Abstract
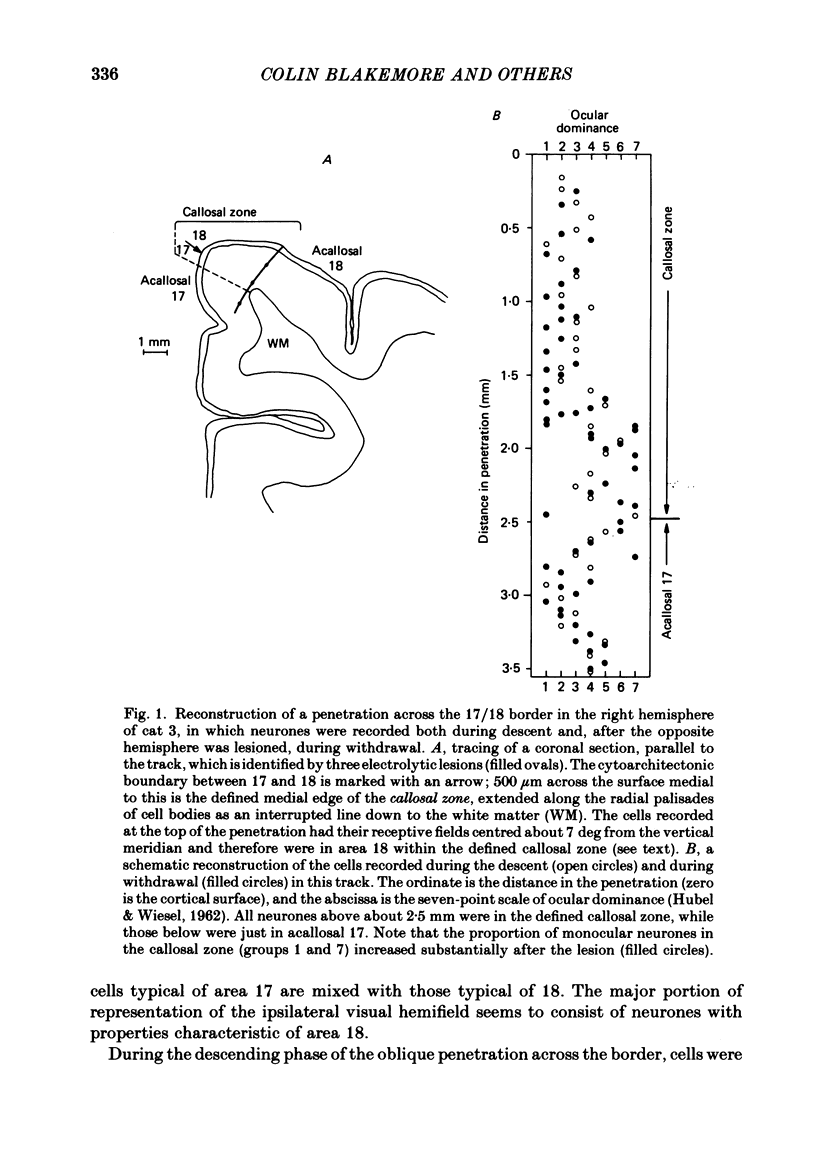
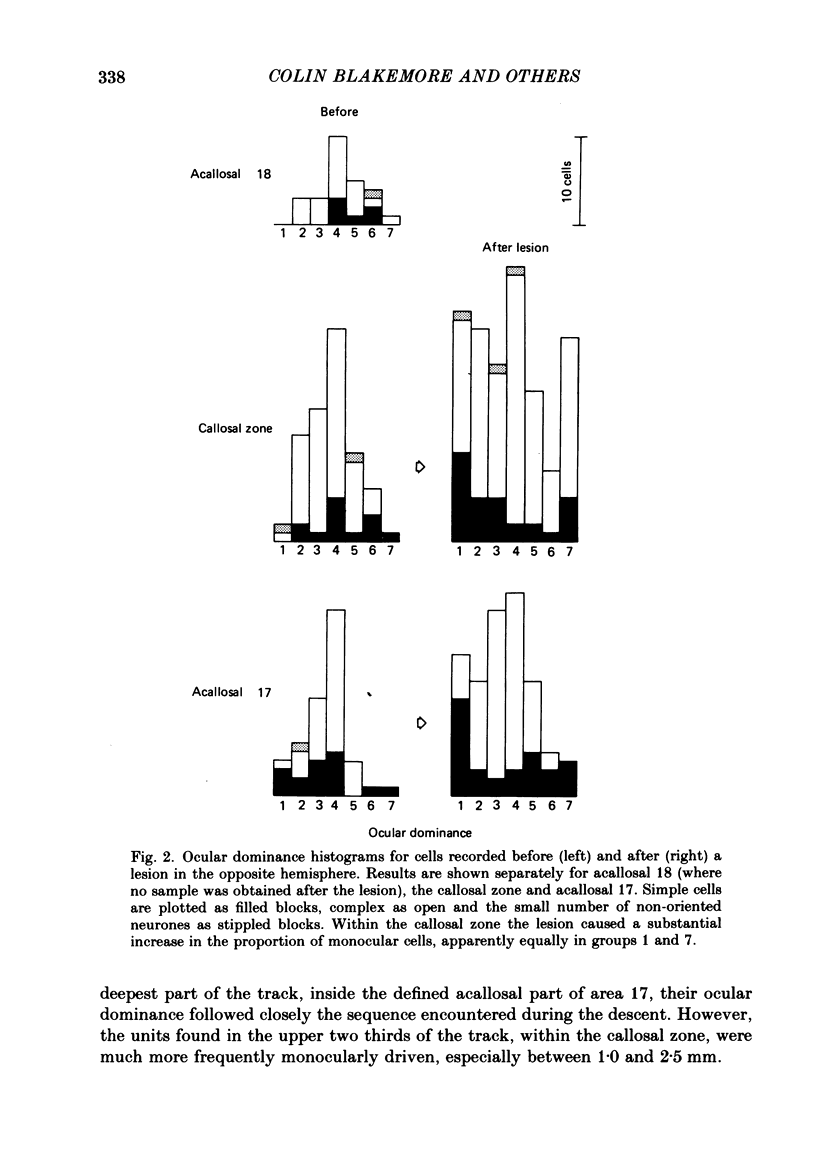
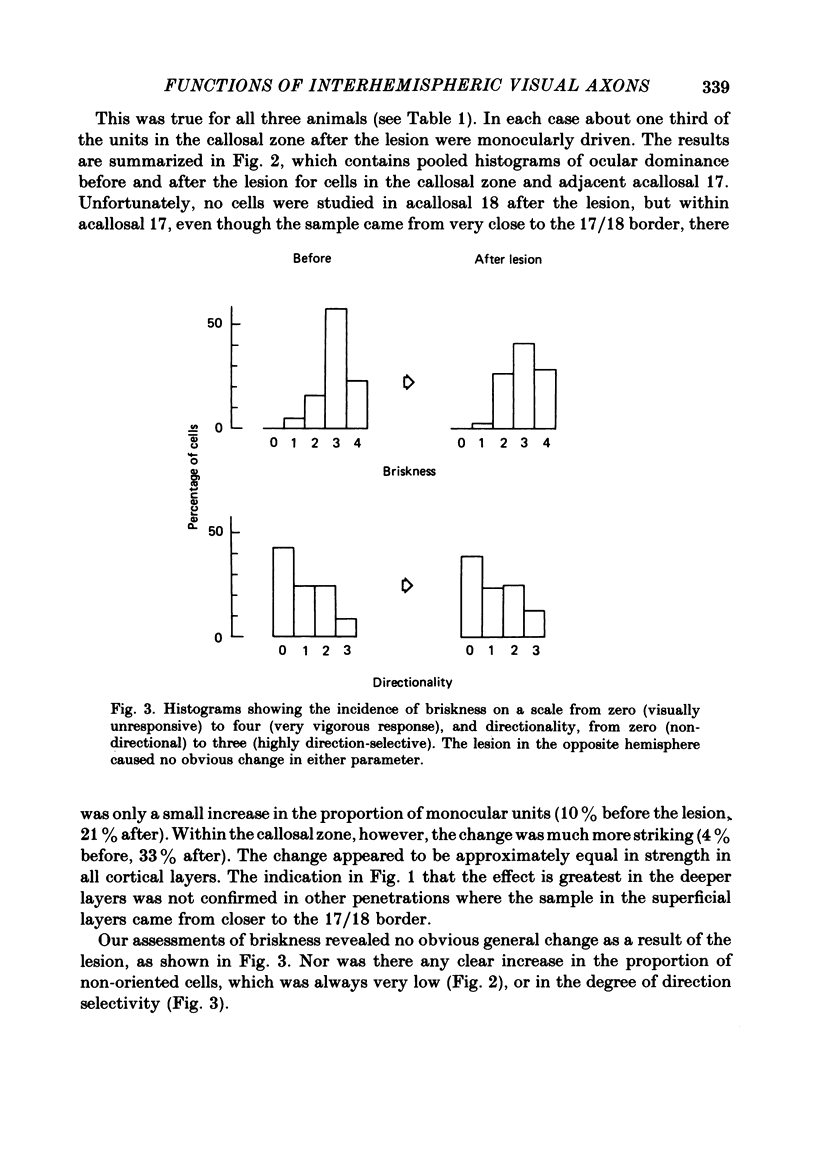
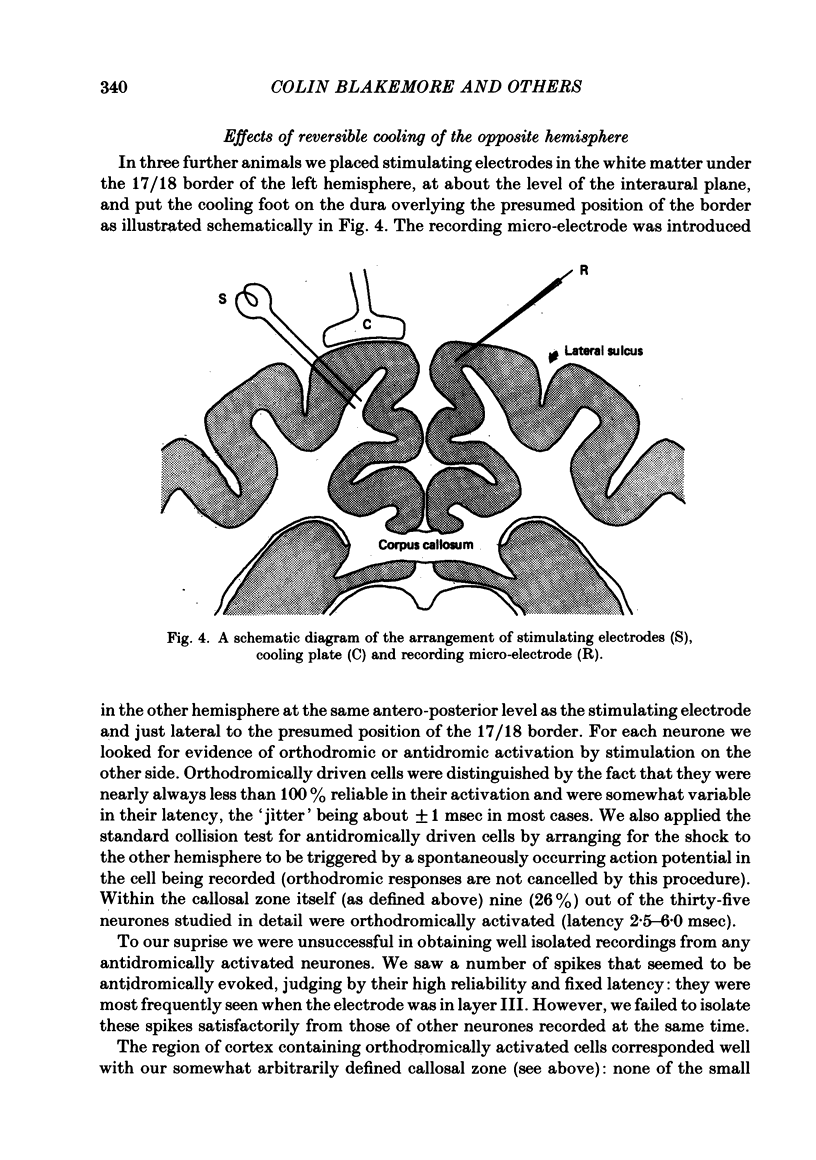
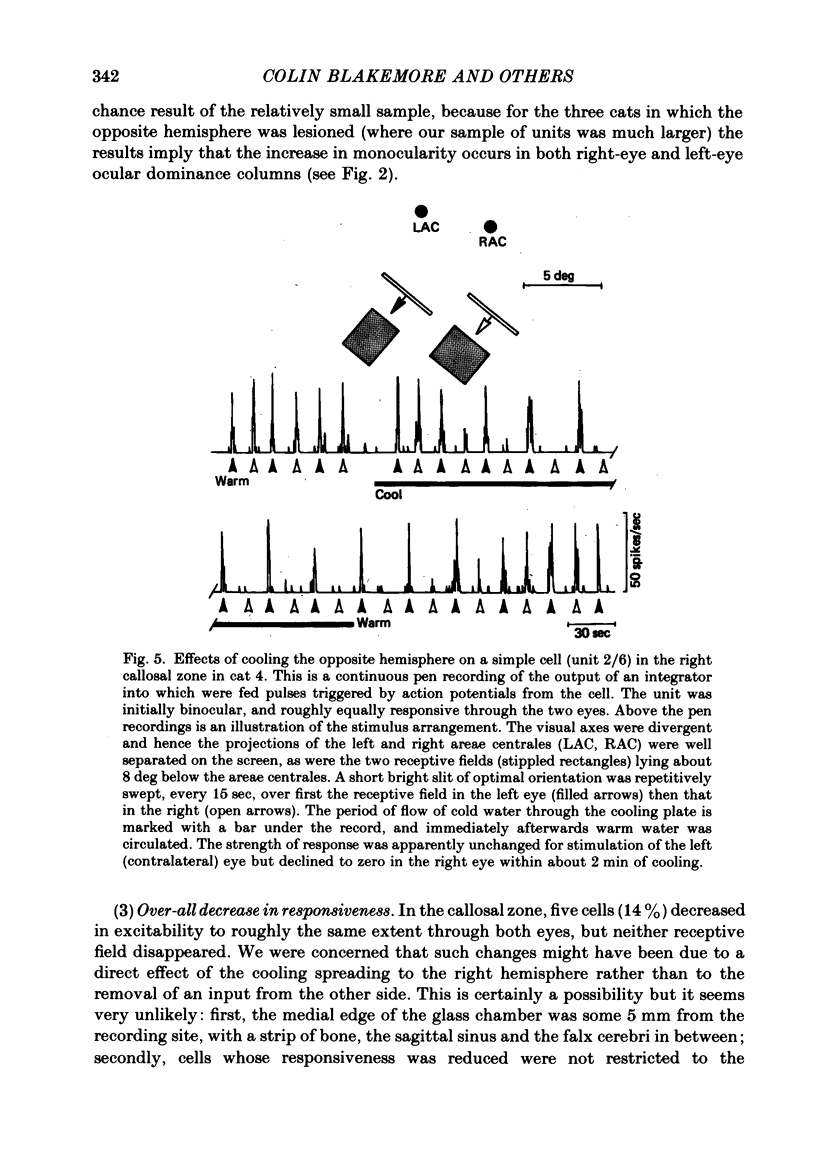
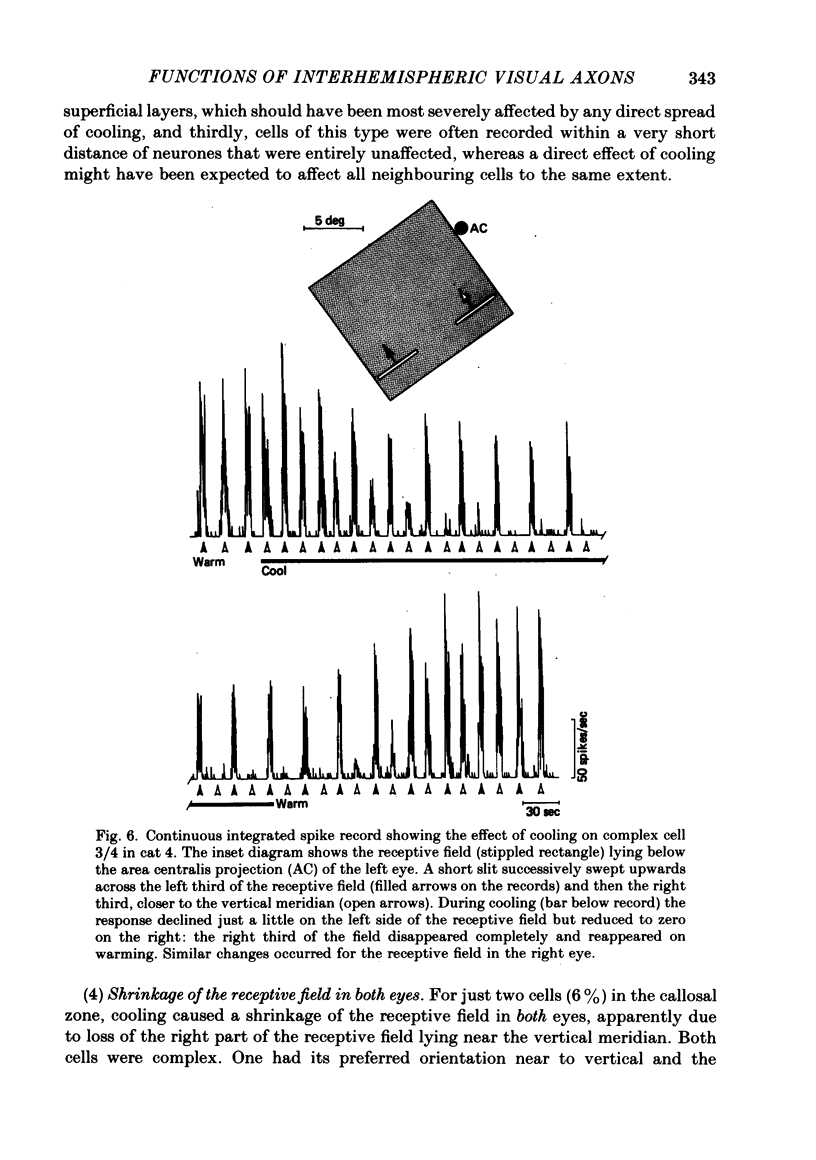
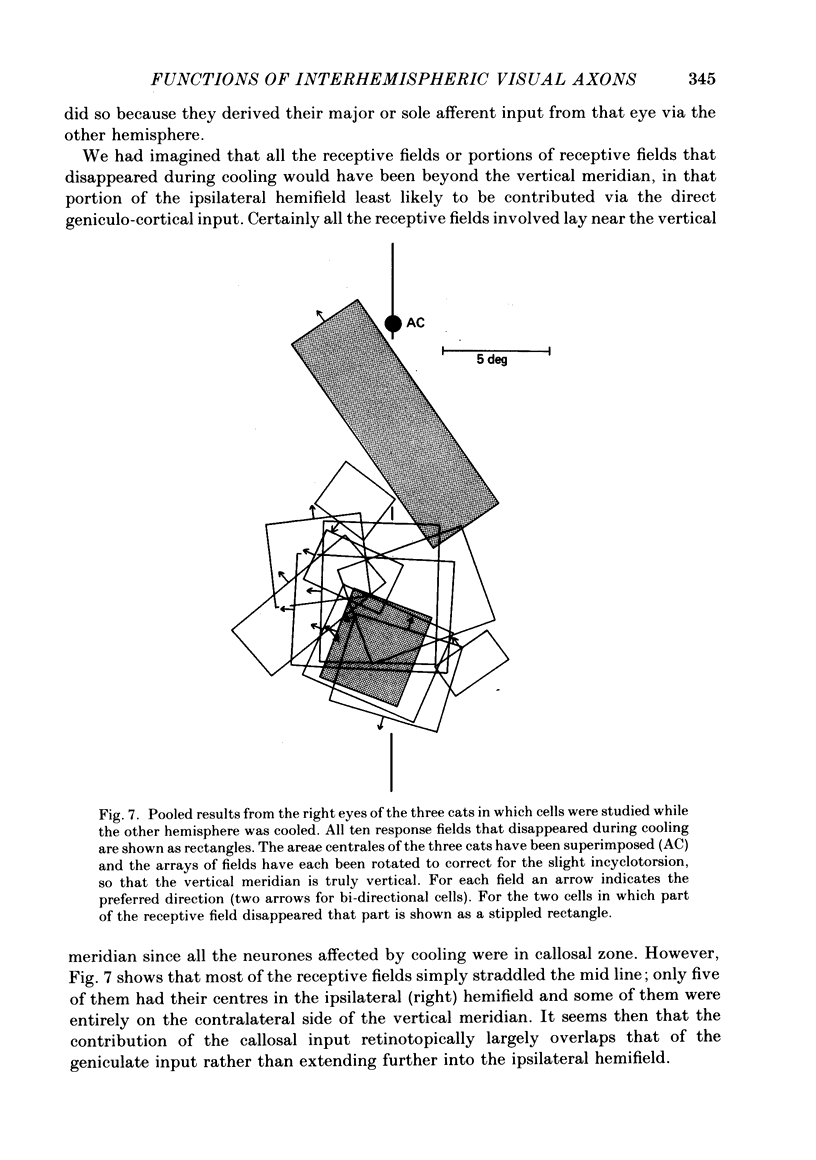
The functions of interhemispheric axons linking the borders between cortical areas 17 and 18 on the two sides of the brain were investigated by two techniques. A well-matched sample of neurones was recorded in the 17/18 border region before and after an extensive lesion was made in the corresponding part of the other hemisphere. The proportion of binocularly driven cells fell from 96% to 67%, confirming the results of Dreher & Cottee (1975). Orientation-and direction-selectivity, as well as the responsiveness of the population of neurones, seemed unaltered. The reduction in binocularity was much less convincing for cells in the body of area 17, even very close to the callosal-recipient zone. Reversible cooling of the 17/18 border had no effect on the few cells recorded outside the callosal zone in the other hemisphere nor on eighteen of the thirty-five cells recorded in the callosal zone. However, in ten cells the receptive field disappeared completely in one eye; in five cells there was a general reduction in responsiveness; two cells lost a portion of the receptive field, on the ipsilateral side, in both eyes. The receptive fields that were apparently transmitted via the corpus callosum lay around the vertical meridian of the visual field and were not restricted to the visual hemifield ipsilateral to the receiving hemisphere: their distribution overlapped that provided by the direct geniculo-cortical input. The principal function of the callosal projection between the 17/18 borders may be to contribute to binocular convergence on cortical cells and perhaps to play a part in stereoscopic vision.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevento L. A., Ebner F. F. The areas and layers of corticocortical terminations in the visual cortex of the Virginia opossum. J Comp Neurol. 1971 Feb;141(2):157–189. doi: 10.1002/cne.901410203. [DOI] [PubMed] [Google Scholar]
- Berlucchi G. Anatomical and physiological aspects of visual functions of corpus callosum. Brain Res. 1972 Feb 25;37(2):371–392. doi: 10.1016/0006-8993(72)90708-1. [DOI] [PubMed] [Google Scholar]
- Berlucchi G., Gazzaniga M. S., Rizzolatti G. Microelectrode analysis of transfer of visual information by the corpus callosum. Arch Ital Biol. 1967 Nov;105(4):583–596. [PubMed] [Google Scholar]
- Berlucchi G., Rizzolatti G. Binocularly driven neurons in visual cortex of split-chiasm cats. Science. 1968 Jan 19;159(3812):308–310. doi: 10.1126/science.159.3812.308. [DOI] [PubMed] [Google Scholar]
- Blakemore C. Binocular depth discrimination and the nasotemporal division. J Physiol. 1969 Nov;205(2):471–497. doi: 10.1113/jphysiol.1969.sp008978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. Binocular depth perception and the optic chiasm. Vision Res. 1970 Jan;10(1):43–47. doi: 10.1016/0042-6989(70)90060-x. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Fiorentini A., Maffei L. A second neural mechanism of binocular depth discrimination. J Physiol. 1972 Nov;226(3):725–749. doi: 10.1113/jphysiol.1972.sp010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Hawken M. J. Rapid restoration of functional input to the visual cortex of the cat after brief monocular deprivation. J Physiol. 1982 Jun;327:463–487. doi: 10.1113/jphysiol.1982.sp014243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOUDHURY B. P., WHITTERIDGE D., WILSON M. E. THE FUNCTION OF THE CALLOSAL CONNECTIONS OF THE VISUAL CORTEX. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:214–219. doi: 10.1113/expphysiol.1965.sp001783. [DOI] [PubMed] [Google Scholar]
- Donaldson I. M., Whitteridge D. The nature of the boundary between cortical visual areas II and III in the cat. Proc R Soc Lond B Biol Sci. 1977 Dec 13;199(1136):445–462. doi: 10.1098/rspb.1977.0153. [DOI] [PubMed] [Google Scholar]
- Dreher B., Cottee L. J. Visual receptive-field properties of cells in area 18 of cat's cerebral cortex before and after acute lesions in area 17. J Neurophysiol. 1975 Jul;38(4):735–750. doi: 10.1152/jn.1975.38.4.735. [DOI] [PubMed] [Google Scholar]
- Ebner F. F., Myers R. E. Distribution of corpus callosum and anterior commissure in cat and raccoon. J Comp Neurol. 1965 Jun;124(3):353–365. doi: 10.1002/cne.901240306. [DOI] [PubMed] [Google Scholar]
- Elberger A. J. Ocular dominance in striate cortex is altered by neonatal section of the posterior corpus callosum in the cat. Exp Brain Res. 1981;41(3-4):280–291. doi: 10.1007/BF00238885. [DOI] [PubMed] [Google Scholar]
- Eldridge J. L. A reversible ophthalmoscope using a corner-cube [proceedings]. J Physiol. 1979 Oct;295:1P–2P. [PubMed] [Google Scholar]
- Garey L. J., Jones E. G., Powell T. P. Interrelationships of striate and extrastriate cortex with the primary relay sites of the visual pathway. J Neurol Neurosurg Psychiatry. 1968 Apr;31(2):135–157. doi: 10.1136/jnnp.31.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Cortical and callosal connections concerned with the vertical meridian of visual fields in the cat. J Neurophysiol. 1967 Nov;30(6):1561–1573. doi: 10.1152/jn.1967.30.6.1561. [DOI] [PubMed] [Google Scholar]
- Hughes A., Wilson M. E. Callosal terminations along the boundary between visual areas I and II in the rabbit. Brain Res. 1969 Jan;12(1):19–25. doi: 10.1016/0006-8993(69)90052-3. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M. The primary visual pathway through the corpus callosum: morphological and functional aspects in the cat. Arch Ital Biol. 1980 May;118(2):124–188. [PubMed] [Google Scholar]
- Kalil R. E., Chase R. Corticofugal influence on activity of lateral geniculate neurons in the cat. J Neurophysiol. 1970 May;33(3):459–474. doi: 10.1152/jn.1970.33.3.459. [DOI] [PubMed] [Google Scholar]
- Leicester J. Projection of the visual vertical meridian to cerebral cortex of the cat. J Neurophysiol. 1968 May;31(3):371–382. doi: 10.1152/jn.1968.31.3.371. [DOI] [PubMed] [Google Scholar]
- Marzi C. A., Antonini A., Di Stefano M., Legg C. R. Callosum-dependent binocular interactions in the lateral suprasylvian area of Siamese cats which lack binocular neurons in areas 17 and 18. Brain Res. 1980 Sep 15;197(1):230–235. doi: 10.1016/0006-8993(80)90450-3. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Blakemore C. Binocular depth perception and the corpus callosum. Vision Res. 1970 Jan;10(1):49–54. doi: 10.1016/0042-6989(70)90061-1. [DOI] [PubMed] [Google Scholar]
- Orban G. A., Kennedy H., Maes H. Functional changes across the 17-18 border in the cat. Exp Brain Res. 1980;39(2):177–186. doi: 10.1007/BF00237548. [DOI] [PubMed] [Google Scholar]
- Payne B. R., Elberger A. J., Berman N., Murphy E. H. Binocularity in the cat visual cortex is reduced by sectioning the corpus callosum. Science. 1980 Mar 7;207(4435):1097–1099. doi: 10.1126/science.7355278. [DOI] [PubMed] [Google Scholar]
- Sanides D., Albus K. The distribution of interhemispheric projections in area 18 of the cat: coincidence with discontinuities of the representation of the visual field in the second visual area (V2). Exp Brain Res. 1980 Jan;38(2):237–240. doi: 10.1007/BF00236745. [DOI] [PubMed] [Google Scholar]
- Sanides D. The retinotopic distribution of visual callosal projections in the suprasylvian visual areas compared to the classical visual areas (17, 18, 19) in the cat. Exp Brain Res. 1978 Nov 15;33(3-4):435–443. doi: 10.1007/BF00235565. [DOI] [PubMed] [Google Scholar]
- Schmielau F., Singer W. The role of visual cortex for binocular interactions in the cat lateral geniculate nucleus. Brain Res. 1977 Jan 21;120(2):354–361. doi: 10.1016/0006-8993(77)90914-3. [DOI] [PubMed] [Google Scholar]
- Shatz C. Abnormal interhemispheric connections in the visual system of Boston Siamese cats: a physiological study. J Comp Neurol. 1977 Jan 15;171(2):229–245. doi: 10.1002/cne.901710207. [DOI] [PubMed] [Google Scholar]
- Sherk H. Area 18 cell responses in cat during reversible inactivation of area 17. J Neurophysiol. 1978 Jan;41(1):204–215. doi: 10.1152/jn.1978.41.1.204. [DOI] [PubMed] [Google Scholar]
- Swadlow H. A., Weyand T. G., Waxman S. G. The cells of origin of the corpus callosum in rabbit visual cortex. Brain Res. 1978 Nov 3;156(1):129–134. doi: 10.1016/0006-8993(78)90088-4. [DOI] [PubMed] [Google Scholar]
- Toyama K., Matsunami K., Ohno T. Antidromic identification of association, commissural and corticofugal efferent cells in cat visual cortex. Brain Res. 1969 Jul;14(2):513–517. doi: 10.1016/0006-8993(69)90127-9. [DOI] [PubMed] [Google Scholar]
- Toyama K., Tokashiki S., Matsunami K. Synaptic action of commissural impulses upon association efferent cells in cat visual cortex. Brain Res. 1969 Jul;14(2):518–520. doi: 10.1016/0006-8993(69)90128-0. [DOI] [PubMed] [Google Scholar]
- Vesbaesya C., Whitteridge D., Wilson M. E. Callosal connexions of the cortex representing the area centralis. J Physiol. 1967 Jul;191(2):79P–80P. [PubMed] [Google Scholar]
- Zeki S. M. Interhemispheric connections of prestriate cortex in monkey. Brain Res. 1970 Apr 1;19(1):63–75. doi: 10.1016/0006-8993(70)90237-4. [DOI] [PubMed] [Google Scholar]
- Zeki S. M., Sandeman D. R. Combined anatomical and electrophysiological studies on the boundary between the second and third visual areas of rhesus monkey cortex. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):555–562. doi: 10.1098/rspb.1976.0094. [DOI] [PubMed] [Google Scholar]
- Zeki S., Fries W. A function of the corpus callosum in the Siamese cat. Proc R Soc Lond B Biol Sci. 1980 Feb 29;207(1167):249–258. doi: 10.1098/rspb.1980.0023. [DOI] [PubMed] [Google Scholar]


