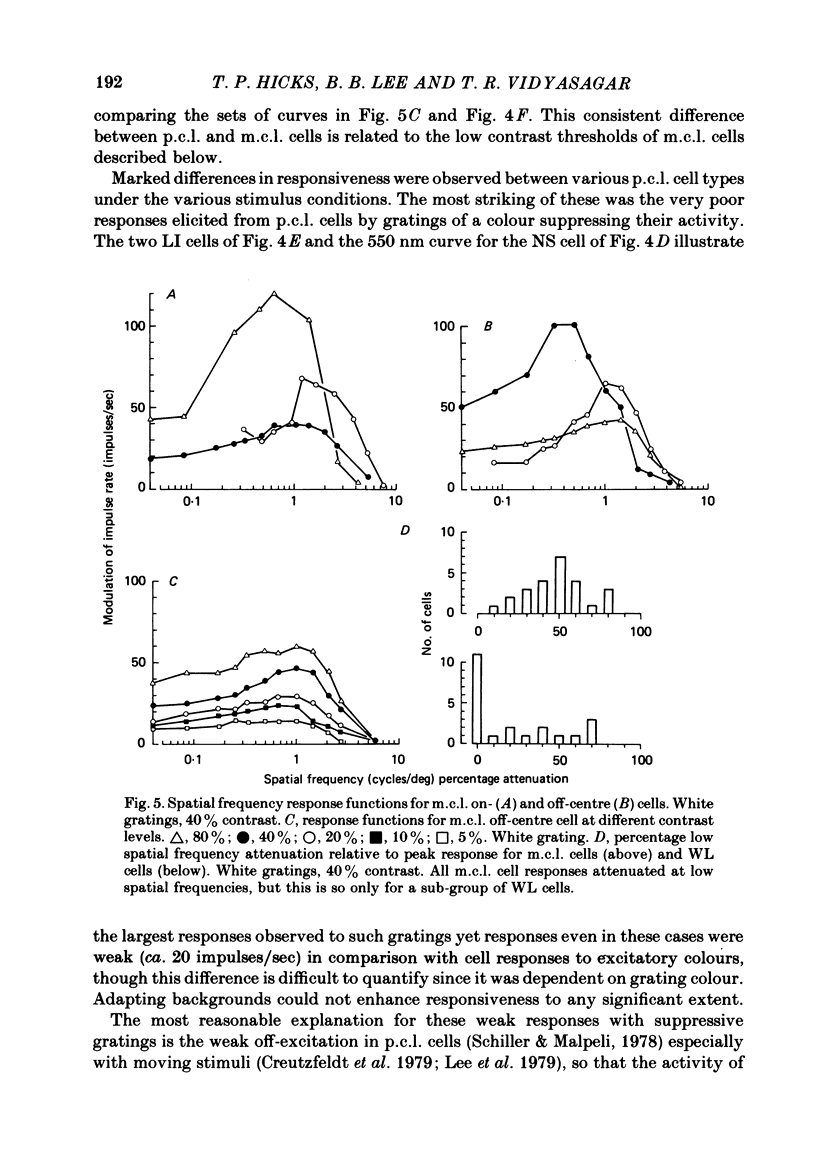
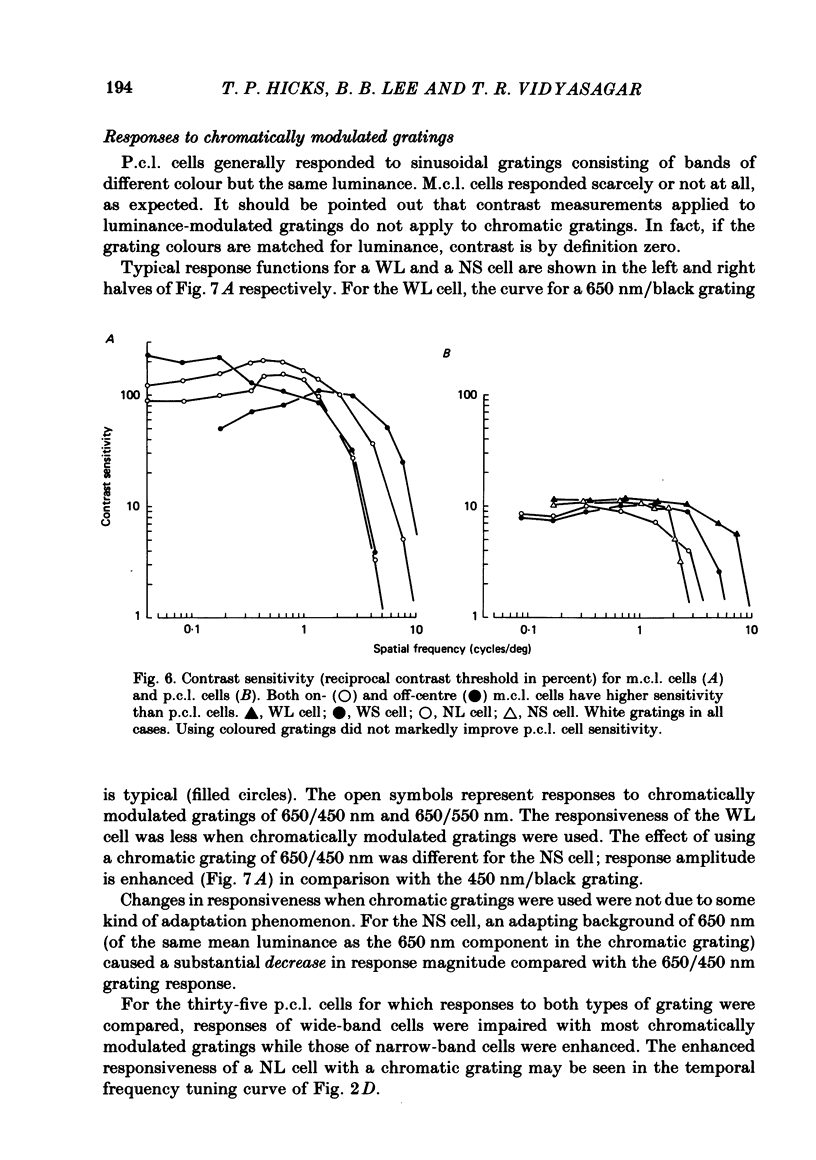
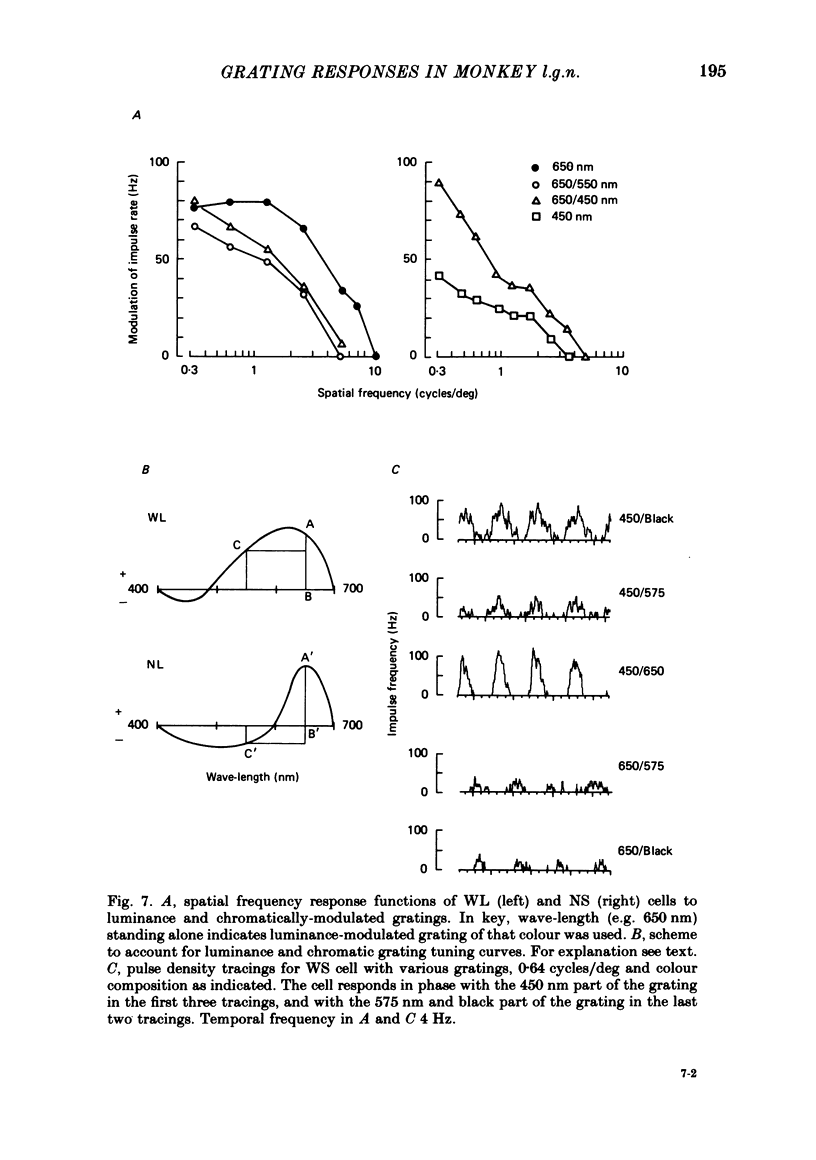
Responses of cells in the parvocellular (p.c.l.) and magnocellular (m.c.l.) layers of the macaque lateral geniculate nucleus to sine-wave gratings were studied. Both p.c.l. and m.c.l. cells responded best at a temporal frequency (drift rate) of 10-20 Hz. P.c.l. cells responded at temporal frequencies lower than 1 Hz; m.c.l. cells did not. With coloured- or white-black luminance-modulated gratings, responses of m.c.l. cells were weaker at low than at medium spatial frequencies. With coloured gratings, p.c.l. cell responses were not attenuated at low spatial frequencies. With white gratings a few p.c.l. cells did show such attenuation. Optimal responses from p.c.l. cells were obtained with coloured gratings; white gratings evoked weaker responses. With a grating of a colour causing suppression of a p.c.l. cell's activity, the modulation of firing was much less than with a grating of a colour excitatory for the cell. M.c.l. on- and off-centre cells responded equally well to moving gratings. The ability of p.c.l. cells to resolve fine gratings was dependent on cell type as well as on the colour of grating used. The ability of m.c.l. cells to resolve fine gratings was comparable to that of p.c.l. cells. The contrast sensitivity of m.c.l. cells was much higher than that of p.c.l. cells. This may account for their ability to resolve fine gratings, despite their larger centre size. In comparison with luminance-modulated gratings, chromatically modulated gratings could evoke larger or smaller responses, depending on p.c.l. cell type and the colours in the grating. M.c.l. cells responded poorly or not at all.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunt A. H., Hendrickson A. E., Lund J. S., Lund R. D., Fuchs A. F. Monkey retinal ganglion cells: morphometric analysis and tracing of axonal projections, with a consideration of the peroxidase technique. J Comp Neurol. 1975 Dec 1;164(3):265–285. doi: 10.1002/cne.901640302. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Robson J. G. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968 Aug;197(3):551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavonius C. R., Schumacher A. W. Human visual acuity measured with colored test objects. Science. 1966 May 27;152(3726):1276–1277. doi: 10.1126/science.152.3726.1276. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Lee B. B., Elepfandt A. A quantitative study of chromatic organisation and receptive fields of cells in the lateral geniculate body of the rhesus monkey. Exp Brain Res. 1979 May 2;35(3):527–545. doi: 10.1007/BF00236770. [DOI] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P., Tolhurst D. J. Concealed colour opponency in ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):217–229. doi: 10.1113/jphysiol.1975.sp011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P., Tolhurst D. J. Trichromatic colour opponency in ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):197–216. doi: 10.1113/jphysiol.1975.sp011087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B., Fukada Y., Rodieck R. W. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976 Jun;258(2):433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P., Zrenner E. Enchancement of luminance flicker by color-opponent mechanisms. Science. 1979 Aug 10;205(4406):587–589. doi: 10.1126/science.109925. [DOI] [PubMed] [Google Scholar]
- Hilz R., Cavonius C. R. Sehschärfe bei Farbunterschieden ohne Helligkeitsunterschiede. Vision Res. 1970 Dec;10(12):1393–1398. doi: 10.1016/0042-6989(70)90090-8. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kelly D. H., van Norren D. Two-band model of heterochromatic flicker. J Opt Soc Am. 1977 Aug;67(8):1081–1091. doi: 10.1364/josa.67.001081. [DOI] [PubMed] [Google Scholar]
- King-Smith P. E., Carden D. Luminance and opponent-color contributions to visual detection and adaptation and to temporal and spatial integration. J Opt Soc Am. 1976 Jul;66(7):709–717. doi: 10.1364/josa.66.000709. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Elepfandt A., Virsu V. Phase of responses to moving sinusoidal gratings in cells of cat retina and lateral geniculate nucleus. J Neurophysiol. 1981 May;45(5):807–817. doi: 10.1152/jn.1981.45.5.807. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Malpeli J. G., Baker F. H. The representation of the visual field in the lateral geniculate nucleus of Macaca mulatta. J Comp Neurol. 1975 Jun 15;161(4):569–594. doi: 10.1002/cne.901610407. [DOI] [PubMed] [Google Scholar]
- Padmos P., Norren D. V. Cone systems interaction in single neurons of the lateral geniculate nucleus of the macaque. Vision Res. 1975 May;15(5):617–619. doi: 10.1016/0042-6989(75)90311-9. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Malpeli J. G. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. J Neurophysiol. 1978 May;41(3):788–797. doi: 10.1152/jn.1978.41.3.788. [DOI] [PubMed] [Google Scholar]
- Shapley R., Kaplan E., Soodak R. Spatial summation and contrast sensitivity of X and Y cells in the lateral geniculate nucleus of the macaque. Nature. 1981 Aug 6;292(5823):543–545. doi: 10.1038/292543a0. [DOI] [PubMed] [Google Scholar]
- Walraven P. L. Color vision. Annu Rev Psychol. 1972;23:347–374. doi: 10.1146/annurev.ps.23.020172.002023. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Properties of concentrically organized X and Y ganglion cells of macaque retina. J Neurophysiol. 1978 Nov;41(6):1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]