MINIREVIEWS
The diagnosis of autoimmune connective tissue diseases (CTDs) depends not only on the identification of patients manifesting disease-associated groups of clinical symptoms and signs but also on the detection of autoantibodies directed against nuclear or cytoplasmic antigens. Clinicians, however, often do not fully understand the appropriate application and limitations of these tests (10, 25).
Over the last decade, new methods of performing immunology tests have been introduced into laboratory practice, principally to facilitate the processing of large numbers of samples. This rapid change has compounded the problems that result when requesting clinicians are unaware of the performance characteristics of laboratory tests. Testing for autoantibodies to extractable nuclear antigens (ENAs) provides a cogent example.
Interpretation of the clinical significance and role of these antibodies in the diagnosis and management of CTDs is based on information gained by using gel-based techniques, such as double immunodiffusion (DID) and counterimmunoelectrophoresis (CIEP) (11). The disease associations linked to the findings may no longer hold true with newer techniques, such as enzyme-linked immunosorbent assays (ELISAs) and immunoblotting (IB) assays. For example, anti-Sm antibodies detected by gel-based techniques are highly specific for systemic lupus erythematosus (SLE) and form part of the revised American Rheumatism Association criteria for the classification of SLE (26). However, the detection of anti-Sm antibodies by ELISAs in some patients who do not have SLE has diluted the strength of this formerly very powerful clinical association (12). Clinicians not aware of this subtle difference in the performance characteristics of the ELISA method may overdiagnose SLE.
In this minireview, we examine the role of anti-ENA antibody testing in the diagnosis of CTDs, compare the performance of tests that are commonly used in the diagnostic immunology laboratory to measure anti-ENA antibodies, and discuss ways in which the laboratory can improve its anti-ENA antibody testing and reporting to make the results more meaningful for clinicians.
INDICATIONS FOR ENA TESTING
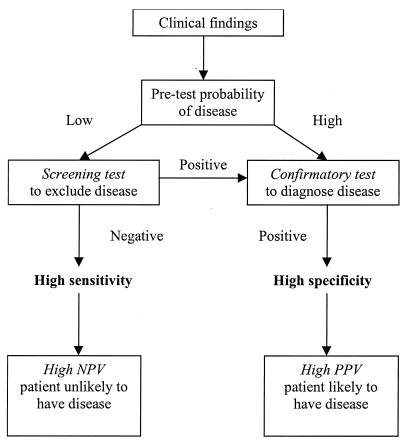
Clinicians order tests for anti-ENA antibodies for one of several reasons. The common indications are to establish a diagnosis of CTDs in patients with suggestive clinical features; to exclude CTDs in patients with few or uncertain clinical findings; to subclassify patients known to have CTDs into prognostic groups; and to monitor disease activity. Each of these indications, however, demands a test with different performance characteristics to yield meaningful information. Clinicians will therefore extract the greatest benefit from the results if they have a clear understanding of the factors that influence test performance. These include the pretest probability of a CTD given the clinical history and physical findings, the prevalence of CTDs in the relevant population, and the method used to perform the assay (Fig. 1). When there are few clinical findings, the pretest probability of a CTD is low; thus, a test with a high sensitivity (and thus a high negative predictive value [NPV]) is required to effectively exclude the presence of a CTD. In this setting, a screening test such as an indirect immunofluorescence (IIF) test for antinuclear antibodies (ANA) with HEp-2 cells offers the appropriate level of sensitivity (27). It would be inappropriate to use relatively insensitive tests, such as those for anti-ENA antibodies, as screening tests to exclude the presence of a CTD. Conversely, the ANA test is not sufficiently specific to establish a diagnosis of a CTD, and a more specific confirmatory test, such as an anti-double-stranded DNA (dsDNA) or an anti-ENA antibody test, is indicated. With the exception of antibodies to cytoplasmic antigens such as Jo-1 (histidyl-tRNA synthetase), it is rare to have a positive anti-ENA antibody test in the absence of a positive ANA test when acetone-fixed HEp-2 cells are used as the substrate for IIF (3, 14, 31). Thus, the finding of ANA-negative SLE due to the use of SS-A antigen-poor substrates such as mouse liver is no longer a valid consideration.
FIG. 1.
The required characteristics of a test are dependent on the indications for which it is performed. The pretest probability of a CTD (disease prevalence) in the general population is low, and therefore a test must have high specificity to yield a high posttest probability of having the disease to be useful in establishing a diagnosis (confirmatory test, such as CIEP for anti-ENA antibodies). A test used to exclude disease (screening test, such as IIF with HEp-2 cells for ANA) must be very sensitive to yield a high posttest probability of not having the disease.
In practice, there is no justification for requesting tests for anti-ENA antibodies unless the ANA test is already known to be positive (or there is a high likelihood that it will be so based on pretest probability). Our practice is to recommend two-stage testing, with an initial ANA screening test followed by confirmatory anti-ENA and anti-dsDNA antibody tests, as needed. The significance of any positive test result for anti-ENA antibodies in the absence of a positive ANA test must be interpreted with caution.
INTERPRETING THE LITERATURE
The generally quoted sensitivities and specificities for the diseases associated with anti-ENA autoantibodies are based on a large body of literature that is difficult to interpret because the studies have been heterogenous with regard to study design, the subjects included, and the ways in which the assays were performed. Most of the studies (e.g., that reported in reference 22) have been retrospective analyses using highly selected populations, a factor which may have significantly biased the results. Further, the details of the types and methods of tests performed varied between studies. For example, the absence of international standards makes comparison of an in-house assay with a commercially produced assay unreliable. The sources of antigens (recombinant or purified) also might have had a significant impact on the performance of tests, and changes to methods and reagents over time make historical comparisons with some earlier studies problematic. The cutoff limits for antibody detection also varied between studies, a factor which could have substantially affected the reported sensitivities and specificities.
Flawed methodology in some of the published studies has resulted in problems with spectrum bias, review bias, and verification bias, which need to be considered in the interpretation of findings. The composition of the study population may affect the test performance, as a study that recruits patients with more severe SLE is more likely to detect anti-ENA antibodies to SS-A, SS-B, and Sm than a study that recruits patients with milder disease. Similarly, the levels of anti-SS-A and anti-SS-B antibodies have been shown to fluctuate with disease activity in both SLE and Sjogren’s syndrome (36); thus, the separation of patients into subgroups by disease spectrum and disease activity would improve the validity of some studies. The lack of an objective independent “gold standard” for the diagnosis of CTDs is another major limitation in the evaluation of any autoantibody test, leading to the use of inappropriate surrogate diagnostic criteria. For example, sensitivity and specificity in some studies have been determined by using the revised American Rheumatism Association classification criteria for SLE. These include the presence of certain marker autoantibodies, such as ANA, anti-Sm, and anti-dsDNA antibodies, an evident source of verification bias. These problems in diagnostic test research are well documented and are not limited to autoantibody testing (20).
It is also crucial to distinguish between the ability of an assay to reproducibly detect an autoantibody when it is present (its precision and accuracy) and the ability of the assay to detect the autoantibody in patients with or without disease (its sensitivity and specificity). For example, the establishment of reference sera and standards by the ANA Subcommittee of the International Union of Immunological Societies Standards Committee (23, 28), the European Consensus Workshops (6, 35) and, more recently, the Association of Medical Laboratory Immunologists (AMLI) (9) has provided invaluable tools for the evaluation of the precision and accuracy of new assays for anti-ENA antibodies, such as those based on ELISAs. However, it is inappropriate to report the results of these evaluations as the sensitivity and specificity of an assay (16, 30, 34).
Similarly, there is also confusion regarding positive predictive value (PPV) and NPV. The sensitivity and specificity of a test for a particular disease are intrinsic to that test and are independent of the disease prevalence. In contrast, PPV and NPV are derived from the application of a test to a particular study population and are therefore related to the disease prevalence in that population as well as to the sensitivity and specificity of the test (Table 1). PPV and NPV quoted for one population (for example, a rheumatology outpatient clinic [15, 22]) are consequently not directly transferable to another population (for example, a private laboratory serving predominantly general practitioners).
TABLE 1.
Table used to calculate the sensitivity, specificity, PPV, and NPV of a testa
| Result | Disease | No disease |
|---|---|---|
| Positive | True positive (a) | False positive (b) |
| Negative | False negative (c) | True negative (d) |
Sensitivity is the proportion of people with disease in whom the test result is positive [a/(a + c)]. Specificity is the proportion of people without disease in whom the test result is negative [d/(b + d)]. PPV is the proportion of people with a positive test result who have disease [a/(a + b)] or (sensitivity × prevalence)/{(sensitivity × prevalence) + [(1 − specificity) × (1 − prevalence)]}. NPV is the proportion of people with a negative test result who do not have disease [d/(c + d)] or [specificity× (1 − prevalence)]/{[(1 − sensitivity) × prevalence] + [specificity × (1 − prevalence)]}.
Some studies have inappropriately reported concordance rates between test methods in comparisons of different methods for anti-ENA detection (2, 19), rather than the more valid kappa statistic, which measures agreement beyond chance (1). Alternatively, as illustrated by Ulvestad et al., the use of the area under the curve of the receiver-operating-characteristic (ROC) plot is a statistically valid method for comparing the diagnostic accuracies of different tests (32).
The choice of appropriate normal and disease control populations also can be critical in determining test performance. For example, Maddison et al. did not include patients with mixed CTD as a control group; therefore, the specificity of anti-U1 RNP antibodies for SLE may have been falsely elevated in their study (13).
Given all these considerations, the sensitivities and specificities presented in Table 2 should be considered only a guide. Clearly, a large cross-sectional study using standardized techniques with well-defined patient and control groups is required to validate these estimates. More importantly, an individual laboratory should be aware of how an assay performs for its referral population and should attempt to establish cutoff values relevant to that population so as to maximize the utility of the test for its referring clinicians.
TABLE 2.
Anti-ENA antibodies most commonly detected by different methods and their performance characteristicsa
| ENA | Major clinical associations | CIEP
|
ELISA
|
IB
|
Comments | |||
|---|---|---|---|---|---|---|---|---|
| Se | Sp | Se | Sp | Se | Sp | |||
| SS-A | Sjogren’s syndrome | 85-95 | 50-60 | 90-97 | 45-50 | 70-85 | 40-50 | Conformational epitopes on 52- and 60-kDa SS-A antigen best detected by CIEP; IB is unreliable because of denaturation of epitopes by SDS-PAGE |
| SLE | 25-30 | 50-60 | 35-60 | 45-50 | 10-15 | 40-50 | ||
| SS-B | Sjogren’s syndrome | 70-80 | 60-70 | 75-85 | 50-60 | 90-95 | 55-65 | Linear epitopes on 48-kDa SS-B antigen best detected by IB; CIEP is less sensitive but more specific |
| SLE | 10-15 | 50-55 | 20-30 | 45-50 | 30-35 | 40-50 | ||
| Sm | SLE | 30-35 | 98-100 | 35-50 | 55-99 | 30-35 | 95-99 | ELISA specificity may be improved by use of highly purified or recombinant antigens; IB is more specific than ELISA |
| U1 RNP | Mixed CTD | 90-95 | 60-75 | 95-98 | 50-60 | 80-85 | 65-75 | ELISA specificity may be improved by use of highly purified or recombinant antigens; IB is more specific than ELISA |
| SLE | 15-35 | 55-75 | 50-60 | 50-55 | 30-40 | 55-70 | ||
| Scl-70 | Scleroderma | 25-35 | 95-99 | 30-45 | 80-90 | 30-45 | 90-95 | CIEP is less sensitive because of the low negative charge on Scl-70 antigen at pH 8.0; this factor can be improved by running gel electrophoresis at pH 8.4 |
| SLE | 0-5 | 0-5 | 20-25 | 15-25 | 10-20 | 5-10 | Anti-Scl-70 antibodies detected by ELISA in SLE may not be “false positives”; rather, they may identify a subgroup of patients at high risk of pulmonary hypertension and renal disease | |
| Jo-1 | PM/DMb | 25-40 | 95-99 | 35-45 | 90-95 | 60-90 | 95-99 | Anti-Jo-1 antibodies stain the cytoplasm of HEp-2 cells and may be reported as“ANA negative” |
Sensitivities (Se) and specificities (Sp) for the associated clinical conditions are given as percentages and are estimates based on interpretation of the available literature.
PM/DM, polymyositis and dermatomyositis.
PRACTICAL ASPECTS OF ASSAY PERFORMANCE
The advantages and disadvantages of the methods commonly used for the detection of anti-ENA antibodies are outlined in Table 3.
TABLE 3.
Comparison of the advantages and disadvantages of the methods commonly used for the detection of anti-ENA antibodies
| Method | Epitopes | Advantages | Disadvantages |
|---|---|---|---|
| DID | Conformational and linear | Inexpensive | Low sensitivity |
| High specificity | Slow turnaround time | ||
| Detects wide range of anti-ENA antibodies | Subjective | ||
| Labour-and skill-intensive | |||
| Requires well-defined reference sera | |||
| CIEP | Conformational and linear | Inexpensive | May miss some antibodies to 52-kDa SS-A, SS-B, and Scl-70 |
| More sensitive and more specific than DID | Same disadvantages as DID | ||
| Faster turnaround time than DID | |||
| ELISA | Conformational and linear | Rapid turnaround time | Relatively expensive (although less expensive than IB) |
| More sensitive than DID and CIEP | Requires purified antigen | ||
| Objective and quantitative | Low specificity | ||
| Less skill and training required | |||
| Capacity for automation | |||
| IB | Linear only | More sensitive than DID and CIEP | Expensive |
| May miss some antibodies to 60-kDa SS-A and Scl-70 | |||
| Labor-and skill-intensive (especially for in-house assays) |
Gel-based techniques.
Gel-based techniques remain the method of choice for the detection of the common anti-ENA antibodies. CIEP has been shown to be generally more sensitive and specific than DID (6, 11). However, unlike DID, CIEP has the disadvantage that the net movement of the antigen across the gel toward the antibody in the serum is determined by the opposing forces of electroendosmotic flow and electrical gradient. For less negatively charged antigens, such as Scl-70, this situation may result in the movement of the antigen in the same direction as the anti-Scl-70 antibodies, resulting in the lack of formation of precipitin lines (5). Gel-based assays also depend on the precipitation of antigen-antibody complexes. The majority of anti-SS-B antibodies recognize linear rather than conformational epitopes (38). These occur at a low frequency in the SS-B ribonucleoprotein and have low antigenicity, resulting in a reduced ability to precipitate immune complexes. Thus, some anti-SS-B antibodies may not be detected by CIEP.
The major advantages of CIEP are that it is relatively inexpensive to perform and a positive result is highly specific for the associated clinical diagnosis. However, it is less sensitive than other techniques, such as ELISAs. The performance and interpretation of CIEP are both labor-intensive and skill dependent, with a relatively slow turnaround time compared to that of ELISA-based kits. Walravens et al. reported that serum prediffusion significantly improved the quality of the precipitin lines in CIEP (37), and Phan et al. showed that this modification can be integrated into the work flow of a diagnostic laboratory with a significant improvement in the turnaround time (18). Another major limitation of gel-based techniques is the need for a well-characterized control antibody to establish an immunological line of identity with the test serum. Fortunately, the establishment of reference sera and standards by the ANA Subcommittee of the International Union of Immunological Societies Standards Committee (23, 28), the European Consensus Workshops (6, 35) and, more recently, the AMLI (9), has assisted in making this goal achievable in all laboratories.
ELISAs.
ELISA-based kits have become the most widely used method for anti-ENA antibody testing, accounting for 54 of 71 laboratories participating in the Royal College of Pathologists of Australasia Quality Assurance Program. This usage is largely due to their relative simplicity of performance, objectivity in interpretation, rapid turnaround time, and capacity for automation. These factors are particularly appealing to large-volume, high-throughput laboratories, in which the higher cost of commercial ELISA-based kits is offset by lower labor and training costs. However, a major problem with ELISAs is their low specificity (2, 6, 13, 30). This characteristic is partly due to antigen impurity, as most ENAs are small nuclear ribonucleoproteins (snRNPs) that form part of multicomponent spliceosomes or nucleosomes and are often closely associated with RNAs and other proteins, such as RNA polymerases. The high-affinity interactions of ENAs with these other components of the spliceosome complex mean that it is almost impossible to purify these antigens to biochemical homogeneity. Indeed, efforts to increase purity by overrigorous purification steps may lead to denaturation of the native protein and loss of conformational epitopes. Further, antigen purification procedures are labor-intensive and require large volumes of starting material to yield a small quantity of pure antigen.
The use of recombinant DNA technology to produce large commercial quantities of pure antigen has significantly improved the performance of ELISAs (24). Most expression systems use the procaryote Escherichia coli as the host, which may result in false-positive results in ELISAs due to the presence of immunoglobulin G antibodies against contaminating E. coli intracellular proteins in some patients (7). The bacteria may also lack the necessary machinery to stabilize and regulate the folding of the synthesized protein into the native secondary and tertiary structures, possibly resulting in the loss of conformational epitopes and giving rise to false-negative results. The use of eucaryotic expression systems may help resolve this problem (24). Another potential source of pure antigen is synthetic peptides; however, these present solely linear epitopes and may therefore be useful only for antigens such as SS-B, where the major known epitopes are linear (38).
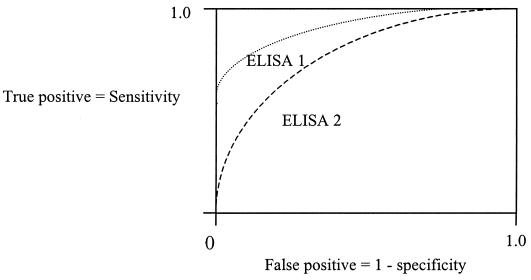
The low specificity of ELISAs cannot be overcome simply by increasing the cutoff limits of detection. Sensitivity and specificity are reciprocally related, and specificity can be increased only at the expense of sensitivity (Fig. 2). Therefore, as mentioned previously, a laboratory using an ELISA should ideally validate the cutoff limits for the assay in the population that it serves. Some laboratories have attempted to resolve the problem of the low specificity of ELISAs by using a combination testing strategy with different assay methods. For example, the ELISA is used to screen for anti-ENA antibodies, and positive samples are further characterized by a more specific test, such as CIEP. Samples that test positive in the ELISA but negative in the CIEP then are reported as negative. This strategy is in keeping with the recommendations of the European Consensus Workshops (35).
FIG. 2.
The ROC curve of a test describes the reciprocal relationship between sensitivity and specificity and is independent of the disease prevalence in the population. Analysis of the area under the curve of the ROC curve allows comparison of the performance characteristics of one test with those of another; in the example shown, ELISA 1 is superior to ELISA 2. The cutoff for disease can be raised to improve specificity, but this gain is offset by a loss of sensitivity; conversely, if the cutoff is lowered, any gain in sensitivity is offset by a loss of specificity. Use of a sensitive screening test followed by a specific confirmatory test overcomes this problem by combining the high sensitivity of the first test with the high specificity of the second test.
Nevertheless, a negative CIEP test result following a positive ELISA test result may not necessarily mean that the latter is positive. As mentioned above, ELISA is more sensitive than CIEP for the detection of anti-SS-B and anti-Scl-70 antibodies. More specifically, antibodies to Scl-70 detected by ELISA have only recently been recognized to occur in a subset of patients who have SLE and who have a higher risk of developing pulmonary hypertension and renal disease (8). These anti-Scl-70 antibodies may recognize different epitopes with a lower avidity than the anti-Scl-70 antibodies found in scleroderma and therefore may not be detected by CIEP.
There is a considerable degree of variability in the performance of the currently available ELISAs (2, 30), partly due to the absence of internationally accepted reference sera and standards for ELISAs, unlike for IIF, gel-based assays, and Western blotting assays (23). This situation is currently being addressed by the AMLI (9).
IB techniques.
The advantage of IB assays over ELISAs is that the antigens do not need to be purified, as the cellular extracts are separated by electrophoretic mobility in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Further, these assays allow characterization of the “fine specificity” of the autoantibody response to multiple subunits of the spliceosome complex, such as the recognition of the U1 RNP A, B, and C proteins and the Sm B, B′, D, and E proteins. However, these assays detect only linear epitopes, as the target autoantigens are denatured by SDS-PAGE. Other problems associated with IB assays include poor transfer of the antigen from the gel across the nitrocellulose membrane and a high level of background staining (33). For example, IB assays are less sensitive than CIEP assays and ELISAs for the detection of anti-SS-A antibodies because the SS-A antigen is a “poor blotter” and 15% of anti-SS-A antibodies recognize only conformational epitopes on the 60-kDa SS-A antigen (4). Similarly, IB assays may not detect some anti-Scl-70 antibodies (2, 19).
Zampieri et al. showed that the choice of blocking agents can significantly affect the performance of IB assays (39). The nonionic detergent Tween 20 was shown to facilitate the renaturation of protein antigens by removing bound SDS and reducing surface tension. This process had the beneficial effect of increasing the intensity of weak bands against the background and thereby increasing assay sensitivity, particularly for antibodies to ENAs such as Sm, Scl-70, U1 RNP, and Jo-1, where the predominant epitopes are conformational (39).
Other methods.
RNA precipitation assays have been shown to be the most accurate methods for the detection of antibodies to SS-A and SS-B (17) and to have the highest sensitivity and specificity (15). Unfortunately, the performance of these assays is complicated, is labor-intensive, demands a high level of skill, and involves exposure to radioactive materials; therefore, these assays are not widely used. Similarly, immunoprecipitation by using radiolabeled substrates is another method that is able to recognize conformational epitopes with high sensitivity and specificity (29). Immunoprecipitation assays share the same problems as RNA precipitation assays, and their use is restricted to research and reference laboratories. Another method that is no longer widely used is the particle agglutination method, which has largely been superseded by ELISA-based methods (12). Finally, several flow cytometry-based assays are being developed for the detection of anti-ENA antibodies by use of beads that have different diameters and that are coated with individual ENAs. However, these assays are likely to share the same limitations as ELISA-based assays, in particular, a specificity lower than that of gel-based assays.
STRATEGIES FOR ANTI-ENA ANTIBODY TESTING
It is clear that no ideal test exists that is both highly sensitive and highly specific for anti-ENA antibodies. The European Consensus Workshops recommended that ENA testing be performed by two or more methods (35). This recommendation is difficult to implement, particularly in the current era of cost containment. Rather, it may be possible to achieve the same outcome by combining the sensitivity of the ANA test with the specificity of a test for anti-ENA antibodies. We consider CIEP to be the most appropriate method for detecting anti-ENA antibodies because of its high specificity (Table 2). In practice, most laboratories have chosen to use an ELISA-based method for its simplicity, convenience, and rapid turnaround time. This strategy introduces the problem of the lower specificity of the result; some laboratories have tried to resolve this problem by supplementing the ELISA with a more specific method. For example, some laboratories screen by ELISA and confirm positive results by CIEP (Table 4). This strategy may have merit and is in keeping with the recommendations of the European Consensus Workshops. However, the need for a screening ELISA is questionable if an ANA test has already been performed. Certainly, the use of a specific but less sensitive test, such as CIEP, followed by a sensitive but less specific test, such as ELISA, is not recommended for the statistical reasons outlined.
TABLE 4.
Comparison of three possible strategies for combination ENA testing
| Testing strategy | Advantages | Disadvantages |
|---|---|---|
| ELISA followed by CIEP | Rapid turnaround time, especially for negative results | Limited range of anti-ENA antibodies screened by ELISA |
| High sensitivity of ELISA combined with high specificity of CIEP | May miss other anti-ENA antibodies, which would be detected as unidentified precipitin lines by CIEP | |
| Cost of ELISA-based kits | ||
| CIEP followed by CIEP | Inexpensive | Low sensitivity for 52-kDa SS-A, SS-B, and Scl-70 |
| High specificity | ||
| Detects wide range of anti-ENA antibodies in addition to the six most common, i.e., anti-SS-A, anti-SS-B, anti-Sm, anti-U1 RNP, anti-Scl-70, and anti-Jo-1 | Slow turnaround time, especially for difficult samples | |
| Need to maintain a “backup” method for difficult samples | ||
| CIEP followed by ELISA | Slow turnaround time | |
| Low sensitivity of CIEP combined with low specificity of ELISA |
MEANINGFUL ENA TEST REPORTING
Regardless of the testing strategy an individual laboratory selects, it must place significant emphasis on the often-neglected postanalytical phase to improve the clinical utility of anti-ENA antibody testing. To achieve this goal, the laboratory must clearly communicate the significance of a positive or negative test result to the clinician. While clinicians do not necessarily need to know all the technical aspects of assay performance involved in the analytical phase, it is important that they are at least aware of the sensitivity and specificity of the test method used. A more meaningful way to report ENA test results may be to quote likelihood ratios, which the clinician can use in conjunction with a nomogram to determine the posttest probability of diseases in their patients (1). For example, the likelihood ratio for SLE of a positive test for anti-Sm antibodies detected by CIEP is 30 (based on the values in Table 2). If a clinician estimates that a patient has a pretest probability of 10% for SLE, it is possible by using the likelihood ratio and a nomogram to determine that the posttest probability of disease is increased to approximately 75%.
CONCLUSIONS
Rondeel et al. determined that an alarmingly high rate of 66% of 104 clinicians surveyed considered the results of testing for ANA and anti-ENA antibodies to be of no clinical value (21). We believe that the anti-ENA antibody test has been devalued because clinicians are often not familiar with the limitations and appropriate indications for the test, and it is the responsibility of the diagnostic laboratory to address this problem.
Attention to the preanalytical phase can improve a clinician’s understanding of the indications for anti-ENA antibody testing and can lead to more appropriate test ordering. In the analytical phase, the laboratory should ideally validate the assay by establishing cutoff limits for its referral population, rather than relying solely on information supplied by the manufacturer or the published literature. The test used should also be optimized for maximal precision and accuracy. In the postanalytical phase, the laboratory should clearly communicate the significance of the anti-ENA antibody test result to the clinician. This communication can be improved if the clinician is made aware of the limitations of the method used by the laboratory. Careful attention to these factors will help make the anti-ENA antibody test more clinically meaningful.
Acknowledgments
We thank Roger Garsia, Andrew Williams, and Melanie Wong for reviewing the manuscript.
REFERENCES
- 1.Altmann, D. G. 1999. Practical statistics for medical research. Chapman & Hall, Ltd., London, United Kingdom.
- 2.Bizzaro, N., R. Tozzoli, E. Tonutti, A. Piazza, F. Manoni, A. Ghirardello, D. Bassetti, D. Villalta, M. Pradella, and P. Rizzotti. 1998. Variability between methods to determine ANA, anti-dsDNA and anti-ENA autoantibodies: a collaborative study with the biomedical industry. J. Immunol. Methods 219:99–107. [DOI] [PubMed] [Google Scholar]
- 3.Blomberg, S., L. Ronnblom, A. C. Wallgren, B. Nilsson, and A. Karlsson-Parra. 2000. Anti-SSA/Ro antibody determination by enzyme-linked immunosorbent assay as a supplement to standard immunofluorescence in antinuclear antibody screening. Scand. J. Immunol. 51:612–617. [DOI] [PubMed] [Google Scholar]
- 4.Boire, G., F. J. Lopez-Longo, S. Lapointe, and H. A. Menard. 1991. Sera from patients with autoimmune disease recognize conformational determinants on the 60-kd Ro/SS-A protein. Arthritis Rheum. 34:722–730. [DOI] [PubMed] [Google Scholar]
- 5.Bunn, C., and T. Kveder. 1996. Counterimmunoelectrophoresis and immunodiffusion for the detection of antibodies to soluble cellular antigens, p.A3.1–A3.12 In W. J. van Venrooij and R. N. Maini (ed.), Manual of biological markers of disease. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 6.Charles, P. J., W. J. van Venrooij, and R. N. Maini. 1992. The consensus workshops for the detection of autoantibodies to intracellular antigens in rheumatic diseases: 1989–1992. Clin. Exp. Rheumatol. 10:507–511. [PubMed] [Google Scholar]
- 7.Gharavi, A. E., J. L. Chu, and K. B. Elkon. 1988. Autoantibodies to intracellular proteins in human systemic lupus erythematosus are not due to random polyclonal B cell activation. Arthritis Rheum. 31:1337–1345. [DOI] [PubMed] [Google Scholar]
- 8.Gussin, H. A., G. P. Ignat, J. Varga, and M. Teodorescu. 2001. Anti-topoisomerase I (anti-Scl-70) antibodies in patients with systemic lupus erythematosus. Arthritis Rheum. 44:376–383. [DOI] [PubMed] [Google Scholar]
- 9.James, K., A. B. Carpenter, L. Cook, R. Marchand, and R. M. Nakamura. 2000. Development of the antinuclear and anticytoplasmic antibody consensus panel by the Association of Medical Laboratory Immunologists. Clin. Diagn. Lab. Immunol. 7:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keren, D. F., and V. A. Hruszczyk. 1997. Clinical significance and cost-effectiveness of clinical immunology testing, p.1206–1212. In N. R. Rose, E. C. de Macario, J. D. Folds, H. Clifford Lane, and R. M. Nakamura (ed.), Manual of clinical and laboratory immunology, 5th ed. American Society for Microbiology, Washington, D.C.
- 11.Kurata, N., and E. M. Tan. 1976. Identification of antibodies to nuclear acidic antigens by counterimmunoelectrophoresis. Arthritis Rheum. 19:574–580. [DOI] [PubMed] [Google Scholar]
- 12.Lock, R. J., and D. J. Unsworth. 2001. Antibodies to extractable nuclear antigens. Has technological drift affected clinical interpretation? J. Clin. Pathol. 54:187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddison, P. J., R. P. Skinner, P. Vlachoyiannopoulos, D. M. Brennand, and D. Hough. 1985. Antibodies to nRNP, Sm, Ro(SSA) and La(SSB) detected by ELISA: their specificity and inter-relations in connective tissue disease sera. Clin. Exp. Immunol. 62:337–345. [PMC free article] [PubMed] [Google Scholar]
- 14.Manoussakis, M. N., K. L. Garalea, A. G. Tzioufas, and H. M. Moutsopoulos. 1988. Testing for antibodies to ENA and to dsDNA is not indicated in FANA-negative sera. Clin. Rheumatol. 7:465–469. [PubMed] [Google Scholar]
- 15.Manoussakis, M. N., K. G. Kistis, X. Liu, V. Aidinis, A. Guialis, and H. M. Moutsopoulos. 1993. Detection of anti-Ro(SSA) antibodies in autoimmune diseases: comparison of five methods. Br. J. Rheumatol. 32:449–455. [DOI] [PubMed] [Google Scholar]
- 16.Meheus, L., W. J. van Venrooij, A. Wiik, P. J. Charles, A. G. Tzioufas, O. Meyer, G. Steiner, D. Gianola, S. Bombardieri, A. Union, S. De Keyser, E. Veys, and F. De Keyser. 1999. Multicenter validation of recombinant, natural and synthetic antigens used in a single multiparameter assay for the detection of specific anti-nuclear autoantibodies in connective tissue disorders. Clin. Exp. Rheumatol. 17:205–214. [PubMed] [Google Scholar]
- 17.Meilof, J. F., I. Bantjes, J. De Jong, A. P. Van Dam, and R. J. Smeenk. 1990. The detection of anti-Ro/SS-A and anti-La/SS-B antibodies. A comparison of counterimmunoelectrophoresis with immunoblot, ELISA, and RNA-precipitation assays. J. Immunol. Methods 133:215–226. [DOI] [PubMed] [Google Scholar]
- 18.Phan, T. G., W. W. Ng, D. Bird, K. Smithers, V. Wong, K. Gallagher, A. Williams, and S. Adelstein. 2001. High-quality, cost-effective strategy for detection of autoantibodies to extractable nuclear antigens. Clin. Diagn. Lab. Immunol. 8:471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prince, H. E., and W. R. Hogrefe. 1998. Evaluation of a line immunoblot assay for detection of antibodies recognizing extractable nuclear antigens. J. Clin. Lab. Anal. 12:320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid, M. C., M. S. Lachs, and A. R. Feinstein. 1995. Use of methodological standards in diagnostic test research. Getting better but still not good. JAMA 274:645–651. [PubMed] [Google Scholar]
- 21.Rondeel, J. M., W. van Gelder, H. van der Leeden, and R. B. Dinkelaar. 1999. Different strategies in the laboratory diagnosis of autoimmune disease: immunofluorescence, enzyme-linked immunosorbent assay or both? Ann. Clin. Biochem. 36:189–195. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Guerrero, J., R. A. Lew, A. H. Fossel, and P. H. Schur. 1996. Utility of anti-Sm, anti-RNP, anti-Ro/SS-A, and anti-La/SS-B (extractable nuclear antigens) detected by enzyme-linked immunosorbent assay for the diagnosis of systemic lupus erythematosus. Arthritis Rheum. 39:1055–1061. [DOI] [PubMed] [Google Scholar]
- 23.Smolen, J. S., B. Butcher, M. J. Fritzler, T. Gordon, J. Hardin, J. R. Kalden, R. Lahita, R. N. Maini, W. Reeves, M. Reichlin, N. Rothfield, Y. Takasaki, W. J. van Venrooij, and E. M. Tan. 1997. Reference sera for antinuclear antibodies. II. Further definition of antibody specificities in international antinuclear antibody reference sera by immunofluorescence and Western blotting. Arthritis Rheum. 40:413–418. [DOI] [PubMed] [Google Scholar]
- 24.St. Clair, E. W. 1996. Recombinant autoantigens, p.668–676. In J. B. Peter and Y. Shoenfeld (ed.), Autoantibodies. Elsevier Science B. V., Amsterdam, The Netherlands.
- 25.Suarez-Almazor, M. E., L. Gonzalez-Lopez, J. I. Gamez-Nava, E. Belseck, C. J. Kendall, and P. Davis. 1998. Utilization and predictive value of laboratory tests in patients referred to rheumatologists by primary care physicians. J. Rheumatol. 25:1980–1985. [PubMed] [Google Scholar]
- 26.Tan, E. M., A. S. Cohen, J. F. Fries, A. T. Masi, D. J. McShane, N. F. Rothfield, J. G. Schaller, N. Talal, and R. J. Winchester. 1982. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25:1271–1277. [DOI] [PubMed] [Google Scholar]
- 27.Tan, E. M., T. E. Feltkamp, J. S. Smolen, B. Butcher, R. Dawkins, M. J. Fritzler, T. Gordon, J. A. Hardin, J. R. Kalden, R. G. Lahita, R. N. Maini, J. S. McDougal, N. F. Rothfield, R. J. Smeenk, Y. Takasaki, A. Wiik, M. R. Wilson, and J. A. Koziol. 1997. Range of antinuclear antibodies in “healthy- ”individuals. Arthritis Rheum. 40:1601–1611. [DOI] [PubMed] [Google Scholar]
- 28.Tan, E. M., M. J. Fritzler, J. S. McDougal, F. C. McDuffie, R. M. Nakamura, M. Reichlin, C. B. Reimer, G. C. Sharp, P. H. Schur, M. R. Wilson, and R. J. Winchester. 1982. Reference sera for antinuclear antibodies. I. Antibodies to native DNA, Sm, nuclear RNP, and SS-B/La. Arthritis Rheum. 25:1003–1005. [DOI] [PubMed] [Google Scholar]
- 29.Tan, E. M., and C. L. Peebles. 1996. Immunoprecipitation of labelled proteins, p.A9.1–A9.13. In W. J. van Venrooij and R. N. Maini (ed.), Manual of biological markers of disease. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 30.Tan, E. M., J. S. Smolen, J. S. McDougal, B. T. Butcher, D. Conn, R. Dawkins, M. J. Fritzler, T. Gordon, J. A. Hardin, J. R. Kalden, R. G. Lahita, R. N. Maini, N. F. Rothfield, R. Smeenk, Y. Takasaki, W. J. van Venrooij, A. Wiik, M. Wilson, and J. A. Koziol. 1999. A critical evaluation of enzyme immunoassays for detection of antinuclear autoantibodies of defined specificities. I. Precision, sensitivity, and specificity. Arthritis Rheum. 42:455–464. [DOI] [PubMed] [Google Scholar]
- 31.Thomson, K. F., A. Murphy, M. J. Goodfield, and S. A. Misbah. 2001. Is it useful to test for antibodies to extractable nuclear antigens in the presence of a negative antinuclear antibody on Hep-2 cells? J. Clin. Pathol. 54:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulvestad, E., A. Kanestrom, T. M. Madland, E. Thomassen, H. J. Haga, and S. E. Vollset. 2000. Evaluation of diagnostic tests for antinuclear antibodies in rheumatological practice. Scand. J. Immunol. 52:309–315. [DOI] [PubMed] [Google Scholar]
- 33.Van Dam, A. P., H. G. Van den Brink, and R. J. Smeenk. 1990. Technical problems concerning the use of immunoblots for the detection of antinuclear antibodies. J. Immunol. Methods 129:63–70. [DOI] [PubMed] [Google Scholar]
- 34.Van Duijnhoven, H. L., F. J. Van De Warenburg, R. J. Willems, and A. M. Ermens. 1999. A comparison of ELISA assays as routine diagnostic test for detection of autoantibodies against extractable nuclear antigens. Clin. Biochem. 32:179–183. [DOI] [PubMed] [Google Scholar]
- 35.van Venrooij, W. J., P. Charles, and R. N. Maini. 1991. The consensus workshops for the detection of autoantibodies to intracellular antigens in rheumatic diseases. J. Immunol. Methods 140:181–189. [DOI] [PubMed] [Google Scholar]
- 36.Wahren, M., P. Tengner, I. Gunnarsson, I. Lundberg, E. Hedfors, N. R. Ringertz, and I. Pettersson. 1998. Ro/SS-A and La/SS-B antibody level variation in patients with Sjogren’s syndrome and systemic lupus erythematosus. J. Autoimmun. 11:29–38. [DOI] [PubMed] [Google Scholar]
- 37.Walravens, M. J., H. Vanherrewegen, F. Lacquet, G. Godefridis, G. Korevits, E. Stevens, G. Marien, and G. Molenberghs. 1997. Counterimmunoelectrophoresis with serum prediffusion: an improved method for the detection and identification of antibodies against extractable nuclear and cytoplasmic antigens. J. Immunol. Methods 201:89–98. [DOI] [PubMed] [Google Scholar]
- 38.Yiannaki, E. E., A. G. Tzioufas, M. Bachmann, J. Hantoumi, V. Tsikaris, M. Sakarellos-Daitsiotis, C. Sakarellos, and H. M. Moutsopoulos. 1998. The value of synthetic linear epitope analogues of La/SSB for the detection of autoantibodies to La/SSB; specificity, sensitivity and comparison of methods. Clin. Exp. Immunol. 112:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zampieri, S., A. Ghirardello, A. Doria, M. Tonello, R. Bendo, K. Rossini, and P. F. Gambari. 2000. The use of Tween 20 in immunoblotting assays for the detection of autoantibodies in connective tissue diseases. J. Immunol. Methods 239:1–11. [DOI] [PubMed] [Google Scholar]