Abstract
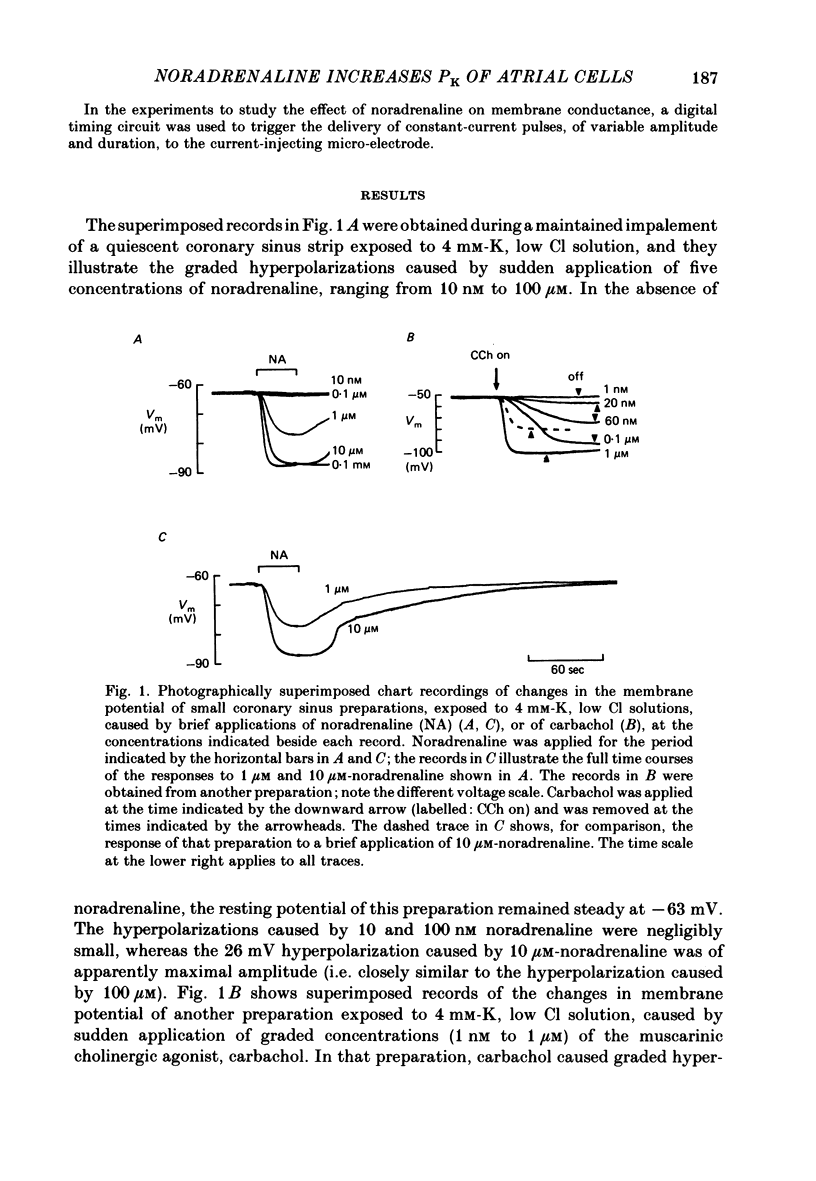
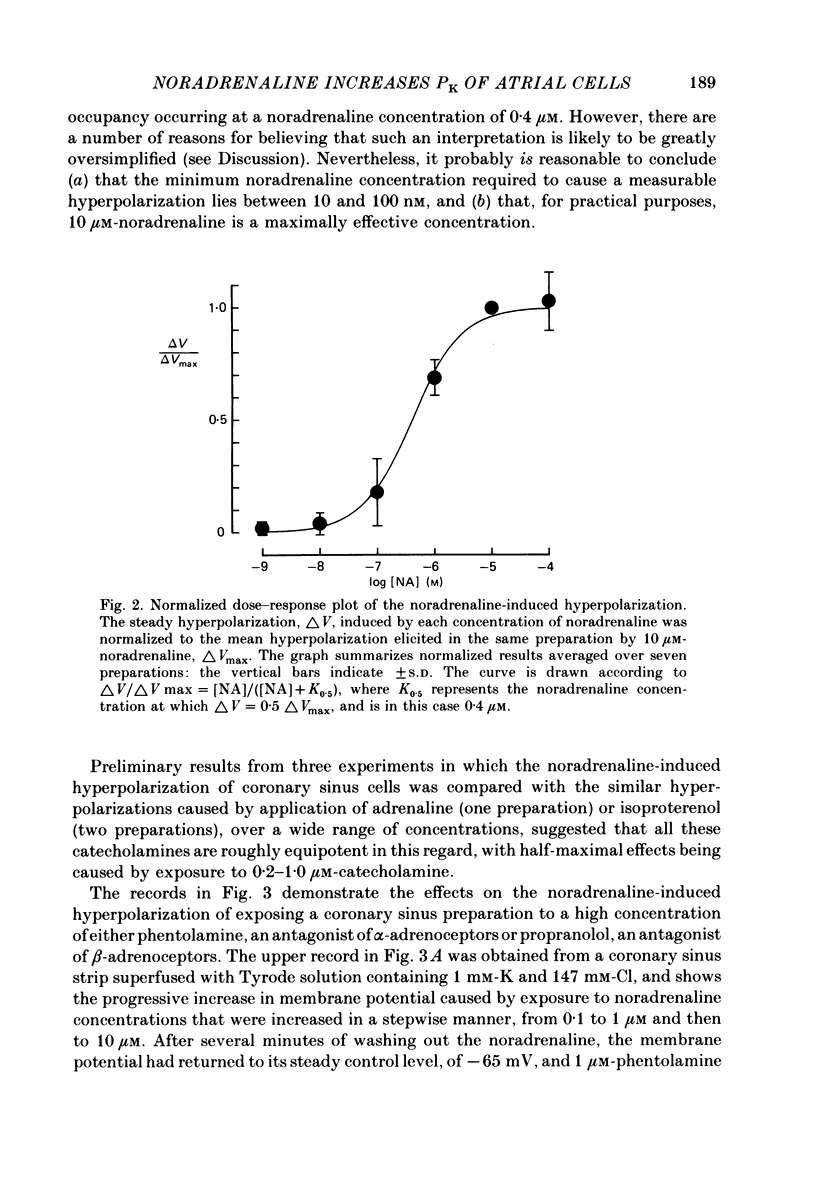
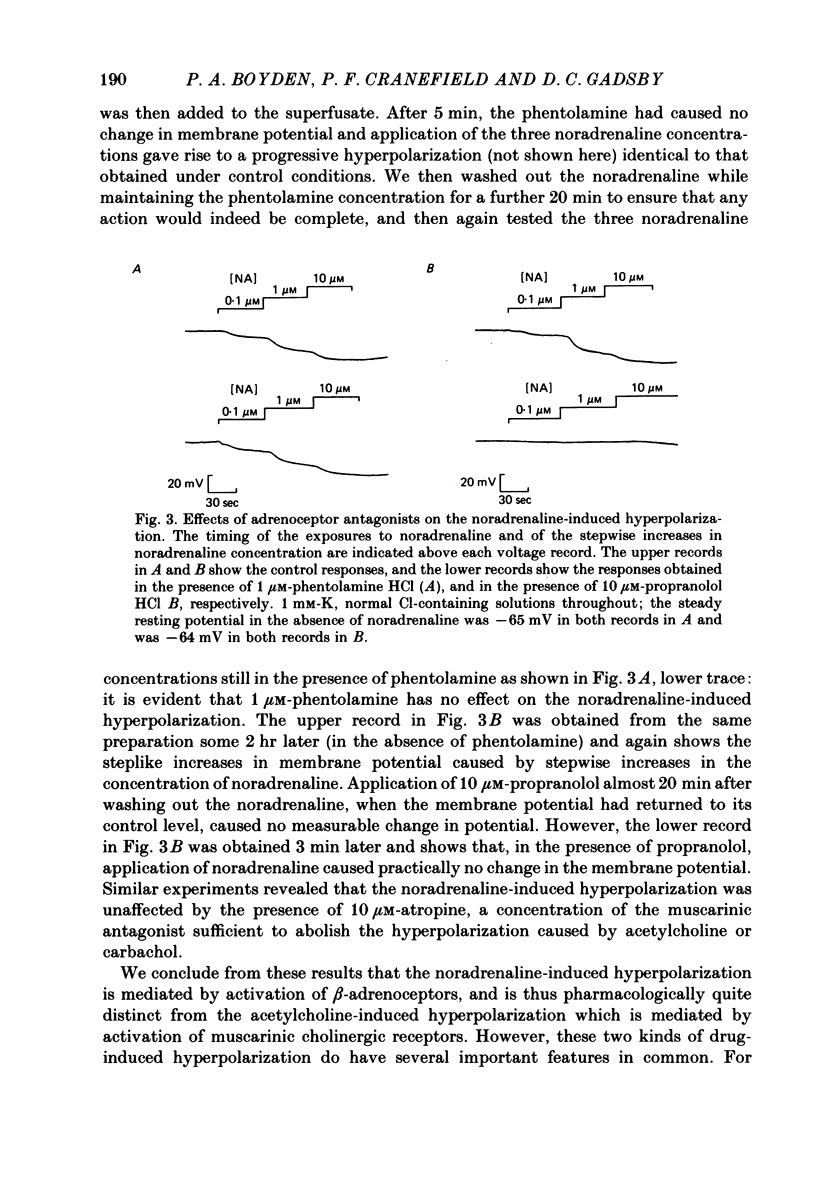
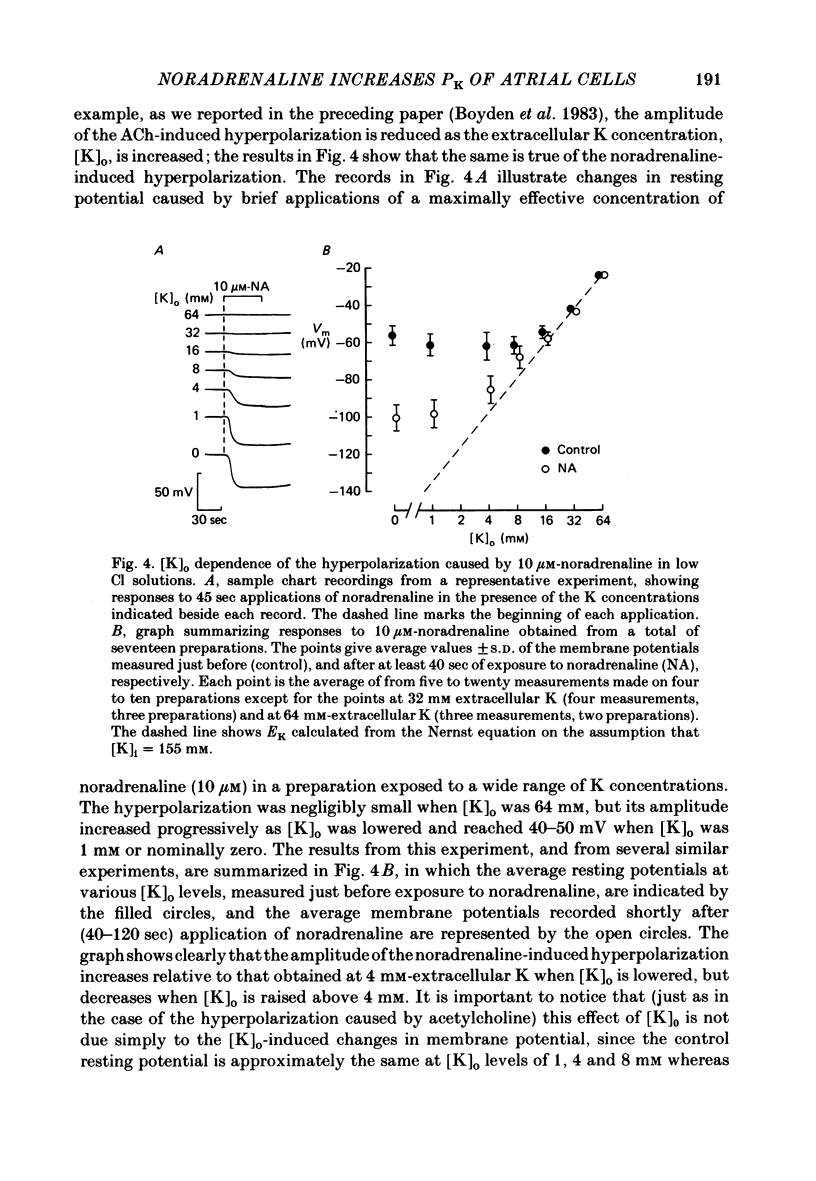
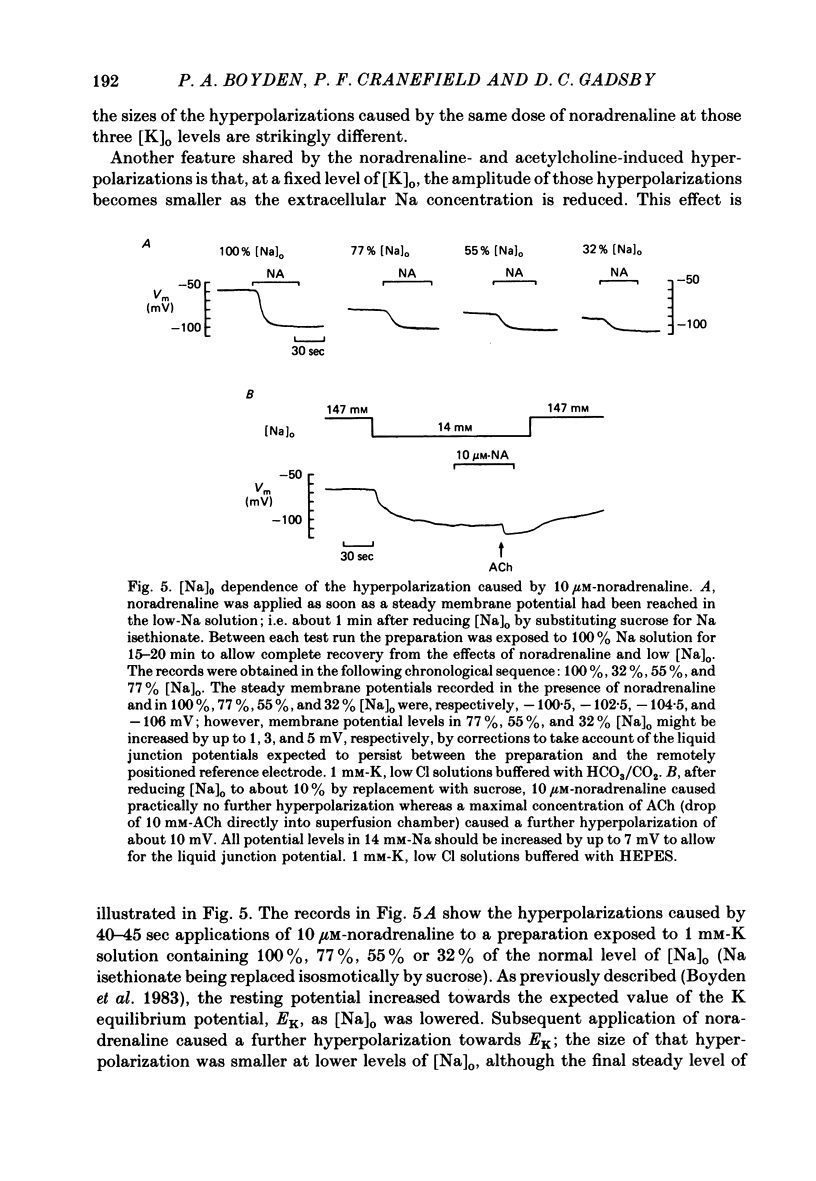
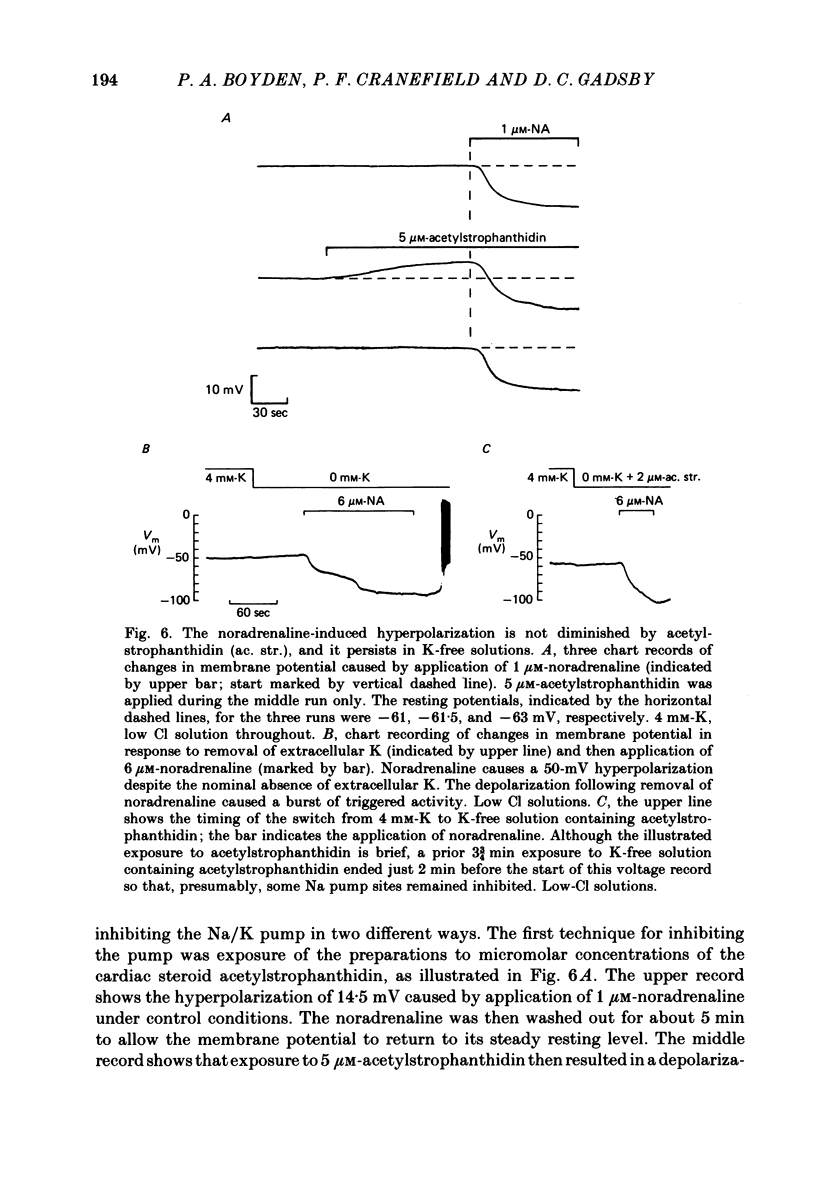
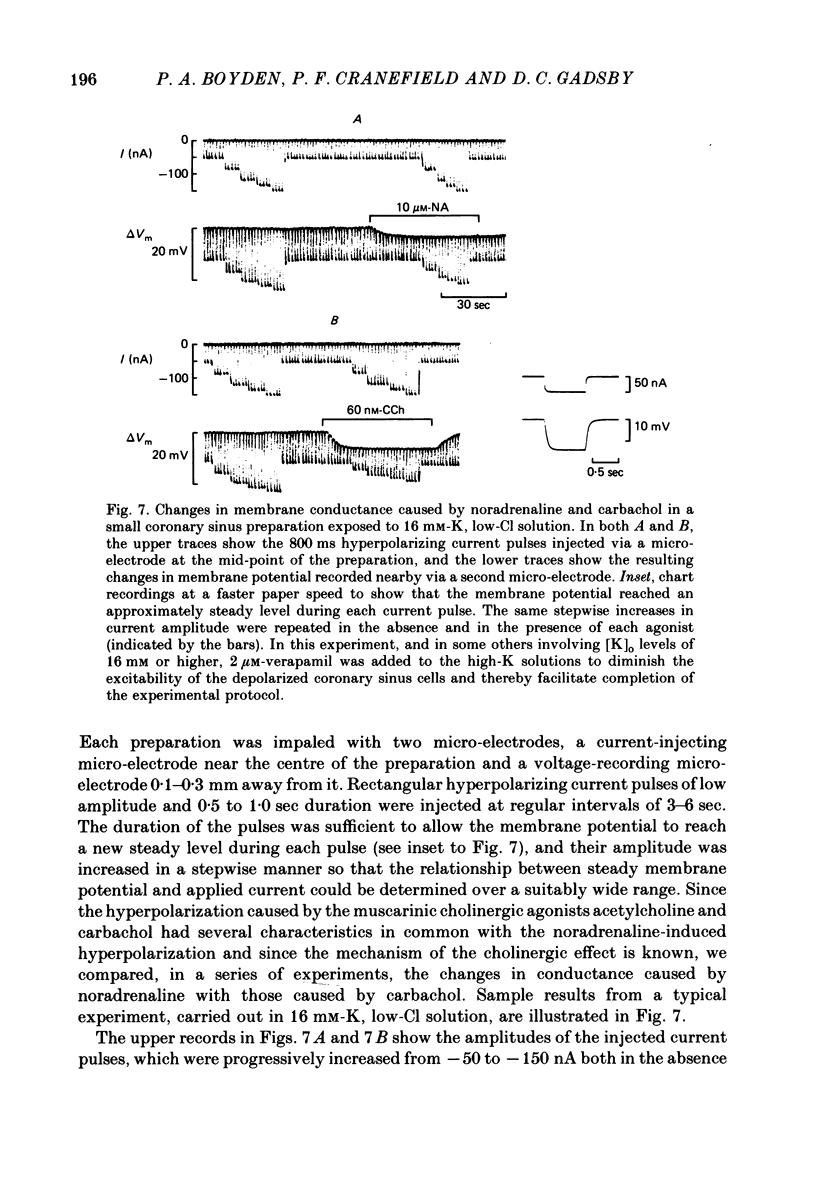
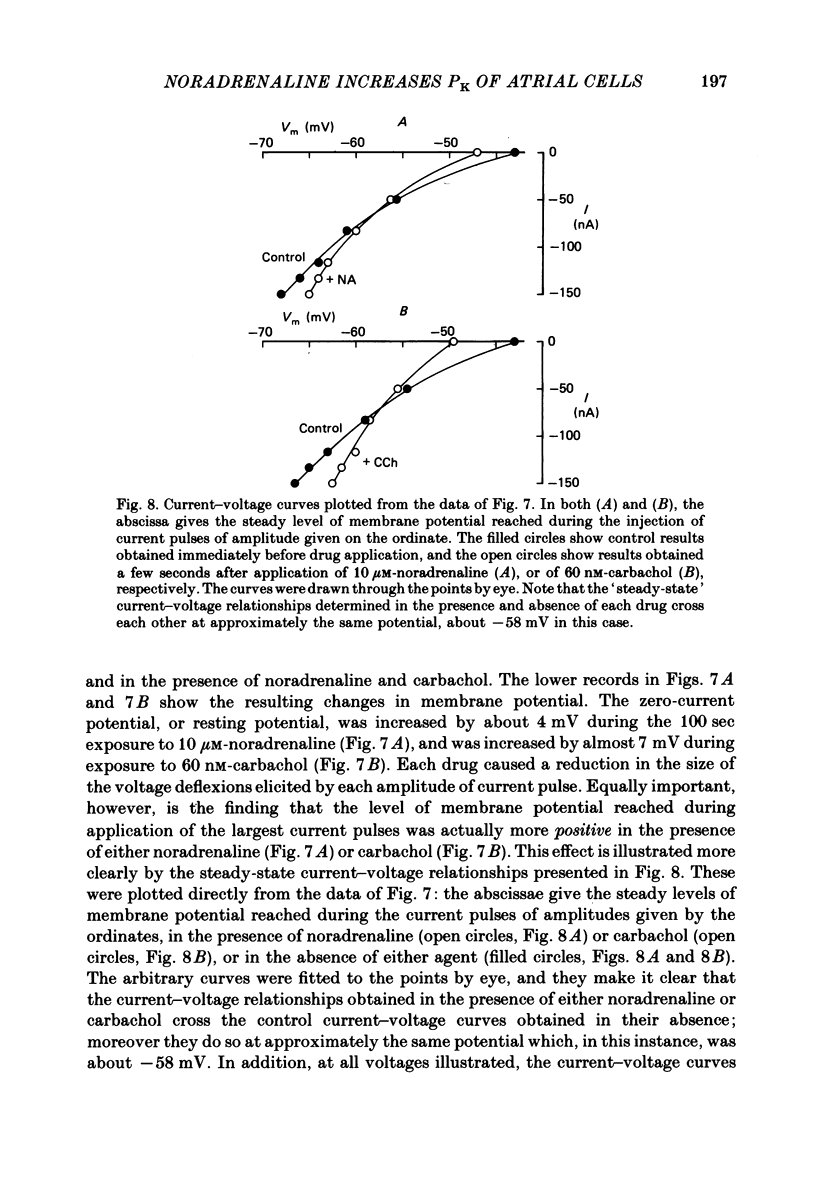
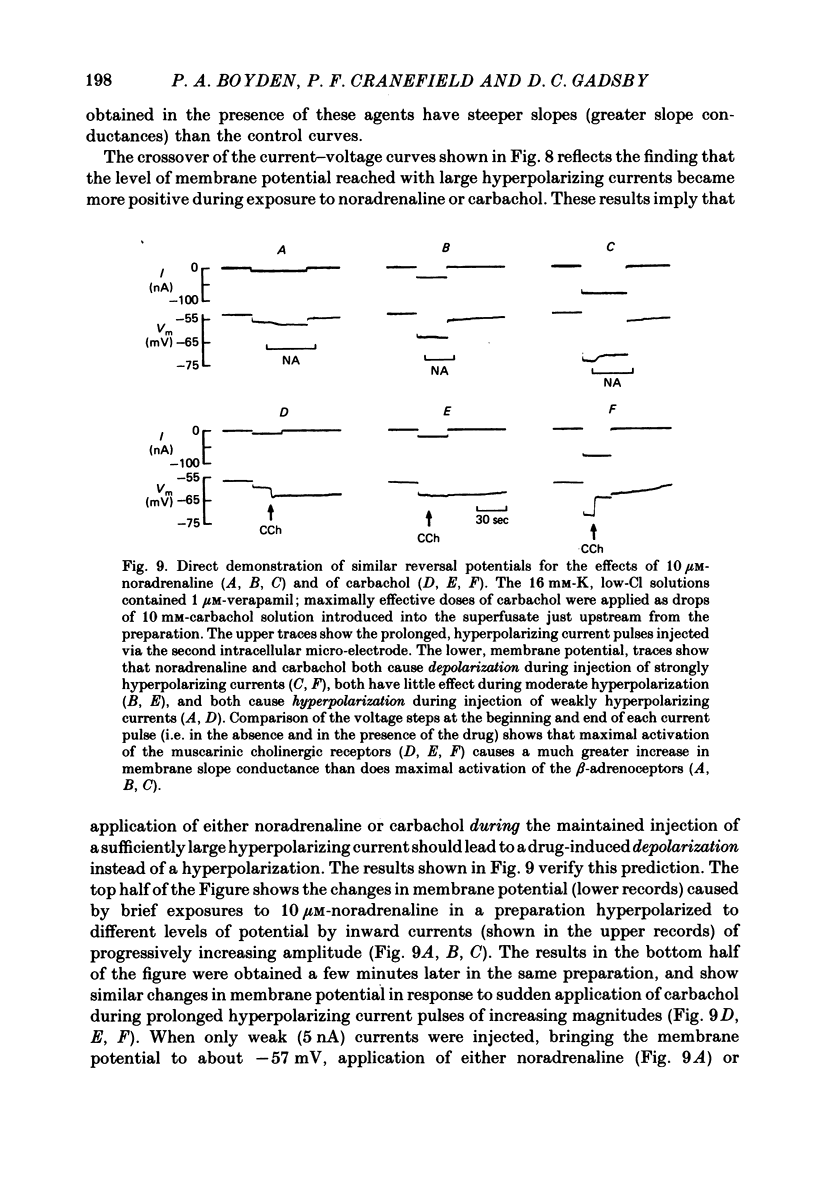
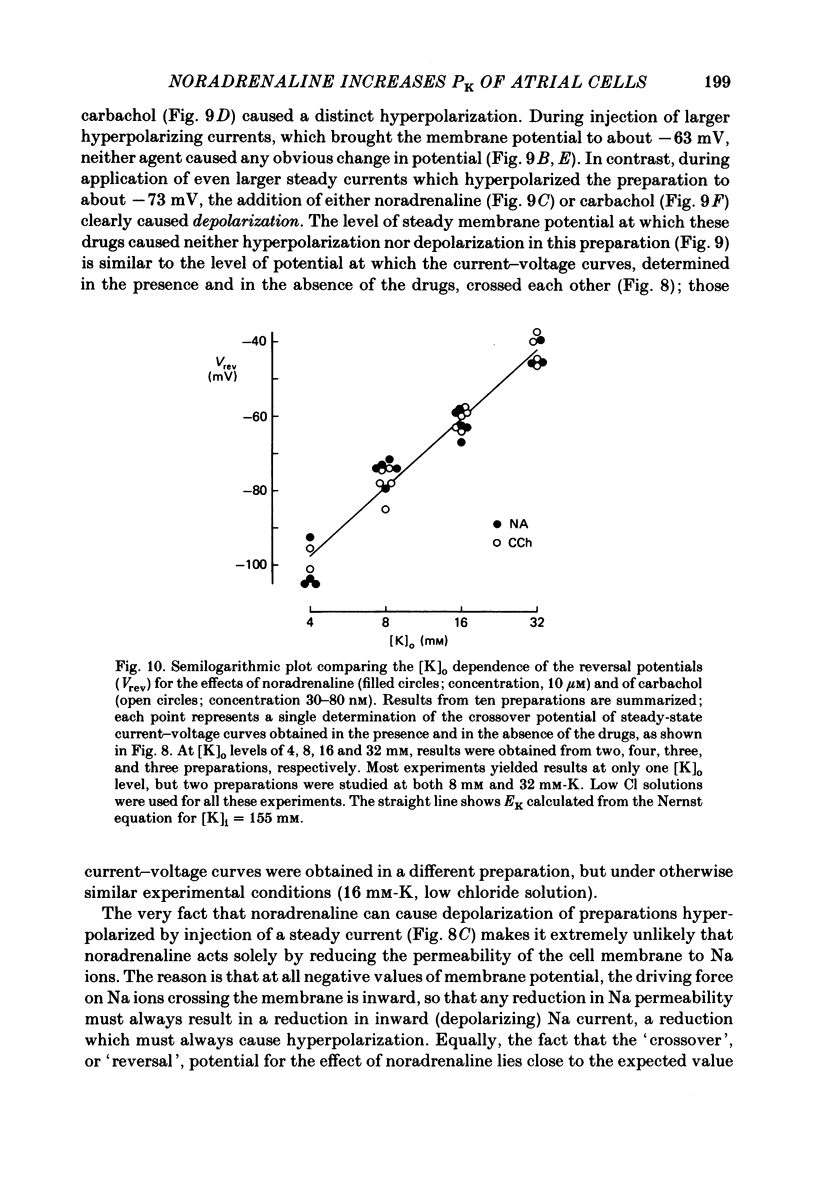
The mechanism of the noradrenaline-induced hyperpolarization was investigated in small strips of coronary sinus tissue mounted in a fast-flow system. The recorded hyperpolarization was negligibly small in response to 10 nM-noradrenaline but was maximal at 10 microM (average amplitude 23 mV, in 4 mM-K solution). The hyperpolarization was unaffected by 1 microM-phentolamine but was abolished by 10 microM-propranolol and so is presumably mediated via beta-adrenoceptors. The noradrenaline-induced hyperpolarization became smaller when the extracellular K concentration ([K]o) was raised or when the extracellular Na concentration was lowered. These results are consistent with two general mechanisms: noradrenaline might cause hyperpolarization by stimulating the Na/K pump to generate more outward current, as previously suggested for other cell types. Alternatively, noradrenaline might lower the permeability ratio, PNa/PK, by reducing the permeability coefficient for Na (PNa) and/or increasing that for K (PK). The noradrenaline-induced hyperpolarization is not diminished during exposure to 5 microM-acetylstrophanthidin, or to K-free solution, or to K-free solution containing acetylstrophanthidin. We conclude that the hyperpolarization does not reflect enhanced electrogenic pump activity. Conductance measurements using two micro-electrodes in very small preparations revealed that, like the muscarinic agonist carbachol, noradrenaline caused an increase in membrane slope conductance. Steady-state current-voltage curves obtained in the presence of noradrenaline, in the presence of carbachol, and in the absence of both drugs all crossed each other at about the same level of membrane potential. During the maintained injection of sufficiently large hyperpolarizing current, application of either noradrenaline or carbachol causes depolarization instead of hyperpolarization. The cross-over or 'reversal' potentials of current-voltage curves, determined with and without the drugs, vary with [K]o approximately as does the K equilibrium potential calculated assuming the intracellular K concentration to be 155 mM. We conclude that, like carbachol and acetylcholine, noradrenaline causes a specific increase in the K permeability of coronary sinus cells.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Ohta Y., Koketsu K. Activation of electrogenic Na+ pump by epinephrine in bullfrog atrium. Jpn Heart J. 1977 Nov;18(6):860–866. doi: 10.1536/ihj.18.860. [DOI] [PubMed] [Google Scholar]
- Akasu T., Ohta Y., Koketsu K. The effect of adrenaline on the electrogenic Na+ pump in cardiac muscle cells. Experientia. 1978 Apr 15;34(4):488–490. doi: 10.1007/BF01935944. [DOI] [PubMed] [Google Scholar]
- Boyden P. A., Cranefield P. F., Gadsby D. C., Wit A. L. The basis for the membrane potential of quiescent cells of the canine coronary sinus. J Physiol. 1983 Jun;339:161–183. doi: 10.1113/jphysiol.1983.sp014710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E., Vereecke J. Adrenaline and the plateau phase of the cardiac action potential. Importance of Ca++, Na+ and K+ conductance. Pflugers Arch. 1969;313(4):300–315. doi: 10.1007/BF00593955. [DOI] [PubMed] [Google Scholar]
- Clausen T., Flatman J. A. The effect of catecholamines on Na-K transport and membrane potential in rat soleus muscle. J Physiol. 1977 Sep;270(2):383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., TRAUTWEIN W. Die Wirkung von Adrenalin auf das Ruhepotential von Myokardfasern des Vorhofs. Experientia. 1956 Oct 15;12(10):396–398. doi: 10.1007/BF02157290. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The dependence of sodium pumping and tension on intracellular sodium activity in voltage-clamped sheep Purkinje fibres. J Physiol. 1981 Aug;317:163–187. doi: 10.1113/jphysiol.1981.sp013819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The effects of rubidium ions and membrane potentials on the intracellular sodium activity of sheep Purkinje fibres. J Physiol. 1981 Aug;317:189–205. doi: 10.1113/jphysiol.1981.sp013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R. F. The pharmacology of vascular smooth muscle. Pharmacol Rev. 1955 Jun;7(2):183–265. [PubMed] [Google Scholar]
- Gadsby D. C. Activation of electrogenic Na+/K+ exchange by extracellular K+ in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4035–4039. doi: 10.1073/pnas.77.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Direct measurement of changes in sodium pump current in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1783–1787. doi: 10.1073/pnas.76.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Electrogenic sodium extrusion in cardiac Purkinje fibers. J Gen Physiol. 1979 Jun;73(6):819–837. doi: 10.1085/jgp.73.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelband H., Rosen M. R., Myerburg R. J., Bush H. L., Bassett A. L., Hoffman B. F. Restorative effect of epinephrine on the electrophysiologic properties of depressed human atrial tissue. J Electrocardiol. 1977;10(4):313–320. doi: 10.1016/s0022-0736(77)80003-4. [DOI] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. Slow inward current and contraction of sheep cardiac Purkinje fibers. J Gen Physiol. 1975 Mar;65(3):367–384. doi: 10.1085/jgp.65.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Pott L. Effects of acetylcholine and parasympathetic nerve stimulation on membrane potential in quiescent guinea-pig atria. J Physiol. 1978 Jun;279:655–668. doi: 10.1113/jphysiol.1978.sp012367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski W., Lüttgau H. C., Schulze J. J. The effects of isoprenaline and a new beta-sympathomimetic amine upon spontaneous activity, diastolic depolarization and plateau height in cardiac Purkinje fibres. Br J Pharmacol. 1978 Jul;63(3):427–434. doi: 10.1111/j.1476-5381.1978.tb07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. F., Singer D. H. Appraisal of the effects of catecholamines on cardiac electrical activity. Ann N Y Acad Sci. 1967 Feb 10;139(3):914–939. doi: 10.1111/j.1749-6632.1967.tb41261.x. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Wiegers S. E. The ionic basis of concentration-related effects of noradrenaline on the action potential of calf cardiac purkinje fibres. J Physiol. 1982 Jan;322:541–558. doi: 10.1113/jphysiol.1982.sp014054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergerke R., Page S. Two physiological agents that appear to facilitate calcium discharge from the sarcoplasmic reticulum in frog heart cells: adrenalin an ATP. Proc R Soc Lond B Biol Sci. 1981 Nov 13;213(1192):325–344. doi: 10.1098/rspb.1981.0069. [DOI] [PubMed] [Google Scholar]
- Pappano A. J., Carmeliet E. E. Epinephrine and the pacemaking mechanism at plateau potentials in sheep cardiac Purkinje fibers. Pflugers Arch. 1979 Oct;382(1):17–26. doi: 10.1007/BF00585899. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M. R., Hordof A. J., Ilvento J. P., Danilo P., Jr Effects of adrenergic amines on electrophysiological properties and automaticity of neonatal and adult canine Purkinje fibers: evidence for alpha- and beta-adrenergic actions. Circ Res. 1977 Apr;40(4):390–400. doi: 10.1161/01.res.40.4.390. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W., SCHMIDT R. F. [On the membrane effect of adrenalin on the myocardial fiber]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:715–726. [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G., Rougier O., Garnier D., Sauviat M. P., Coraboeuf E., Gargouïl Y. M. Effects of adrenaline on membrane inward currents during the cardiac action potential. Pflugers Arch. 1969;309(1):70–81. doi: 10.1007/BF00592283. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Cranefield P. F., Gadsby D. C. Electrogenic sodium extrusion can stop triggered activity in the canine coronary sinus. Circ Res. 1981 Oct;49(4):1029–1042. doi: 10.1161/01.res.49.4.1029. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Cranefield P. F. Triggered and automatic activity in the canine coronary sinus. Circ Res. 1977 Oct;41(4):434–445. doi: 10.1161/01.res.41.4.434. [DOI] [PubMed] [Google Scholar]


