Abstract
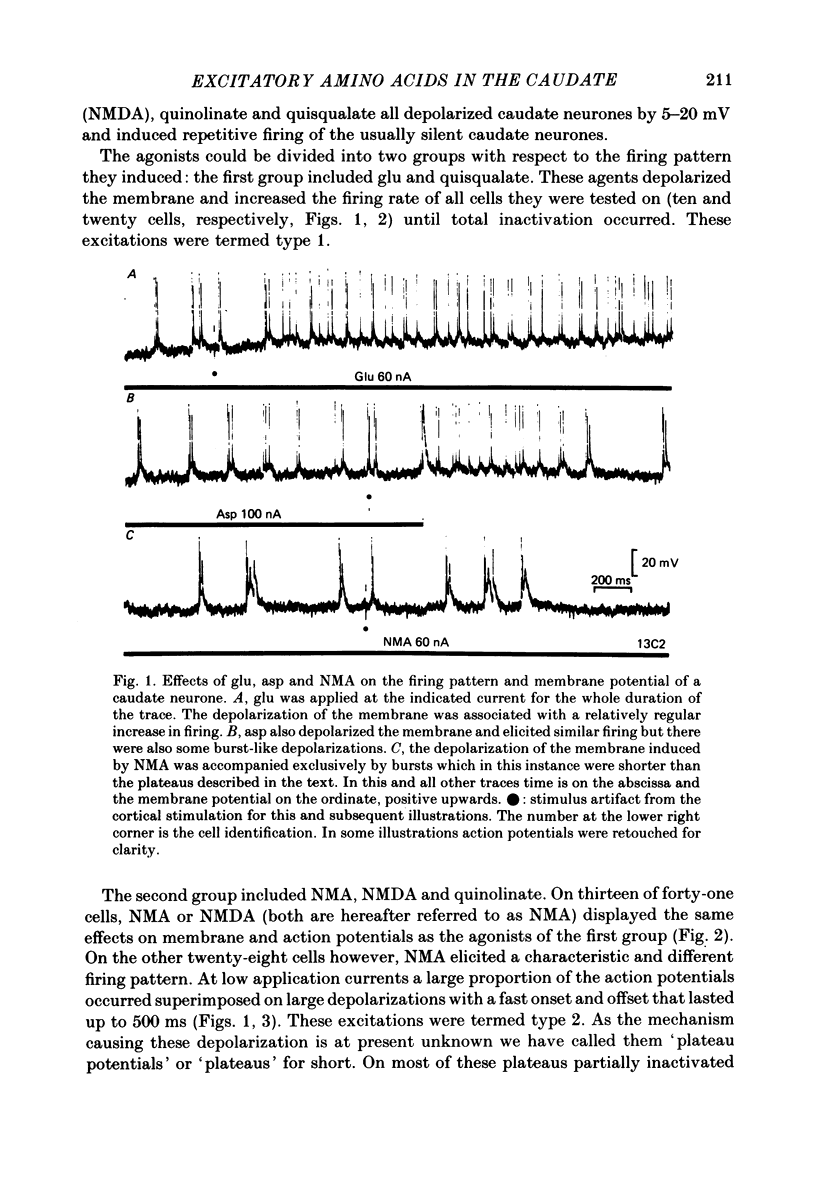
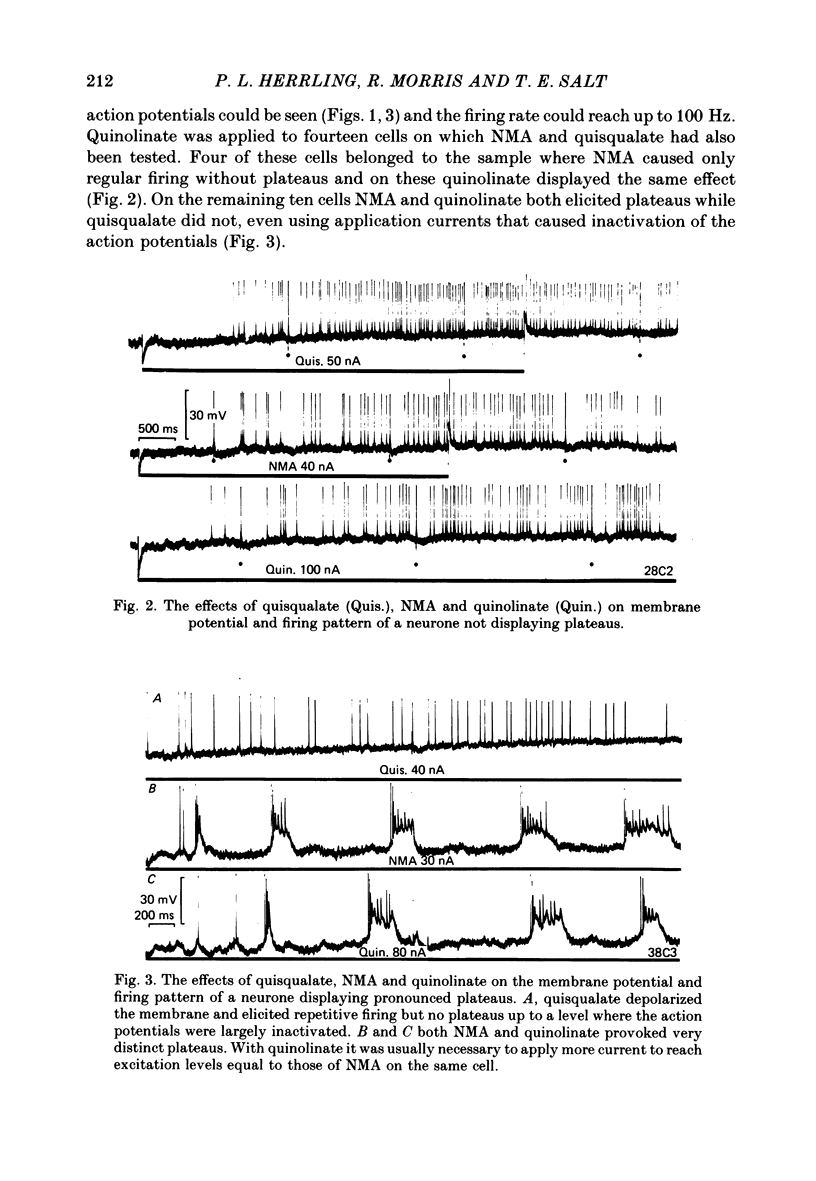
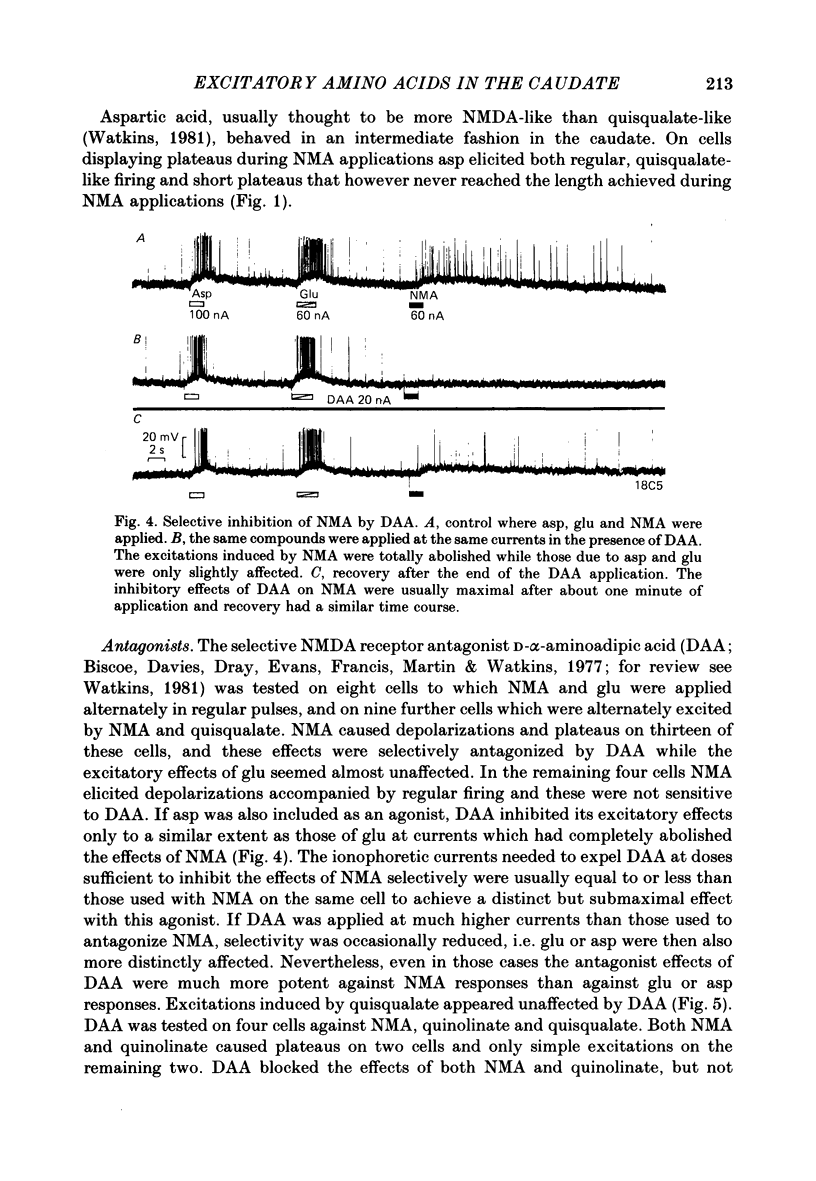
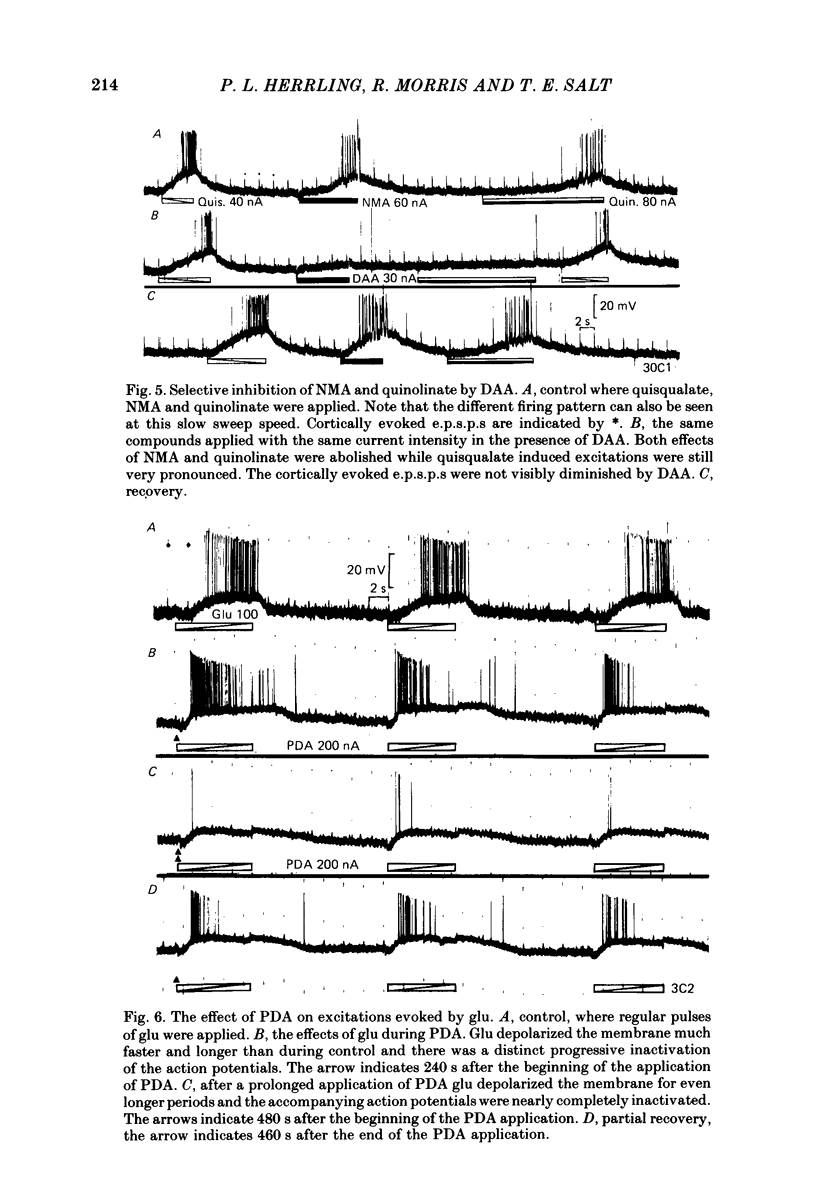
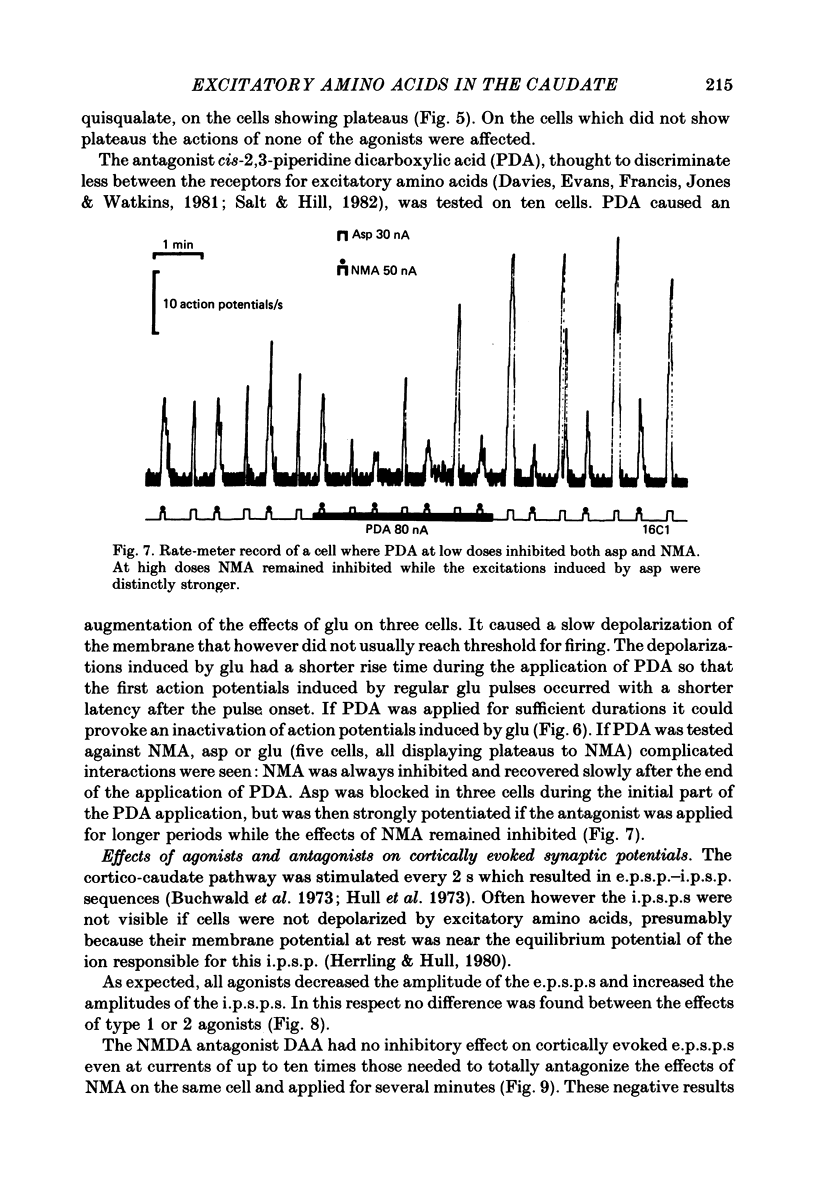
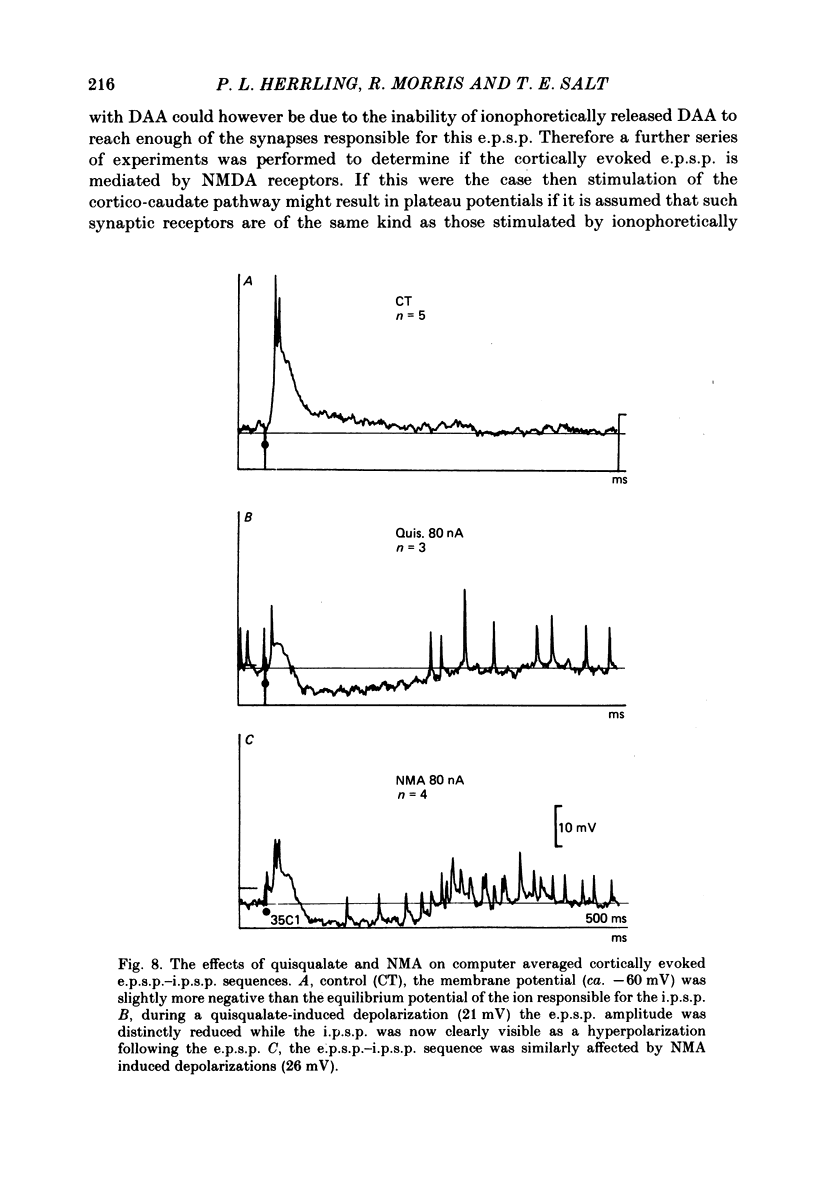
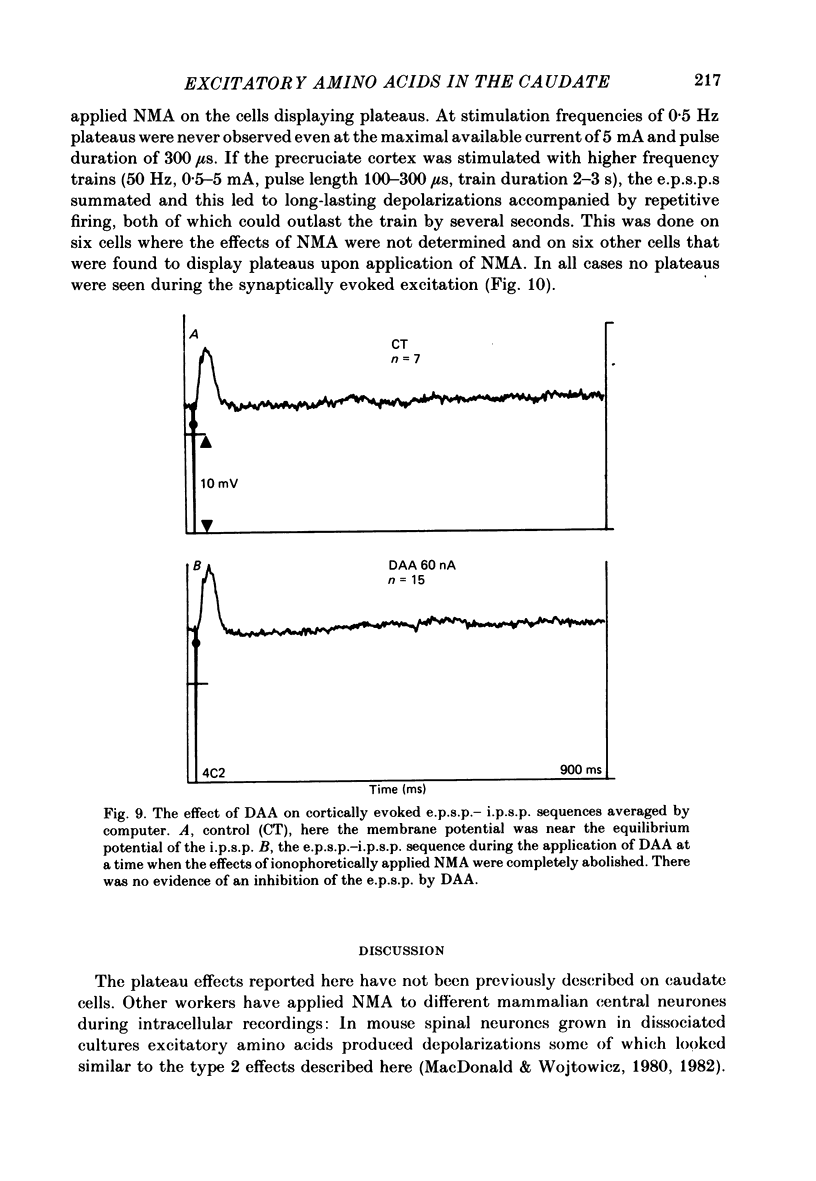
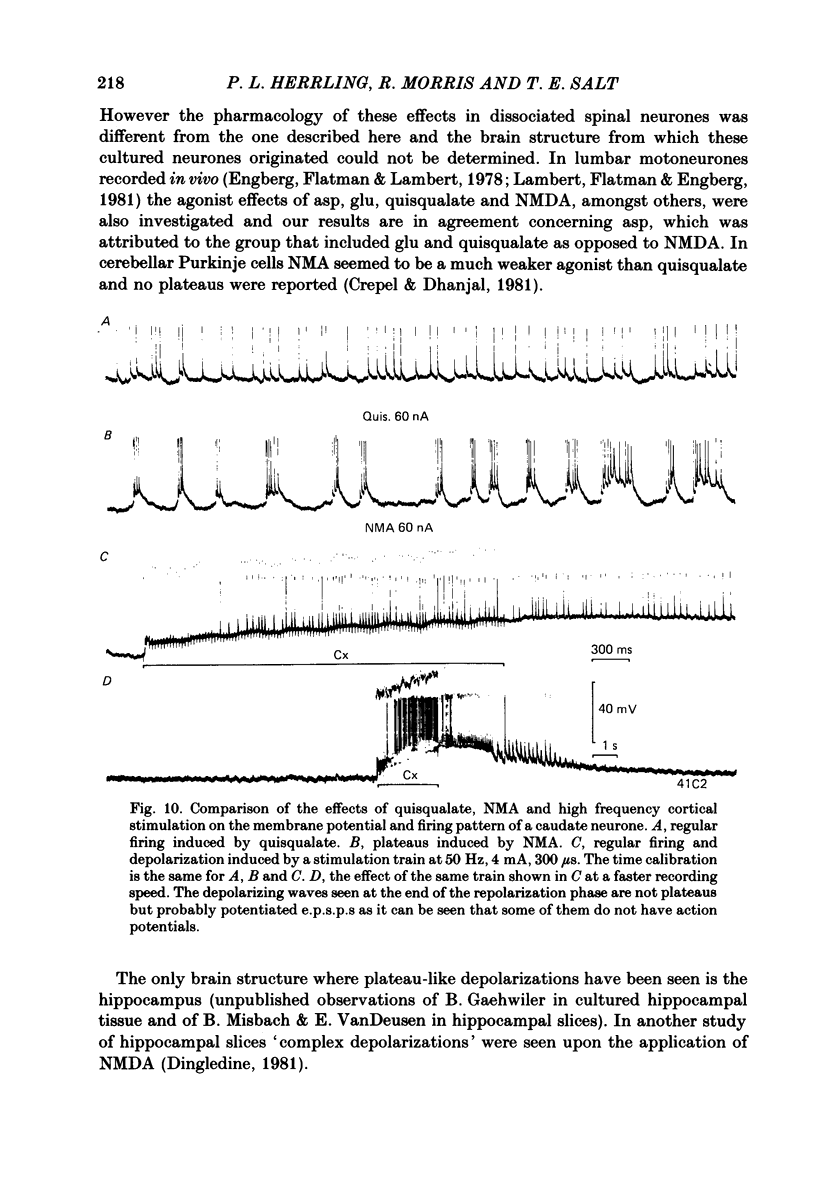
The electrical activity of caudate neurones was recorded with intracellular electrodes in halothane anaesthetized cats. Agonists and antagonists of excitatory amino acid receptors were applied by micro-ionophoresis and their effects on membrane- and action potentials and on cortically evoked synaptic potentials evaluated. The agonists, L-aspartate (asp), L-glutamate (glu), N-methyl-DL-aspartate (NMA), quinolinate and quisqualate all depolarized the membrane, caused repetitive firing, reduced the apparent amplitude of the cortically evoked excitatory post-synaptic potentials (e.p.s.p.s) and increased the amplitude of the associated inhibitory post-synaptic potential. Two of the agonists, NMA and quinolinate, additionally caused the appearance of up to 500 ms long depolarizations (plateaus) on the falling phase of action potentials. These plateaus were seen in about two-thirds of the cells in this sample while in the other third the excitatory effects of NMA and quinolinate were indistinguishable from those of glu and quisqualate. The N-methyl-D-aspartate (NMDA) receptor antagonist D-alpha-aminoadipate (DAA) reversibly inhibited the effects of NMA and quinolinate but only on those cells where these two agents evoked action potential plateaus while on the same cells the effects of asp, glu and quisqualate were either only weakly antagonized or not affected. On cells not displaying plateaus to NMA or quinolinate none of the effects of the agonists could be antagonized by DAA. DAA applications that completely antagonized the effects of NMA never reduced the amplitudes of cortically evoked e.p.s.p.s. Cis-2,3-piperidine dicarboxylate also blocked the effects of NMA and asp at low application currents while at higher currents it enhanced the effects of glu or asp although still retaining its NMA antagonistic activity. High-frequency stimulation of the cortico-caudate pathway resulted in long-lasting depolarizations and repetitive firing, but plateaus of the type caused by NMA or quinolinate were not seen.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ari Y., Kelly J. S. Dopamine evoked inhibition of single cells of the feline putamen and basolateral amygdala. J Physiol. 1976 Mar;256(1):1–21. doi: 10.1113/jphysiol.1976.sp011308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi G., Floris V., Marciani M. G., Morocutti C., Stanzione P. The action of acetylcholine and L-glutamic acid on rat caudate neurons. Brain Res. 1976 Sep 10;114(1):134–138. doi: 10.1016/0006-8993(76)91014-3. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Davies J., Dray A., Evans R. H., Francis A. A., Martin M. R., Watkins J. C. Depression of synaptic excitation and of amino acid induced excitatory responses of spinal neurones by D-alpha-aminoadipate, alpha,epsilon-diaminopimelic acid and HA-966. Eur J Pharmacol. 1977 Oct 1;45(3):315–316. doi: 10.1016/0014-2999(77)90017-6. [DOI] [PubMed] [Google Scholar]
- Bishop G. A., Chang H. T., Kitai S. T. Morphological and physiological properties of neostriatal neurons: an intracellular horseradish peroxidase study in the rat. Neuroscience. 1982 Jan;7(1):179–191. doi: 10.1016/0306-4522(82)90159-2. [DOI] [PubMed] [Google Scholar]
- Buchwald N. A., Price D. D., Vernon L., Hull C. D. Caudate intracellular response to thalamic and cortical inputs. Exp Neurol. 1973 Feb;38(2):311–323. doi: 10.1016/0014-4886(73)90155-6. [DOI] [PubMed] [Google Scholar]
- Cornwall M. C., Thomas M. V. Glass microelectrode tip capacitance: its measurement and a method for its reduction. J Neurosci Methods. 1981 Feb;3(3):225–232. doi: 10.1016/0165-0270(81)90057-1. [DOI] [PubMed] [Google Scholar]
- Davies J., Evans R. H., Francis A. A., Jones A. W., Watkins J. C. Antagonism of excitatory amino acid-induced and synaptic excitation of spinal neurones by cis-2,3-piperidine dicarboxylate. J Neurochem. 1981 Mar;36(3):1305–1307. doi: 10.1111/j.1471-4159.1981.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Davies J., Tongroach P. Neuropharmacological studies on the nigro-striatal and raphe-striatal system in the rat. Eur J Pharmacol. 1978 Sep 15;51(2):91–100. doi: 10.1016/0014-2999(78)90333-3. [DOI] [PubMed] [Google Scholar]
- Davies J., Tongroach P. Tetanus toxin and synaptic inhibition in the substantia nigra and striatum of the rat. J Physiol. 1979 May;290(2):23–36. doi: 10.1113/jphysiol.1979.sp012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divac I., Fonnum F., Storm-Mathisen J. High affinity uptake of glutamate in terminals of corticostriatal axons. Nature. 1977 Mar 24;266(5600):377–378. doi: 10.1038/266377a0. [DOI] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. The action of N-methyl-D-aspartic and kainic acids on motoneurones with emphasis on conductance changes [proceedings]. Br J Pharmacol. 1978 Nov;64(3):384P–385P. [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. The actions of excitatory amino acids on motoneurones in the feline spinal cord. J Physiol. 1979 Mar;288:227–261. [PMC free article] [PubMed] [Google Scholar]
- Fonnum F., Storm-Mathisen J., Divac I. Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibres in rat brain. Neuroscience. 1981;6(5):863–873. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Fry J. P., Zieglgänsberger W., Herz A. Development of acute opioid tolerance and dependence in rat striatal neurones. Naunyn Schmiedebergs Arch Pharmacol. 1980 Aug;313(2):145–149. doi: 10.1007/BF00498571. [DOI] [PubMed] [Google Scholar]
- Herrling P. L., Hull C. D. Iontophoretically applied dopamine depolarizes and hyperpolarizes the membrane of cat caudate neurons. Brain Res. 1980 Jun 23;192(2):441–462. doi: 10.1016/0006-8993(80)90896-3. [DOI] [PubMed] [Google Scholar]
- Hull C. D., Bernardi G., Price D. D., Buchwald N. A. Intracellular responses of caudate neurons to temporally and spatially combined stimuli. Exp Neurol. 1973 Feb;38(2):324–336. doi: 10.1016/0014-4886(73)90156-8. [DOI] [PubMed] [Google Scholar]
- Jones R. S. Specific enhancement of neuronal responses to catecholamine by p-tyramine. J Neurosci Res. 1981;6(1):49–61. doi: 10.1002/jnr.490060106. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Kim J. S., Hasller R., Hau P., Paik K. S. Effect of frontal cortex ablation on striatal glutamic acid level in rat. Brain Res. 1977 Aug 26;132(2):370–374. doi: 10.1016/0006-8993(77)90430-9. [DOI] [PubMed] [Google Scholar]
- Kitai S. T., Kocsis J. D., Preston R. J., Sugimori M. Monosynaptic inputs to caudate neurons identified by intracellular injection of horseradish peroxidase. Brain Res. 1976 Jun 18;109(3):601–606. doi: 10.1016/0006-8993(76)90039-1. [DOI] [PubMed] [Google Scholar]
- Kocsis J. D., Sugimori M., Kitai S. T. Convergence of excitatory synaptic inputs to caudate spiny neurons. Brain Res. 1977 Apr 1;124(3):403–413. doi: 10.1016/0006-8993(77)90942-8. [DOI] [PubMed] [Google Scholar]
- Lambert J. D., Flatman J. A., Engberg I. Actions of excitatory amino acids on membrane conductance and potential in motoneurones. Adv Biochem Psychopharmacol. 1981;27:205–216. [PubMed] [Google Scholar]
- MacDonald J. F., Wojtowicz J. M. The effects of L-glutamate and its analogues upon the membrane conductance of central murine neurones in culture. Can J Physiol Pharmacol. 1982 Mar;60(3):282–296. doi: 10.1139/y82-039. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G., Scherer U., Singh K. A glutamatergic corticostriatal path? Brain Res. 1977 Jun 10;128(2):369–373. doi: 10.1016/0006-8993(77)91003-4. [DOI] [PubMed] [Google Scholar]
- McLennan H., York D. H. Cholinergic mechanisms in the caudate nucleus. J Physiol. 1966 Nov;187(1):163–175. doi: 10.1113/jphysiol.1966.sp008080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross K., Spehlmann R. Microiontophoretic study of the GABA receptor in the feline caudate nucleus. Brain Res. 1981 Aug 31;219(2):345–353. doi: 10.1016/0006-8993(81)90297-3. [DOI] [PubMed] [Google Scholar]
- Oka H. Organization of the cortico-caudate projections. A horseradish peroxidase study in the cat. Exp Brain Res. 1980;40(2):203–208. doi: 10.1007/BF00237538. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., McBean G. J., Sharif N. A., Thomas E. M. Striatal glutamatergic function: modifications following specific lesions. Brain Res. 1982 Mar 4;235(1):83–91. doi: 10.1016/0006-8993(82)90197-4. [DOI] [PubMed] [Google Scholar]
- Royce G. J. Laminar origin of cortical neurons which project upon the caudate nucleus: a horseradish peroxidase investigation in the cat. J Comp Neurol. 1982 Feb 10;205(1):8–29. doi: 10.1002/cne.902050103. [DOI] [PubMed] [Google Scholar]
- Salt T. E., Hill R. G. Differentiation of excitatory amino acid receptors in the rat caudal trigeminal nucleus: a microiontophoretic study. Neuropharmacology. 1982 May;21(5):385–390. doi: 10.1016/0028-3908(82)90020-x. [DOI] [PubMed] [Google Scholar]
- Salt T. E., Hill R. G. Excitatory amino acids as transmitter candidates of vibrissae afferent fibres to the rat trigeminal nucleus caudalis. Neurosci Lett. 1981 Mar 10;22(2):183–187. doi: 10.1016/0304-3940(81)90085-9. [DOI] [PubMed] [Google Scholar]
- Scatton B., Lehmann J. N-methyl-C-aspartate-type receptors mediate striatal 3H-acetylcholine release evoked by excitatory amino acids. Nature. 1982 Jun 3;297(5865):422–424. doi: 10.1038/297422a0. [DOI] [PubMed] [Google Scholar]
- Spencer H. J. Antagonism of cortical excitation of striatal neurons by glutamic acid diethyl ester: evidence for glutamic acid as an excitatory transmitter in the rat striatum. Brain Res. 1976 Jan 30;102(1):91–101. doi: 10.1016/0006-8993(76)90577-1. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Amino acids as neurotransmitters of corticofugal neurones in the rat: a comparison of glutamate and aspartate. Br J Pharmacol. 1979 Dec;67(4):545–551. doi: 10.1111/j.1476-5381.1979.tb08700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W., Perkins M. N. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol. 1981 Jul 10;72(4):411–412. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Goldberg O., Luini A. The stimulation of ion fluxes in brain slices by glutamate and other excitatory amino acids. Mol Cell Biochem. 1981 Sep 25;39:281–295. doi: 10.1007/BF00232580. [DOI] [PubMed] [Google Scholar]
- Vandermaelen C. P., Kitai S. T. Intracellular analysis of synaptic potentials in rat neostriatum following stimulation of the cerebral cortex, thalamus, and substantia nigra. Brain Res Bull. 1980 Nov-Dec;5(6):725–733. doi: 10.1016/0361-9230(80)90212-9. [DOI] [PubMed] [Google Scholar]
- WEBSTER K. E. THE CORTICO-STRIATAL PROJECTION IN THE CAT. J Anat. 1965 Apr;99:329–337. [PMC free article] [PubMed] [Google Scholar]
- Woodruff G. N., McCarthy P. S., Walker R. J. Studies on the pharmacology of neurones in the nucleus accumbens of the rat. Brain Res. 1976 Oct 15;115(2):233–242. doi: 10.1016/0006-8993(76)90509-6. [DOI] [PubMed] [Google Scholar]


