Abstract
1. Age changes in spontaneous and evoked transmitter release, in receptor number and in ultrastructure at the neuromuscular junction were studied in the CBF-1 mouse strain, which stays physically active and relatively free of organ pathology into advanced age.
2. Spontaneous miniature end-plate potentials (m.e.p.p.s) were recorded in the following young (8-12 months) and old (29-33 months) mouse muscles: extensor digitorum longus (e.d.l.), soleus (sol.), gluteus maximus (g.m.), diaphragm (diaph.) and extensor digitorum communis (e.d.c.).
3. M.e.p.p. amplitudes were unchanged with age in four muscle groups despite increases in input resistance (in e.d.l., sol. and g.m.). M.e.p.p. amplitude in old diaph. increased 54% with no change in input resistance. Bimodal distributions of m.e.p.p. amplitudes were observed in 6-23% of muscle fibres but were not more prevalent in old mice. There was little or no change in resting membrane potential with age.
4. Numbers of junctional acetylcholine receptors (measured with 125I-α-bungarotoxin) were the same in all young and old muscles except e.d.l., where a 30% decrease was noted. Extrajunctional receptors and other indicators of denervation (decreases in resting potential, twitch tension or muscle fibre diameter) were absent or minimal.
5. M.e.p.p. frequency decreased in e.d.l., sol. and e.d.c. but not in g.m. or diaph. There was no correlated change in the cholinesterase-positive end-plate area.
6. It is concluded that m.e.p.p. amplitude is maintained in old muscles by a combination of compensatory changes. The decline in m.e.p.p. frequency varies between muscle groups and is independent of the length of the motoneurone axon or level of innervation.
7. Evoked end-plate potentials (e.p.p.s) were recorded in e.d.l., sol. and diaph. from young (11-13 months) and old (29-30 or 34-35 months) male CBF-1 mice in curarized preparations stimulated at 2 or 20 Hz. The amplitude of the initial e.p.p. of the trains was increased by 122% in old e.d.l. and 93% in old sol., and plateau e.p.p. amplitudes were also increased by about 100% (e.d.l.) and 67% (sol.). This, combined with the absence of change in m.e.p.p. amplitude with age, suggests that the number of quanta released per nerve impulse was increased. In diaph. there was no change with age.
8. In all muscle groups, the threshold for initiation of the muscle action potential was unchanged with age. Thus, the relative safety factor of transmission was increased in curarized old e.d.l. and sol. (but not diaph.).
9. Depression of the indirect twitch in solutions with a decreased calcium: magnesium ratio was also used as a relative measure of synaptic efficacy. Old sol. and e.d.l. but not diaph. muscles showed less depression of indirect twitch amplitude than did young muscle under these conditions.
10. In cut-fibre preparations of sol. and diaph. stimulated at 20 Hz, there was no age-dependent difference in e.p.p. amplitude, in directly measured quantal content, or in curare sensitivity. In view of other results, these findings require careful interpretation.
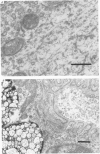
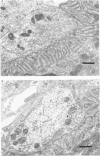
11. Ultrastructural morphometry was carried out in e.d.l. The nerve terminals in old (30 and 34 months) e.d.l. muscles exhibited pronounced loss of synaptic vesicles. In 34-month animals, decreased nerve terminal area and post-synaptic folds devoid of nerve terminals were often observed. Since no evidence of denervation was found by physiological criteria, it is concluded that in 34-month mice, nerve terminals withdraw from some synaptic gutters but do not abandon any junction entirely. The large presynaptic ultrastructural changes contrast with the physiological data showing no deficit and even increases in transmitter release. Therefore, under these conditions, these profound structural changes are either not functionally significant or are well compensated.
Full text
PDF
























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Thesleff S. A comparative study of membrane properties of innervated and chronically denervated fast and slow skeletal muscles of the rat. Acta Physiol Scand. 1968 Aug;73(4):471–480. doi: 10.1111/j.1365-201x.1968.tb10886.x. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. The effect of calcium ions on the secretion of quanta evoked by an impulse at nerve terminal release sites. J Gen Physiol. 1979 Oct;74(4):429–456. doi: 10.1085/jgp.74.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia M. R., Harris J. B., Johnson M. A. Morphology and physiology of skeletal muscle in aging rodents. Muscle Nerve. 1979 May-Jun;2(3):202–212. doi: 10.1002/mus.880020308. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P., Mauro A. Depletion of vesicles from frog neuromuscular junctions by prolonged tetanic stimulation. J Cell Biol. 1972 Jul;54(1):30–38. doi: 10.1083/jcb.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Courtney J., Steinbach J. H. Age changes in neuromuscular junction morphology and acetylcholine receptor distribution on rat skeletal muscle fibres. J Physiol. 1981 Nov;320:435–447. doi: 10.1113/jphysiol.1981.sp013960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim M. A., Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol. 1982 Aug;11(4):641–656. doi: 10.1007/BF01262429. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Frank E., Gautvik K., Sommerschild H. Cholinergic receptors at denervated mammalian motor end-plates. Acta Physiol Scand. 1975 Sep;95(1):66–76. doi: 10.1111/j.1748-1716.1975.tb10026.x. [DOI] [PubMed] [Google Scholar]
- Fujisawa K. Some observations on the skeletal musculature of aged rats-III. Abnormalities of terminal axons found in motor end-plates. Exp Gerontol. 1976;11(1-2):43–47. doi: 10.1016/0531-5565(76)90010-3. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. An analysis of the relationship between the current and potential generated by a quantum of acetylcholine in muscle fibers without transverse tubules. J Membr Biol. 1973;12(3):247–272. doi: 10.1007/BF01870004. [DOI] [PubMed] [Google Scholar]
- Gertler R. A., Robbins N. Differences in neuromuscular transmission in red and white muscles. Brain Res. 1978 Feb 17;142(1):160–164. doi: 10.1016/0006-8993(78)90186-5. [DOI] [PubMed] [Google Scholar]
- Glavinović M. I. Voltage clamping of unparalysed cut rat diaphragm for study of transmitter release. J Physiol. 1979 May;290(2):467–480. doi: 10.1113/jphysiol.1979.sp012784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Carmignoto G., Facci L., Finesso M. Motor nerve sprouting induced by ganglioside treatment. Possible implications for gangliosides on neuronal growth. Brain Res. 1980 Sep 15;197(1):236–241. doi: 10.1016/0006-8993(80)90451-5. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Herrera A. A. Physiological regulation of synaptic effectiveness at frog neuromuscular junctions. J Physiol. 1980 Oct;307:301–317. doi: 10.1113/jphysiol.1980.sp013436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Hanzlíková V., Vysokocil F. Age changes in cross striated muscle of the rat. J Physiol. 1971 Jul;216(2):331–343. doi: 10.1113/jphysiol.1971.sp009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Ribchester R. R. The relationship between end-plate size and transmitter release in normal and dystrophic muscles of the mouse. J Physiol. 1979 Nov;296:245–265. doi: 10.1113/jphysiol.1979.sp013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennekens F. G., Tomlinson B. E., Walton J. N. Histochemical aspects of five limb muscles in old age. An autopsy study. J Neurol Sci. 1971 Nov;14(3):259–276. doi: 10.1016/0022-510x(71)90216-4. [DOI] [PubMed] [Google Scholar]
- Jones S. F., Kwanbunbumpen S. The effects of nerve stimulation and hemicholinium on synaptic vesicles at the mammalian euromuscular junction. J Physiol. 1970 Mar;207(1):31–50. doi: 10.1113/jphysiol.1970.sp009046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpati G., Engel W. K. "Type grouping" in skeletal muscles after experimental reinnervation. Neurology. 1968 May;18(5):447–455. doi: 10.1212/wnl.18.5.447. [DOI] [PubMed] [Google Scholar]
- Kelly S. S. The effect of age on neuromuscular transmission. J Physiol. 1978 Jan;274:51–62. doi: 10.1113/jphysiol.1978.sp012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y. Slowing with age of the rate of slow axonal flow in bifurcating axons of rat dorsal root ganglion cells. Brain Res. 1980 Feb 10;183(2):477–480. doi: 10.1016/0006-8993(80)90484-9. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J. Complex end-plate potentials at the regenerating neuromuscular junction of the rat. Exp Neurol. 1975 Dec;49(3):629–638. doi: 10.1016/0014-4886(75)90048-5. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The statistics of transmitter release at chemical synapses. Int Rev Physiol. 1978;17:49–117. [PubMed] [Google Scholar]
- Meiss D. E., Govind C. K. Heterogeneity of excitatory synapses at the ends of single muscle fibers in lobster, Homarus americanus. J Neurobiol. 1980 Jul;11(4):381–395. doi: 10.1002/neu.480110405. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B., Griffin J. W. Effects of aging on nerve sprouting and regeneration. Exp Neurol. 1980 Oct;70(1):65–82. doi: 10.1016/0014-4886(80)90006-0. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N., Olek A., Kelly S. S., Takach P., Christopher M. Quantitative study of motor endplates in muscle fibres dissociated by a simple procedure. Proc R Soc Lond B Biol Sci. 1980 Oct 15;209(1177):555–562. doi: 10.1098/rspb.1980.0112. [DOI] [PubMed] [Google Scholar]
- Smith D. O. Reduced capabilities of synaptic transmission in aged rats. Exp Neurol. 1979 Dec;66(3):650–666. doi: 10.1016/0014-4886(79)90210-3. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Gutmann E. Spontaneous transmitter release from nerve endings and contractile properties in the soleus and diaphragm muscles of senile rats. Experientia. 1972 Mar 15;28(3):280–281. doi: 10.1007/BF01928688. [DOI] [PubMed] [Google Scholar]