Abstract
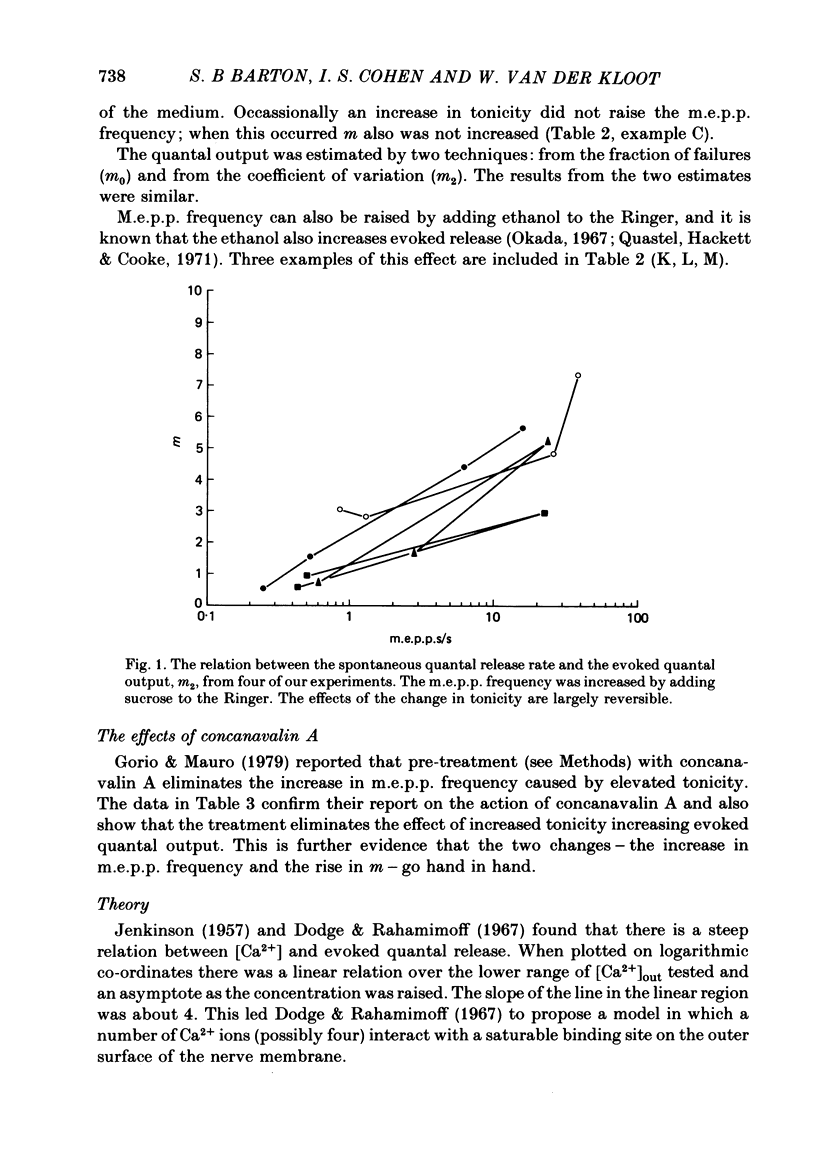
1. The quantal output from stimulated nerve terminals in the frog sciatic nerve—sartorius muscle preparation in low-Ca2+ Ringer solution was measured by the coefficient of variation and the failures methods. Adding sucrose to the Ringer to increase the tonicity or adding ethanol increased miniature end-plate potential (m.e.p.p.) frequency and also the end-plate potential (e.p.p.) amplitude. Earlier reports suggested that increases in tonicity did not increase evoked quantal release.
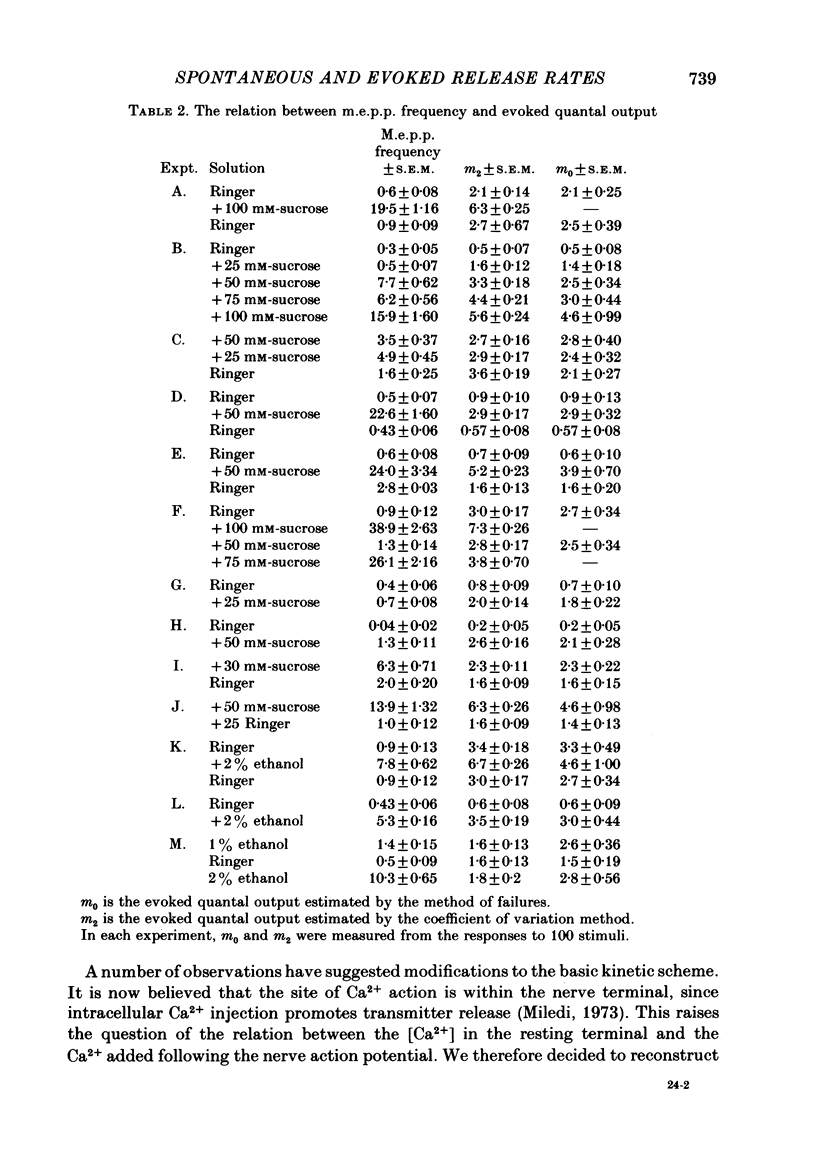
2. Concanavalin A has been reported to block the increase in m.e.p.p. frequency caused by increasing the tonicity of the Ringer (Gorio & Mauro, 1979). This effect was confirmed. The lectin-treated preparations also failed to show an increase in evoked quantal release when the tonicity was increased.
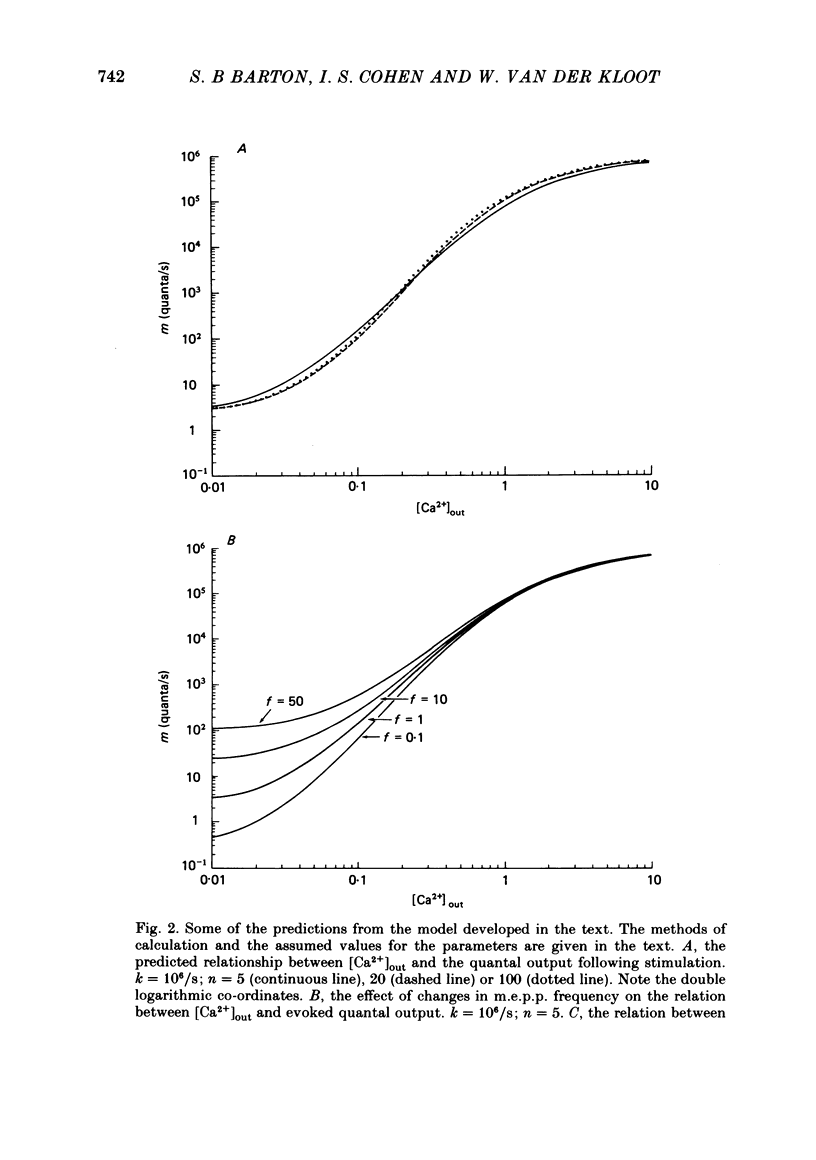
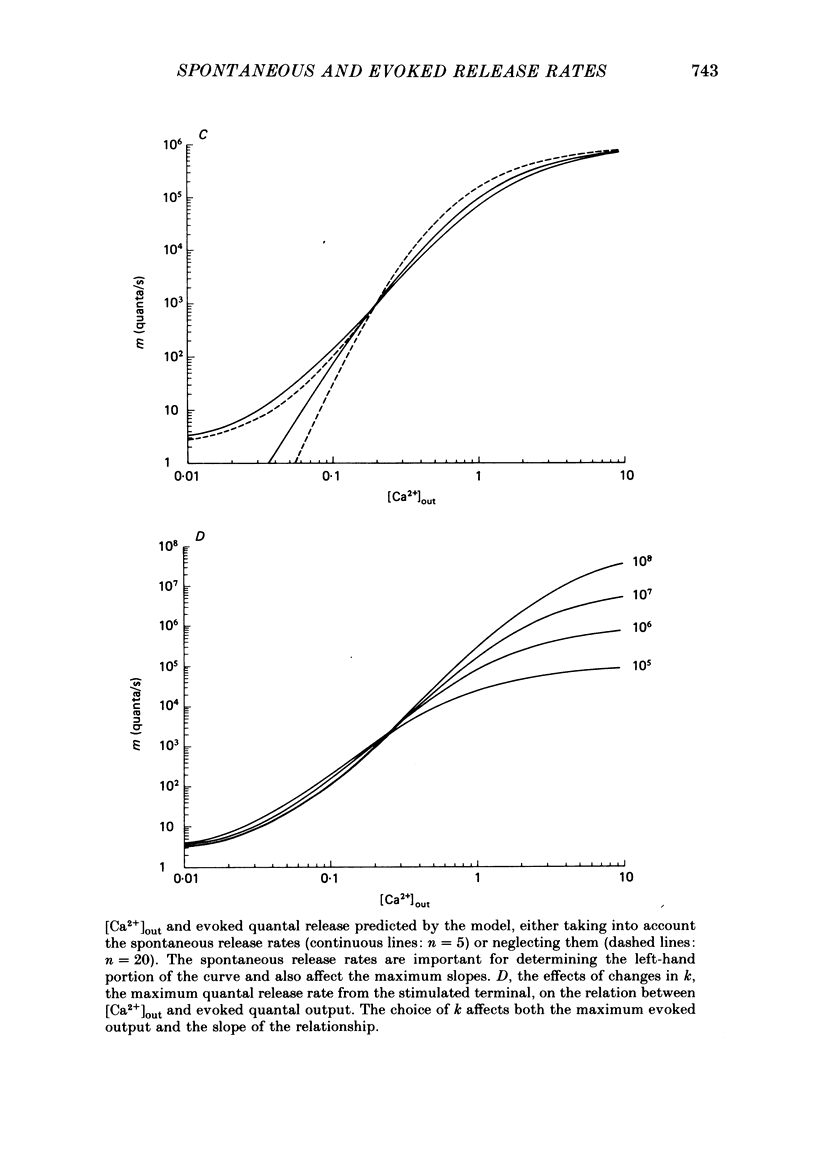
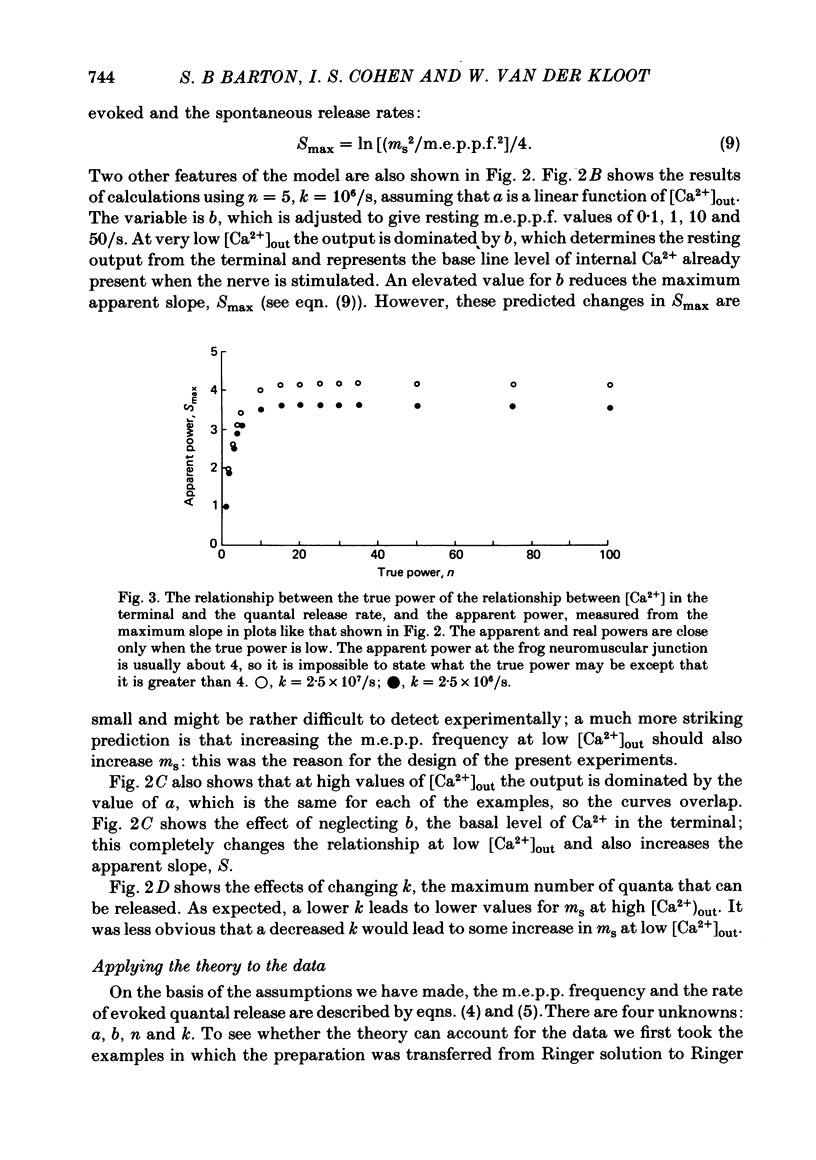
3. A model in which both spontaneous and evoked quantal releases depend on some power of the intracellular [Ca2+] is presented. The model predicts that rises in m.e.p.p. frequency will be accompanied by increased quantal output from stimulated nerve terminals. The maximum slope of the relationship between log (evoked quantal output) and log ([Ca2+]out) will be less than the true power. A theoretical analysis shows that, as the true power approaches infinity, the maximum slope will be slightly above 4. The value for the slope usually found experimentally at the frog neuromuscular junction is also about 4.
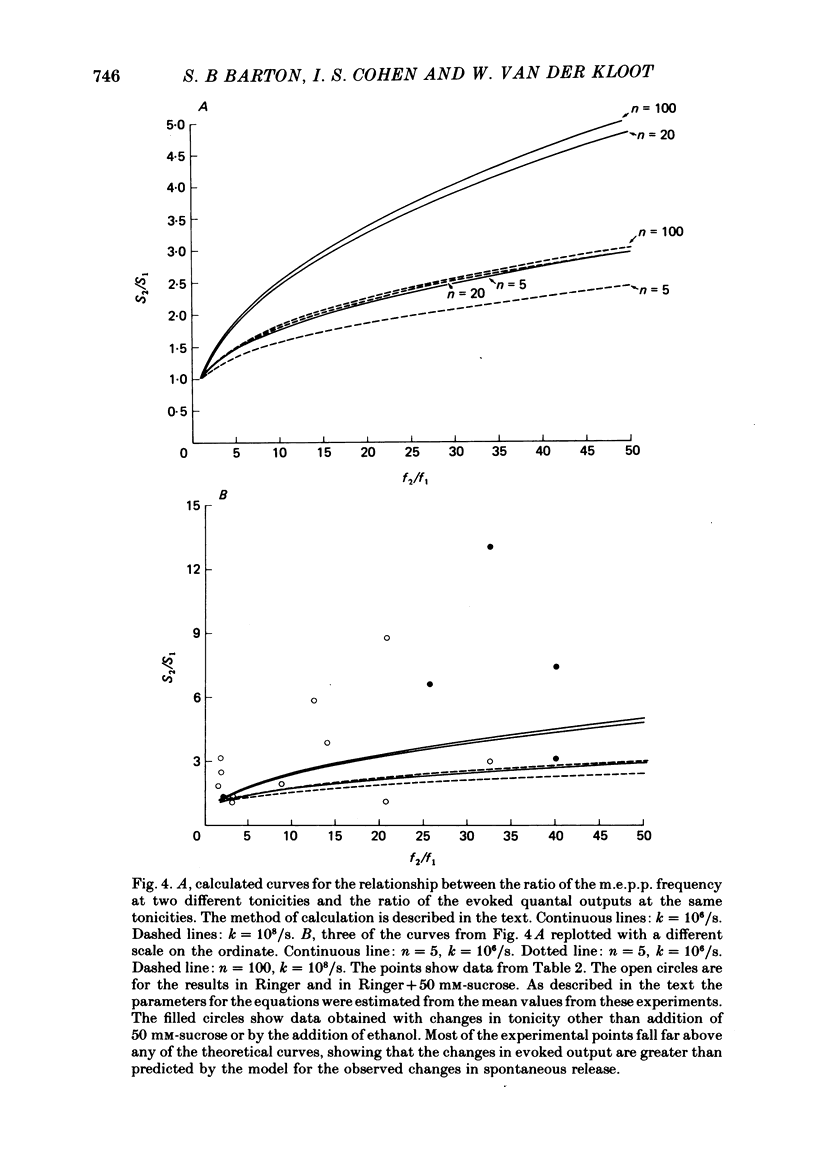
4. The model does not fit the experimental data. The observed increases in evoked quantal release are higher than those predicted for the observed increases in spontaneous release. There are several possible explanations for the discrepancy. Treatments that increase m.e.p.p. frequency may also increase Ca2+ influx into the stimulated terminal. However, we prefer the explanation that there is a fraction of spontaneous release that is independent of the [Ca2+] in the terminal; if this is true the model might account for the data.
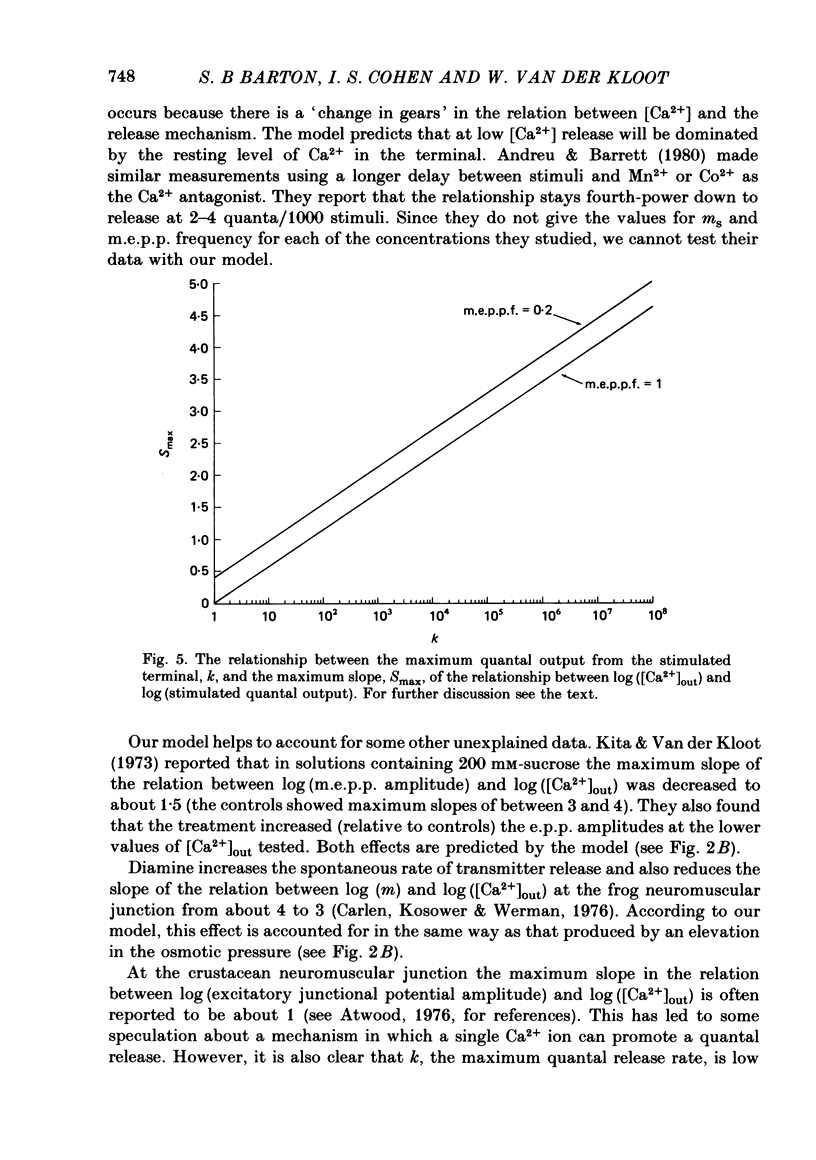
5. The model can account for a variety of puzzling experimental observations, including: (a) the effect of hypertonic solutions and of diamine in decreasing the slope in the relation between log (evoked quantal output) and log ([Ca2+]out); (b) the slope of near 1 observed at the crustacean neuromuscular junction; (c) the decrease in the slope produced by treatment with botulinum toxin.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu R., Barrett E. F. Calcium dependence of evoked transmitter release at very low quantal contents at the frog neuromuscular junction. J Physiol. 1980 Nov;308:79–97. doi: 10.1113/jphysiol.1980.sp013463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood H. L. Organization and synaptic physiology of crustacean neuromuscular systems. Prog Neurobiol. 1976;7(Pt 4):291–391. doi: 10.1016/0301-0082(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Carlen P. L., Kosower E. M., Werman R. Diamide acts intracellularly to enhance transmitter release: the differential permeation of diamide, DIP, DIP+1 and DIP+2 across the nerve terminal membrane. Brain Res. 1976 Nov 26;117(2):277–285. doi: 10.1016/0006-8993(76)90735-6. [DOI] [PubMed] [Google Scholar]
- Crawford A. C. Evoked transmitter release at the frog neuromuscular junction in very low calcium solutions. J Physiol. 1973 May;231(1):47P–48P. [PubMed] [Google Scholar]
- Crawford A. C. Lithium ions and the release of transmitter at the frog neuromuscular junction. J Physiol. 1975 Mar;246(1):109–142. doi: 10.1113/jphysiol.1975.sp010882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C. The dependence of evoked transmitter release on external calcium ions at very low mean quantal contents. J Physiol. 1974 Jul;240(2):255–278. doi: 10.1113/jphysiol.1974.sp010609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Lundh H., Thesleff S. Effects of botulinum toxin on neuromuscular transmission in the rat. J Physiol. 1976 Aug;260(1):177–203. doi: 10.1113/jphysiol.1976.sp011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datyner M. E., Gage P. W. Presynaptic and postsynaptic effects of the venom of the Australian tiger snake at the neuromuscular junction. Br J Pharmacol. 1973 Oct;49(2):340–354. doi: 10.1111/j.1476-5381.1973.tb08381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Effects of sodium pump inhibitors on spontaneous acetylcholine release at the neuromuscular junction. J Physiol. 1965 Dec;181(3):498–505. doi: 10.1113/jphysiol.1965.sp007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956 Dec 28;134(3):689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Mauro A. Reversibility and mode of action of Black Widow spider venom on the vertebrate neuromuscular junction. J Gen Physiol. 1979 Feb;73(2):245–263. doi: 10.1085/jgp.73.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Loyning Y. The effects of hypoxia on neuromuscular transmission in a mammalian preparation. J Physiol. 1966 Jul;185(1):205–223. doi: 10.1113/jphysiol.1966.sp007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Miledi R. The effect of calcium-ionophores on acetylcholine release from Schwann cells. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):51–58. doi: 10.1098/rspb.1977.0028. [DOI] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D. H., Stamenović B. A., Whitaker B. D. The effect of noradrenaline on the end-plate potential in twitch fibres of the frog. J Physiol. 1968 Apr;195(3):743–754. doi: 10.1113/jphysiol.1968.sp008486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Estimates of quantal content during 'chemical potentiation' of transmitter release. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Kita H., Narita K., Van der Kloot W. The relation between tonicity and impulse-evoked transmitter release in the frog. J Physiol. 1982 Apr;325:213–222. doi: 10.1113/jphysiol.1982.sp014146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Van Der Kloot W. Effects of the ionophore X-537A on acetylcholine release at the frog neuromuscular junction. J Physiol. 1976 Jul;259(1):177–198. doi: 10.1113/jphysiol.1976.sp011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Calcium ionophore X-537A increases spontaneous and phasic quantal release of acetylcholine at frog neuromuscular junction. Nature. 1974 Aug 23;250(5468):658–660. doi: 10.1038/250658a0. [DOI] [PubMed] [Google Scholar]
- Kita H., van der Kloot W. The quantitative relation between extracellular calcium and acetylcholine release at the frog neuromuscular junction. Brain Res. 1973 Jan 15;49(1):205–207. doi: 10.1016/0006-8993(73)90415-0. [DOI] [PubMed] [Google Scholar]
- Kita H., van der Kloot W. Time course and magnitude of effects of changes in tonicity on acetylcholine release at frog neuromuscular junction. J Neurophysiol. 1977 Mar;40(2):212–224. doi: 10.1152/jn.1977.40.2.212. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden K. S., van der Kloot W. At the frog neuromuscular junction prostaglandin synthase inhibitors depress and PGE partially restores quantal acetylcholine release. Brain Res. 1982 Feb 25;234(2):464–468. doi: 10.1016/0006-8993(82)90888-5. [DOI] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Drapeau P. A buffering model for calcium-dependent neurotransmitter release. Biophys J. 1982 May;38(2):205–208. doi: 10.1016/S0006-3495(82)84548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles W. D., Smith D. O. Effects of hypertonic solutions on quantal transmitter release at the crayfish neuromuscular junction. J Physiol. 1982 Aug;329:185–202. doi: 10.1113/jphysiol.1982.sp014297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K. Effects of alcohols and acetone on the neuromuscular junction of frog. Jpn J Physiol. 1967 Jun;17(3):245–261. doi: 10.2170/jjphysiol.17.245. [DOI] [PubMed] [Google Scholar]
- Onodera K. Effect of caffeine on the neuromuscular junction of the frog, and its relation to external calcium concentration. Jpn J Physiol. 1973 Dec;23(6):587–597. doi: 10.2170/jjphysiol.23.587. [DOI] [PubMed] [Google Scholar]
- Parnas H., Segel L. A. A theoretical study of calcium entry in nerve terminals, with application to neurotransmitter release. J Theor Biol. 1981 Jul 7;91(1):125–169. doi: 10.1016/0022-5193(81)90378-7. [DOI] [PubMed] [Google Scholar]
- Quastel D. M., Hackett J. T., Cooke J. D. Calcium: is it required for transmitter secretion? Science. 1971 Jun 4;172(3987):1034–1036. doi: 10.1126/science.172.3987.1034. [DOI] [PubMed] [Google Scholar]
- Shimoni Y., Alnaes E., Rahamimoff R. Is hyperosmotic neurosecretion from motor nerve endings a calcium-dependent process? Nature. 1977 May 12;267(5607):170–172. doi: 10.1038/267170a0. [DOI] [PubMed] [Google Scholar]
- Statham H. E., Duncan C. J. The action of ionophores at the frog neuromuscular junction. Life Sci. 1975 Nov 1;17(9):1401–1406. doi: 10.1016/0024-3205(75)90159-9. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Motor end-plate 'desensitization' by repetitive nerve stimuli. J Physiol. 1959 Oct;148:659–664. doi: 10.1113/jphysiol.1959.sp006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W., Kita H. The possible role of fixed membrane surface charges in acetylcholine release at the frog neuromuscular junction. J Membr Biol. 1973;14(4):365–382. doi: 10.1007/BF01868085. [DOI] [PubMed] [Google Scholar]
- Werman R., Carlen P. L., Kushnir M., Kosower E. M. Effect of the thiol-oxidizing agent, diamide, on acetylcholine release at the frog endplate. Nat New Biol. 1971 Sep 22;233(38):120–121. doi: 10.1038/newbio233120a0. [DOI] [PubMed] [Google Scholar]


