Abstract
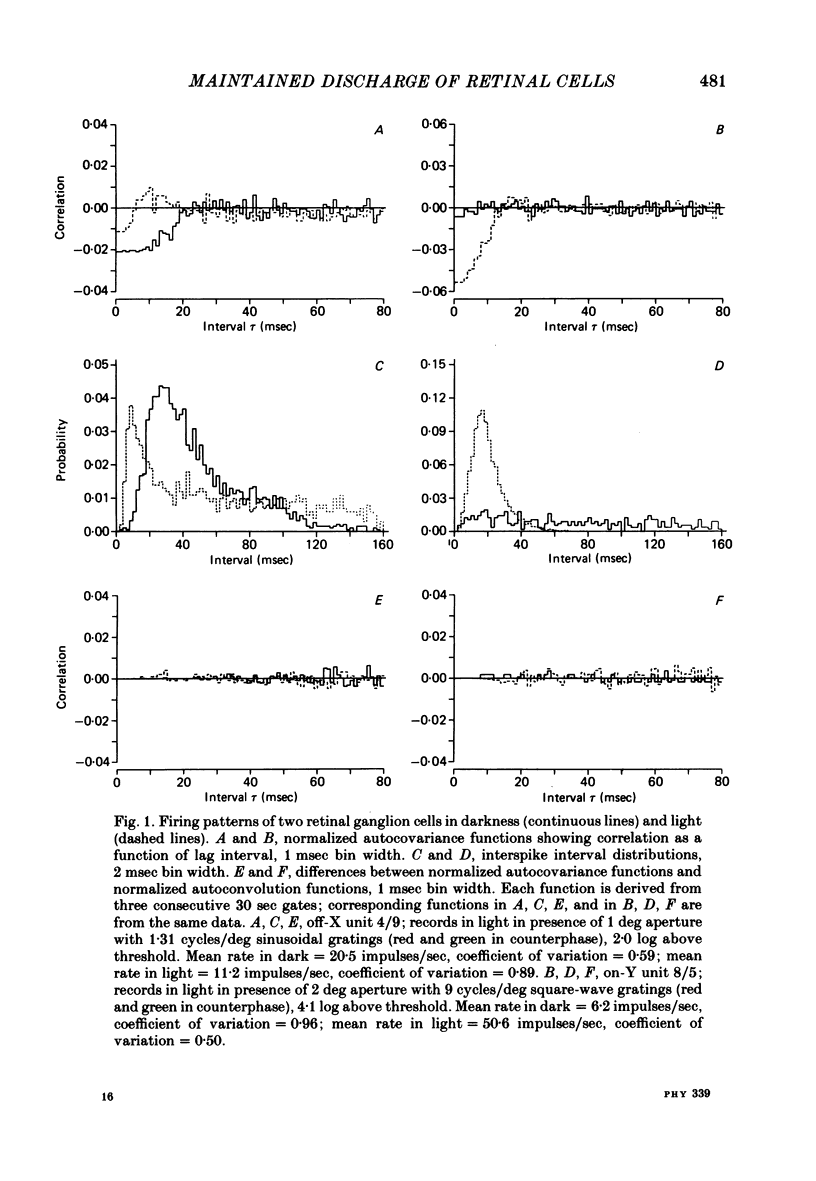
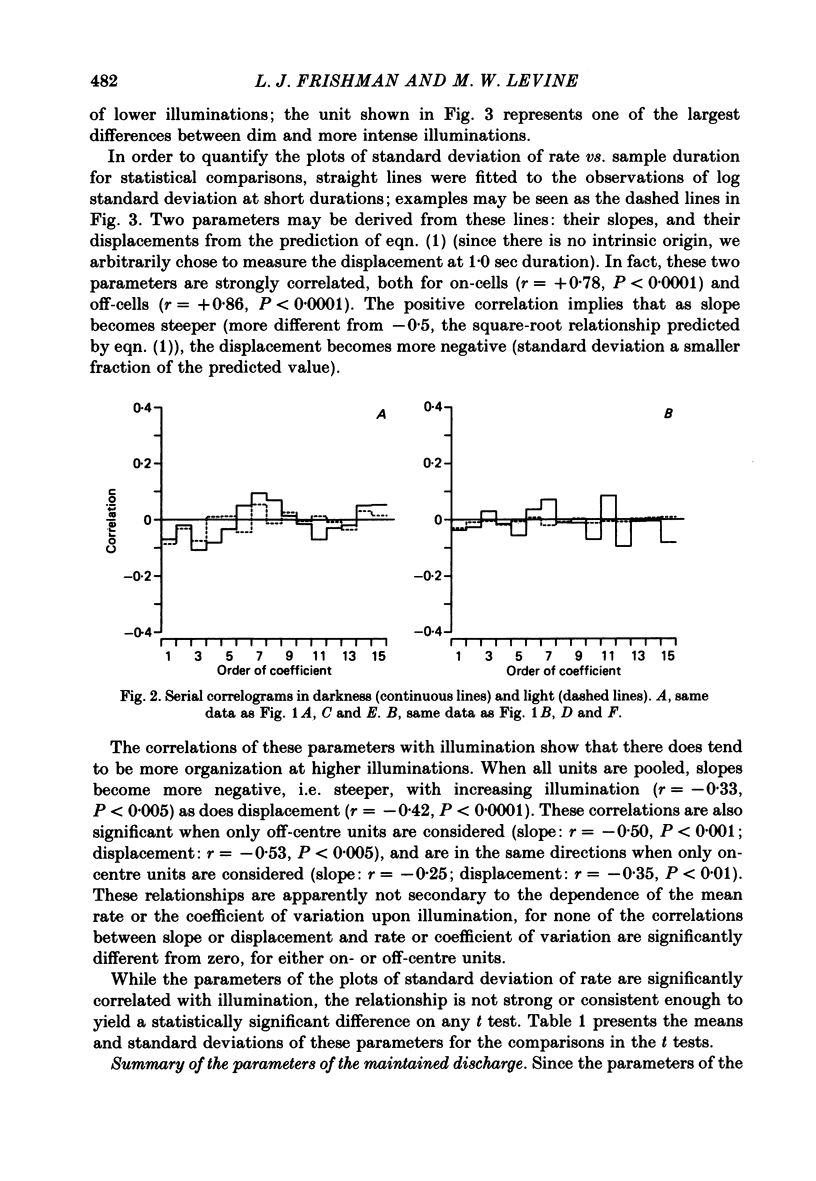
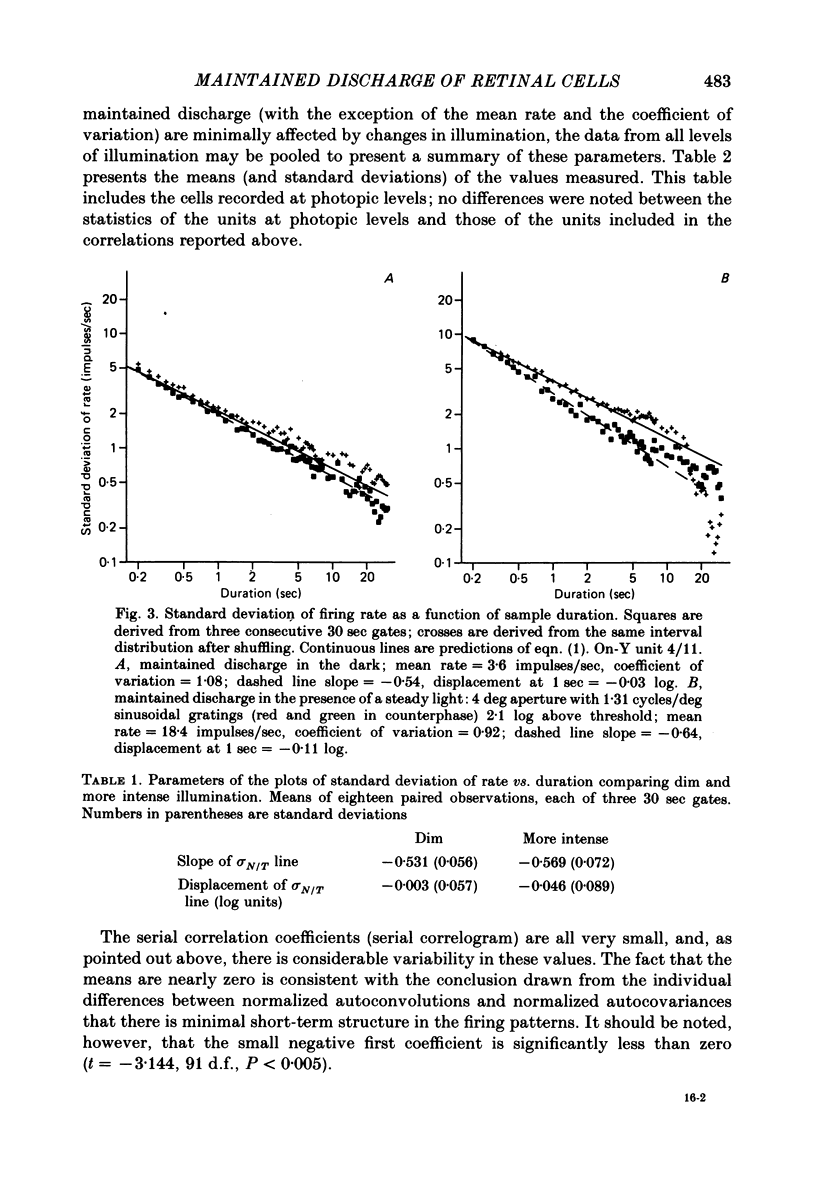
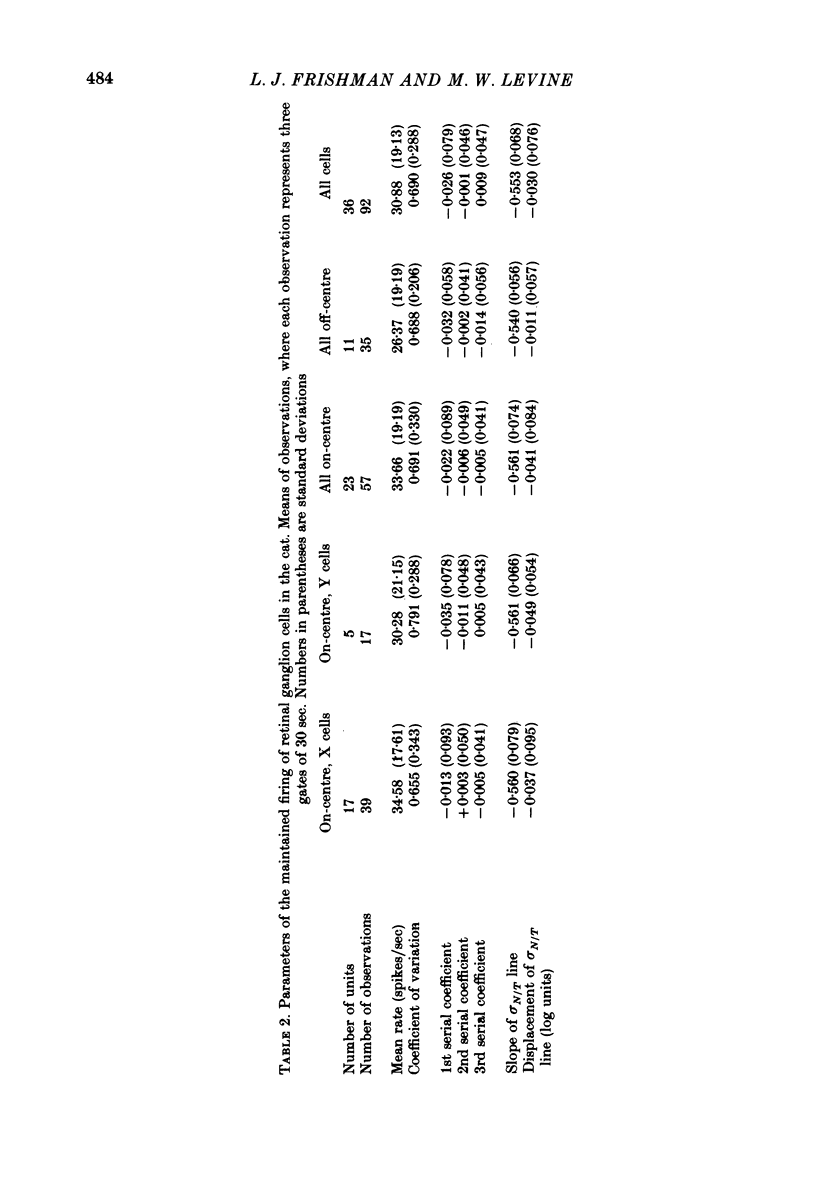
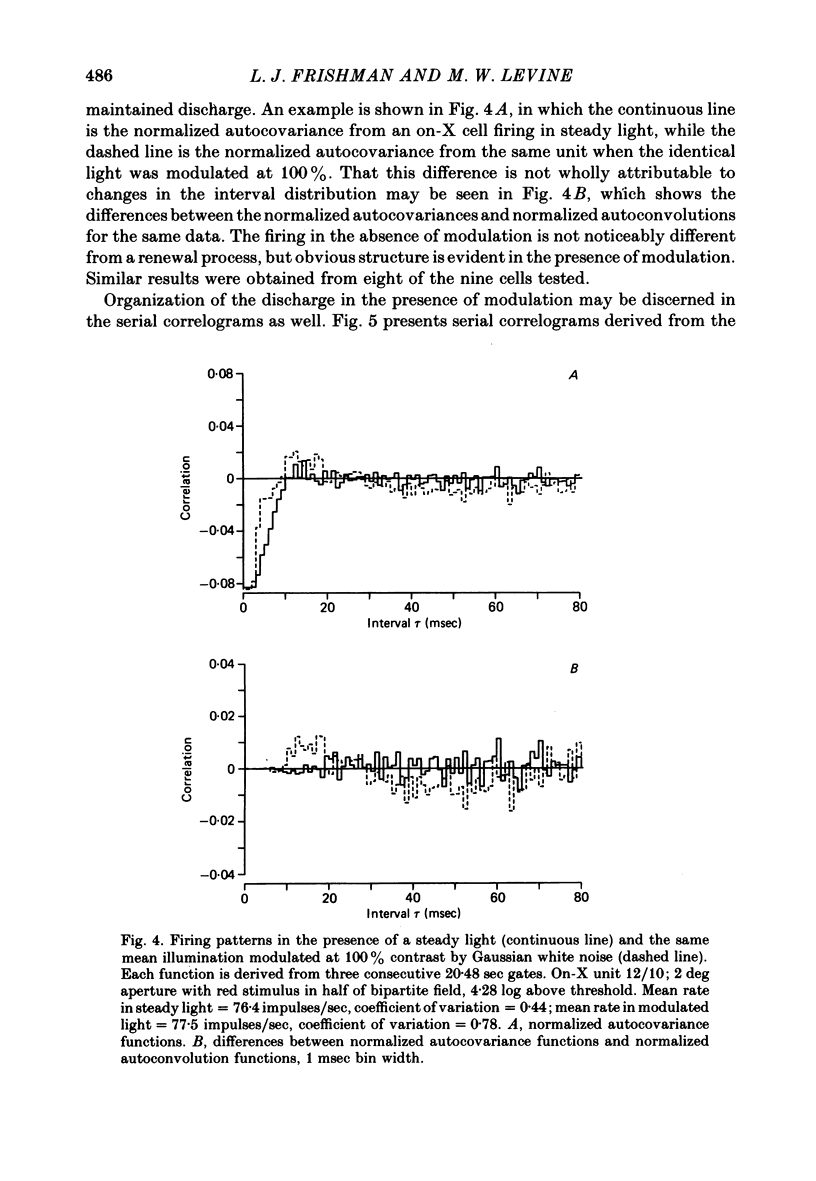
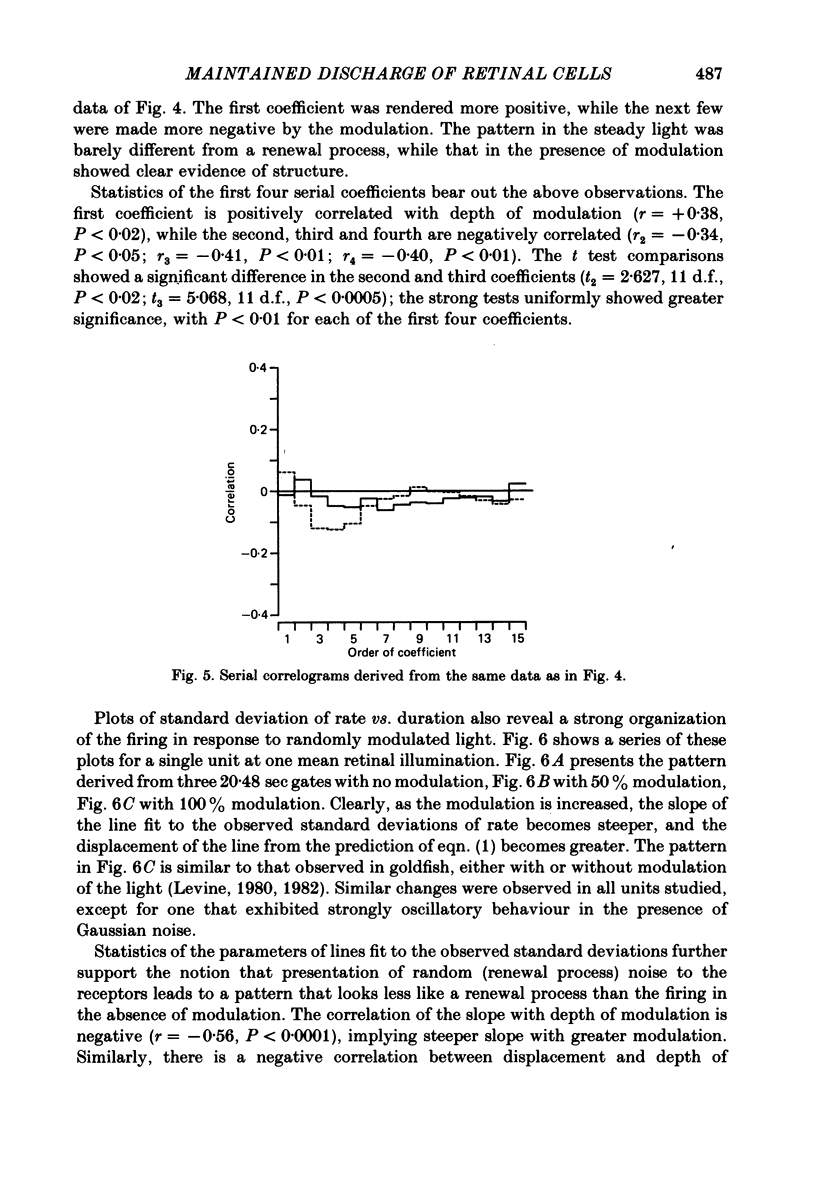
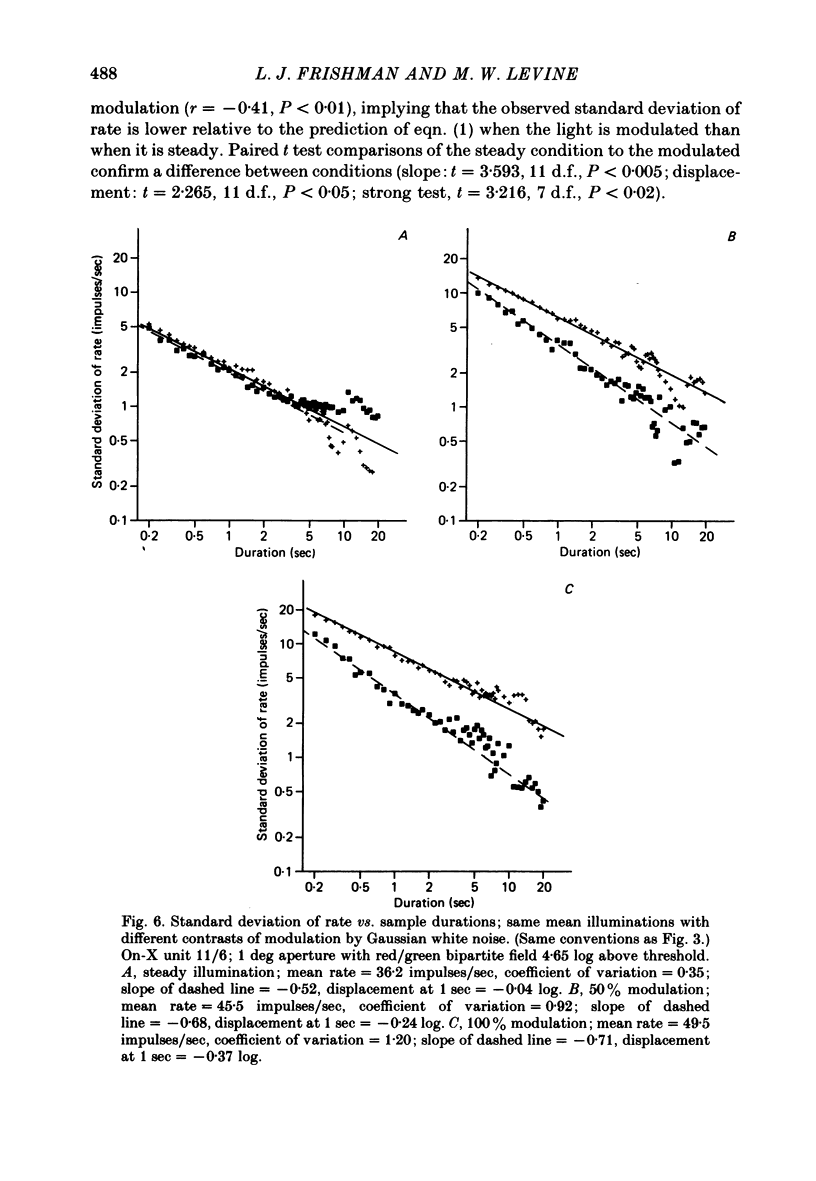
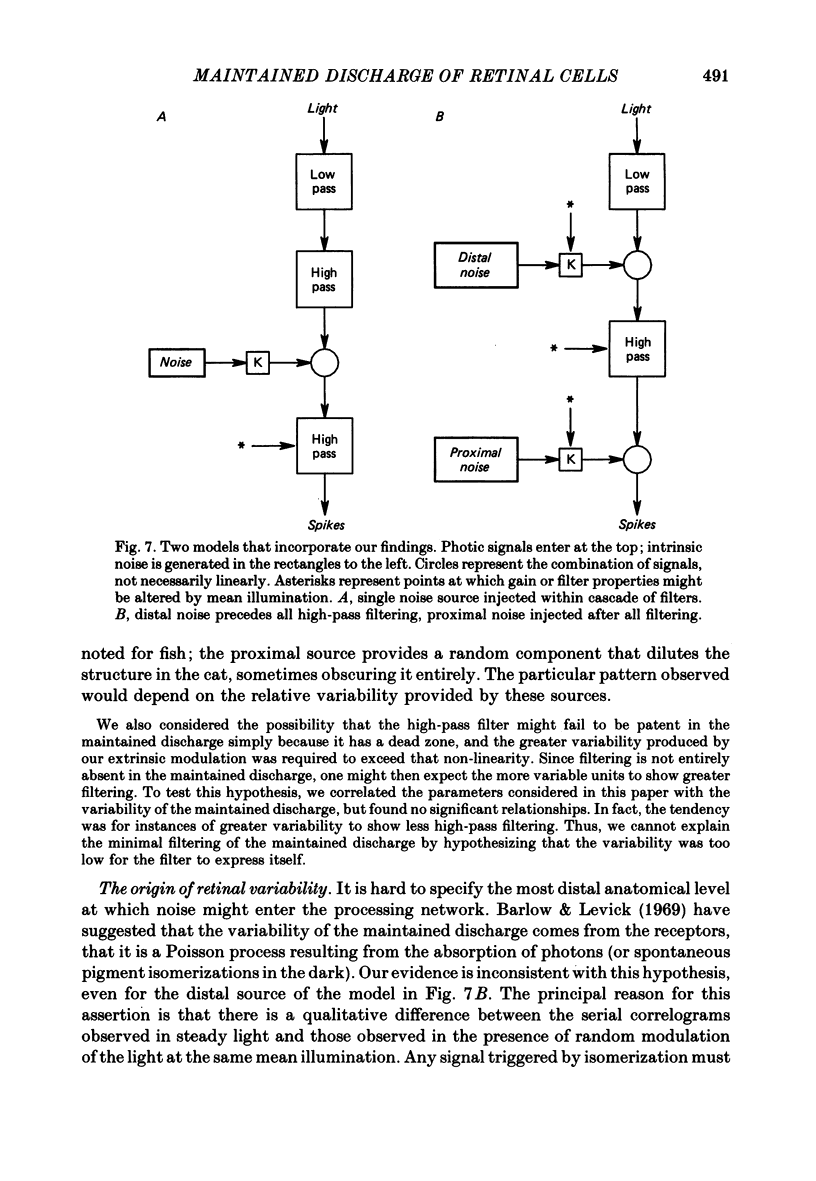
Action potentials were recorded from single fibres in the optic tracts of anaesthetized cats. Continuous records were obtained at various levels of scotopic and mesopic retinal illumination. In some cases, the light intensity was modulated by a pseudorandom Gaussian white-noise signal. The maintained discharge of on-centre neurones increased while the maintained discharge of off-centre neurons decreased with increased illumination of the receptive field centre. For both cell types, the coefficient of variation declined with increased rate of discharge. There was minimal short-term dependency in the firing patterns, and it was unaffected by the level of retinal illumination. Virtually all of the structure revealed by the normalized autocovariance functions could be attributed to the shape of the interval distributions. The first few coefficients of the serial correlogram were slightly negative. The magnitude of this negativity was not related to illumination. Long-term dependency in the firing pattern was also quite small; the standard deviations of the mean rate in samples of about 1 sec duration were only slightly less than would be predicted from the interval distributions. This dependency tended to increase at higher retinal illuminations. Neural discharges elicited by Gaussian modulation of the light were strikingly different from those elicited by steady light. Modulation caused the first coefficient of the serial correlogram to become more positive, while the next several coefficients became more negative. A corresponding pattern could be seen in the normalized autocovariance functions, and in the differences between the normalized autocovariance and normalized autoconvolution. Long-term dependency also increased dramatically, such that the standard deviations of mean rate were about 60% of what would be expected given the interval distributions observed. These results place a number of constraints upon the ways in which intrinsic noise in the retina may enter the visual processing network. Two alternative models consistent with the data are presented.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Copenhagen D. R. Different postsynaptic events in two types of retinal bipolar cell. Nature. 1980 Nov 6;288(5786):84–86. doi: 10.1038/288084a0. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R., Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res. 1971;Suppl 3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Baron W. S., Boynton R. M. Response of primate cones to sinusoidally flickering homochromatic stimuli. J Physiol. 1975 Mar;246(2):311–331. doi: 10.1113/jphysiol.1975.sp010892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. Reconstruction of the electrical responses of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):759–791. doi: 10.1113/jphysiol.1974.sp010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Goldstick T. K., Linsenmeier R. A. The contrast sensitivity of cat retinal ganglion cells at reduced oxygen tensions. J Physiol. 1980 Jul;304:59–81. doi: 10.1113/jphysiol.1980.sp013310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz G., Lennie P. Cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):273–296. doi: 10.1113/jphysiol.1977.sp011902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinston W. J., Vadas M. A., Bishop P. O. Multiple projection of the visual field to the medical portion of the dorsal lateral geniculate nucleus and the adjacent nuclei of the thalamus of the cat. J Comp Neurol. 1969 Jul;136(3):295–315. doi: 10.1002/cne.901360304. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. The relation between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol. 1976 Dec;263(2):257–286. doi: 10.1113/jphysiol.1976.sp011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levine M. W. Firing rate of a retinal neuron are not predictable from interspike interval statistics. Biophys J. 1980 Apr;30(1):9–25. doi: 10.1016/S0006-3495(80)85073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. W. Retinal processing of intrinsic ad extrinsic noise. J Neurophysiol. 1982 Oct;48(4):992–1010. doi: 10.1152/jn.1982.48.4.992. [DOI] [PubMed] [Google Scholar]
- Nygaard R. W., Frumkes T. E. Calibration of the retinal illuminance provided by Maxwellian views. Vision Res. 1982;22(4):433–434. doi: 10.1016/0042-6989(82)90189-4. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J. 1967 Jul;7(4):391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond S. A. Effects of nerve impulses on threshold of frog sciatic nerve fibres. J Physiol. 1979 May;290(2):273–303. doi: 10.1113/jphysiol.1979.sp012771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W. Maintained activity of cat retinal ganglion cells. J Neurophysiol. 1967 Sep;30(5):1043–1071. doi: 10.1152/jn.1967.30.5.1043. [DOI] [PubMed] [Google Scholar]
- Sanderson A. C., Kozak W. M., Calvert T. W. Distribution coding in the visual pathway. Biophys J. 1973 Mar;13(3):218–244. doi: 10.1016/S0006-3495(73)85982-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Yamamoto M., Nakahama H. Variability of inter-spike intervals of cat's on-center optic tract fibres activated by steady light spot: a comparative study on X- and Y-fibres. Exp Brain Res. 1976 Jan 26;24:285–293. doi: 10.1007/BF00235016. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Voltage noise observed in rods of the turtle retina. J Physiol. 1977 Nov;272(2):217–246. doi: 10.1113/jphysiol.1977.sp012042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. How the contrast gain control modifies the frequency responses of cat retinal ganglion cells. J Physiol. 1981 Sep;318:161–179. doi: 10.1113/jphysiol.1981.sp013856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., Fukuda Y. Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. J Neurophysiol. 1974 Jul;37(4):722–748. doi: 10.1152/jn.1974.37.4.722. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Coles J. A. Rod response to sinusoidally flickering light. Vision Res. 1975 Aug-Sep;15:981–983. doi: 10.1016/0042-6989(75)90240-0. [DOI] [PubMed] [Google Scholar]
- Zeevi Y. Y., Mangoubi S. S. Noise suppression in photoreceptors and its relevance to incremental intensity thresholds. J Opt Soc Am. 1978 Dec;68(12):1772–1776. doi: 10.1364/josa.68.001772. [DOI] [PubMed] [Google Scholar]


