Abstract
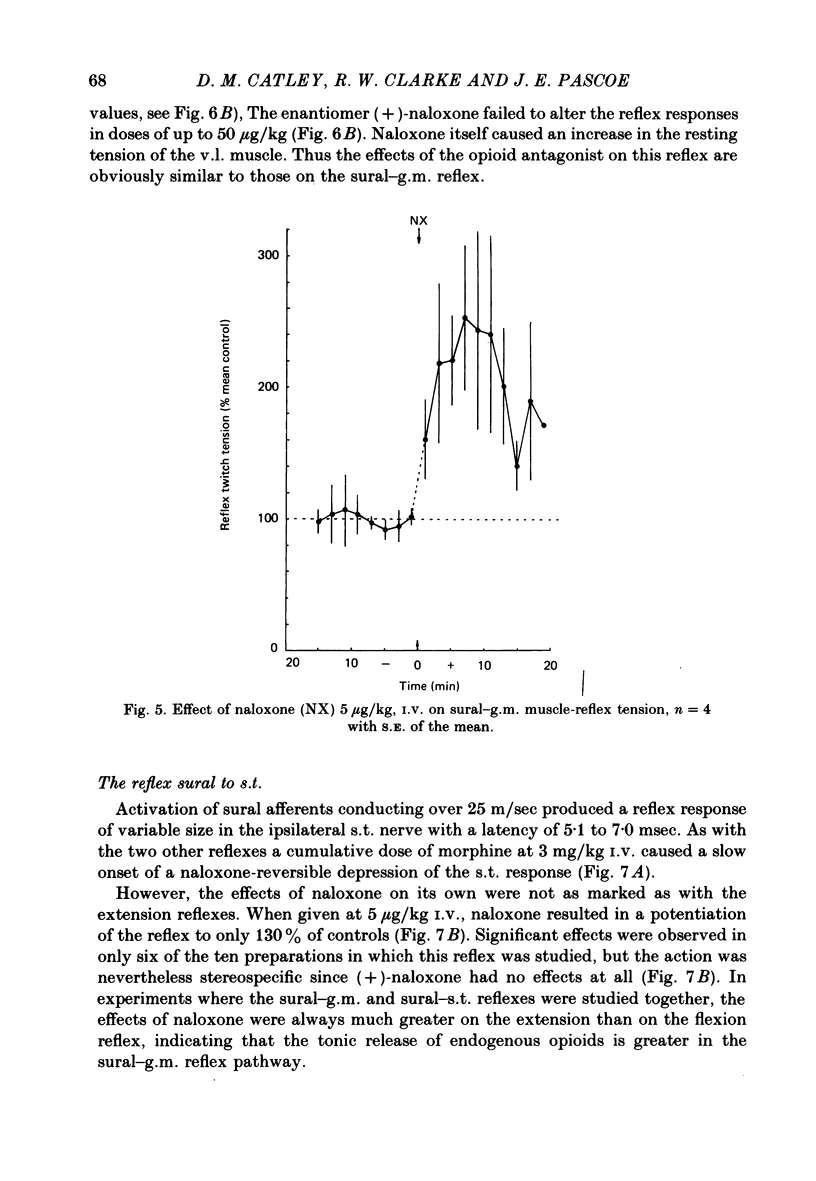
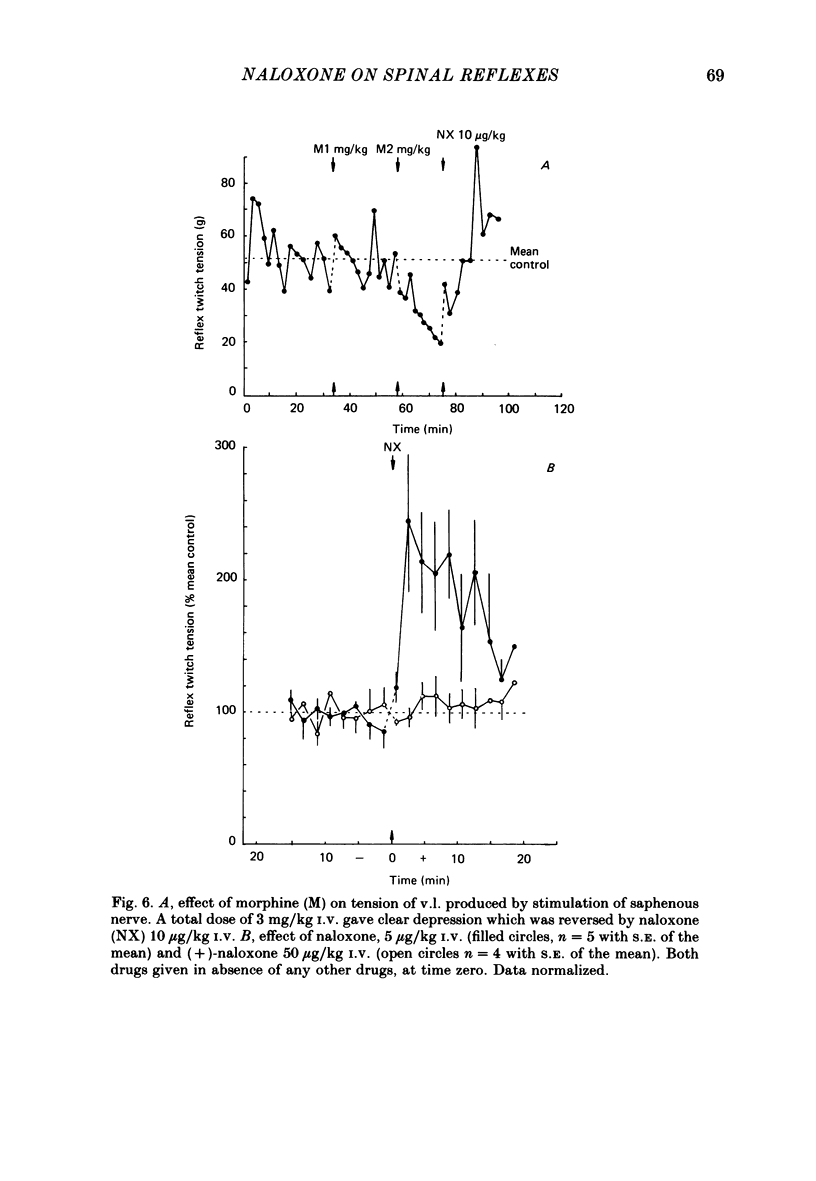
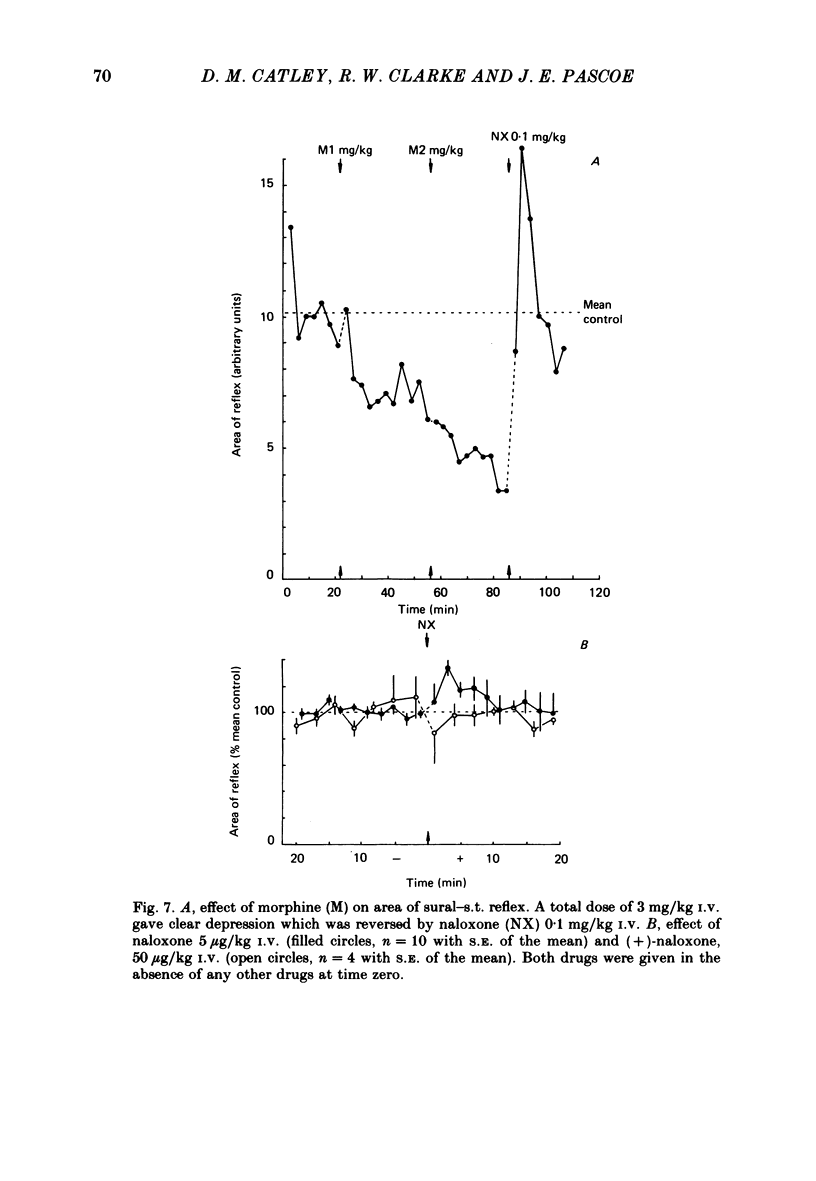
The effects of intravenous morphine, (-)-naloxone and (+)-naloxone have been studied on three ipsilateral cutaneo-muscular reflexes in spinal rabbits. Morphine, 3 mg/kg caused a slow-onset depression of all three reflexes. This effect was naloxone reversible. The ipsilateral extensor reflexes, sural to gastrocnemius medialis and saphenous to vastus lateralis were both enhanced to more than double control size following a 5 micrograms/kg dose of naloxone given in the absence of morphine. For the sural-gastrocnemius reflex, naloxone potentiated the reflex drive from all groups of myelinated afferent fibres. The ipsilateral flexion reflex, sural to semitendinosus, was only weakly enhanced by naloxone, the 5 micrograms dose leading to an increase in the size of the reflex to 130% of control. All observed actions of naloxone were stereospecific as the enantiomer (+)-naloxone failed to affect any of the reflexes even in a dose of 50 micrograms/kg. We conclude from these findings that opioid peptides are tonically released in rabbit spinal cord, and that they have differential effects in control of flexion and extension reflexes. It is suggested that the ipsilateral extension reflexes are held under a more powerful opioid-mediated depression than that operating upon the flexion reflexes, and that this difference may be related to the greater inhibitory inflow to extensor motoneurones from ipsilateral skin areas.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt J. O., Freye E. Opiate antagonist reverses the cardiovascular effects of inhalation anaesthesia. Nature. 1979 Feb 1;277(5695):399–400. doi: 10.1038/277399a0. [DOI] [PubMed] [Google Scholar]
- Bell J. A., Martin W. R. The effect of the narcotic antagonists naloxone, naltrexone and nalorphine on spinal cord C-fiber reflexes evoked by electrical stimulation or radiant heat. Eur J Pharmacol. 1977 Mar 21;42(2):147–154. doi: 10.1016/0014-2999(77)90354-5. [DOI] [PubMed] [Google Scholar]
- Berkowitz B. A., Ngai S. H., Hempstead J., Spector S. Disposition of naloxone: use of a new radioimmunoassay. J Pharmacol Exp Ther. 1975 Dec;195(3):499–504. [PubMed] [Google Scholar]
- Catley D. M., Pascoe J. E. Long-term depression of a reflex and the possible involvement of an opioid [proceedings]. J Physiol. 1978 Aug;281:31P–32P. [PubMed] [Google Scholar]
- Catley D. M., Pascoe J. E. The reflex effects of sural nerve stimulation upon gastrocnemius fusimotor neurones of the rabbit [proceedings]. J Physiol. 1978 Mar;276:32P–32P. [PubMed] [Google Scholar]
- Dingledine R., Iversen L. L., Breuker E. Naloxone as a GABA antagonist: evidence from iontophoretic, receptor binding and convulsant studies. Eur J Pharmacol. 1978 Jan 1;47(1):19–27. doi: 10.1016/0014-2999(78)90369-2. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Hall J. G., Headley P. M. Enkephalins and dorsal horn neurones of the cat: effects on responses to noxious and innocuous skin stimuli. Br J Pharmacol. 1977 Nov;61(3):399–408. doi: 10.1111/j.1476-5381.1977.tb08432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry J. P., Herz A., Zieglgänsberger W. A demonstration of naloxone-precipitated opiate withdrawal on single neurones in the morphine-tolerant/dependent rat brain. Br J Pharmacol. 1980 Mar;68(3):585–592. doi: 10.1111/j.1476-5381.1980.tb14574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry J. P., Zieglgänsberger W., Herz A. Specific versus non-specific actions of opioids on hippocampal neurones in the rat brain. Brain Res. 1979 Mar 16;163(2):295–305. doi: 10.1016/0006-8993(79)90357-3. [DOI] [PubMed] [Google Scholar]
- Goldfarb J., Hu J. W. Enhancement of reflexes by naloxone in spinal cats. Neuropharmacology. 1976 Dec;15(12):785–792. doi: 10.1016/0028-3908(76)90009-5. [DOI] [PubMed] [Google Scholar]
- HAGBARTH K. E. Excitatory and inhibitory skin areas for flexor and extensor motoneurons. Acta Physiol Scand Suppl. 1952;26(94):1–58. [PubMed] [Google Scholar]
- Iijima I., Minamikawa J., Jacobson A. E., Brossi A., Rice K. C. Studies in the (+)-morphinan series. 5. Synthesis and biological properties of (+)-naloxone. J Med Chem. 1978 Apr;21(4):398–400. doi: 10.1021/jm00202a018. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Paterson S. J. Characterization of opioid receptors in nervous tissue. Proc R Soc Lond B Biol Sci. 1980 Oct 29;210(1178):113–122. doi: 10.1098/rspb.1980.0122. [DOI] [PubMed] [Google Scholar]
- Markowitz R., Jacobson J., Bain G., Kornetsky C. Naloxone blockade of morphine analgesia: a dose-effect study of duration and magnitude. J Pharmacol Exp Ther. 1976 Nov;199(2):385–388. [PubMed] [Google Scholar]
- McClane T. K., Martin W. R. Effects of morphine, nalorphine, cyclazocine, and naloxone on the flexor reflex. Int J Neuropharmacol. 1967 Mar;6(2):89–98. doi: 10.1016/0028-3908(67)90057-3. [DOI] [PubMed] [Google Scholar]
- Rossier J., French E. D., Rivier C., Ling N., Guillemin R., Bloom F. E. Foot-shock induced stress increases beta-endorphin levels in blood but not brain. Nature. 1977 Dec 15;270(5638):618–620. doi: 10.1038/270618a0. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh T. L. Spinal opiate analgesia: characteristics and principles of action. Pain. 1981 Dec;11(3):293–346. doi: 10.1016/0304-3959(81)90633-3. [DOI] [PubMed] [Google Scholar]


