Abstract
Epidemiological studies have implicated periodontal disease (PD) as a risk factor for the development of cardiovascular disease (CVD). These studies addressed the premise that local infection may perturb the levels of systemic inflammatory mediators, thereby promoting mechanisms of atherosclerosis. Levels of inflammatory mediators in the sera of subjects with only PD, only CVD, both diseases, or neither condition were compared. Subjects were assessed for levels of C-reactive protein (CRP), serum amyloid A (SAA), ceruloplasmin, α1-acid-glycoprotein (AAG), α1-antichymotrypsin (ACT), and the soluble cellular adhesion molecules sICAM-1 and sVCAM by enzyme-linked immunoabsorbent and/or radial immunodiffusion assays. CRP levels in subjects with either condition alone were elevated twofold above subjects with neither disease, whereas a threefold increase was noted in subjects with both diseases (P = 0.0389). Statistically significant increases in SAA and ACT were noted in subjects with both conditions compared to those with one or neither condition (P = 0.0162 and 0.0408, respectively). Ceruloplasmin levels were increased in subjects with only CVD (P = 0.0001). Increases in sVCAM levels were noted in all subjects with CVD (P = 0.0054). No differences in sICAM levels were noted among subject groups. A trend toward higher levels of AAG was noted in subjects with both conditions and for ACT in subjects with only PD. Immunohistochemical examination of endarterectomy specimens of carotid arteries from subjects with atherosclerosis documented SAA and CRP deposition in association with atheromatous lesions. The data support the hypothesis that localized persistent infection may influence systemic levels of inflammatory mediators. Changes in inflammatory mediator levels potentially impact inflammation-associated atherosclerotic processes.
Hypercholesterolemia, lipid metabolism imbalances, hypertension, age, gender, body mass index, diabetes mellitus, homocysteine, stress, and smoking are widely accepted as “classic” risk factors for development of cardiovascular disease (CVD). However, it has also become clear that the contribution of these factors cannot be supported in 25 to 50% of subjects who develop CVD and cerebrovascular disease (39, 53). These observations have intensified initiatives to identify additional risk factors for the development of atherosclerosis and vascular ischemic diseases. Recent evidence indicates that the development of CVD correlates with elevated levels of C-reactive protein (CRP) (25, 30, 35, 45, 54, 55) and soluble cellular adhesion molecules (CAM) (27, 48, 56, 76) which are produced in response to cellular damage. These observations have led to a reexamination of one of the earliest theories regarding the etiology of atherosclerosis and coronary heart disease (CHD): inflammatory processes which may arise in association with infection (39, 42).
In the mid-1800s, Virchow drew attention to the presence of two distinct lesions along arterial vessel walls: the classical “fatty streak” and a second lesion which was characterized as a fatty deposit developing at sites exhibiting characteristic inflammation-associated features (66). Other studies in experimental animal models demonstrated the development of atherosclerotic lesions in response to bacterial infection, especially in the presence of a high-cholesterol diet (12-14). Studies in animal models appear to support an association between infection with Chlamydia pneumoniae and the incidence of CHD (5, 46, 73). Recent studies also suggested that infection of Apo e−/e+ mice with Porphyromonas gingivalis, a periodontal pathogen, can enhance coronary atheroma formation and calcification (34). The presence of DNA from organisms such as C. pneumoniae (9, 16, 33, 46), Helicobacter pylori (9, 47, 49), and several periodontal pathogens (24) has recently been documented in atheromatous lesions of carotid arterial walls. Included among these were the following periodontal pathogens: Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Bacteroides forsythus, and Prevotella intermedia. A higher incidence of nonhemorrhagic, but not hemorrhagic, stroke was also reported for subjects with periodontal disease (PD) in a 21-year study of 9,962 adults (71). A growing number of studies suggest that the presence of PD is at least a moderate risk factor for the development of heart (1, 3, 4, 12, 15, 19, 28, 41) or cerebrovascular disease (21, 71). The development of CVD and PD appears to overlap with regard to several important risk factors. Included among these are elevations in systemic levels of proinflammatory acute-phase reactants such as CRP (14, 59, 72) and fibrinogen (37, 72). Molecular studies delineating the role of acute-phase proteins (APP), cytokines, and CAM in inflammatory processes have provided insight into their potential to contribute to atherogenic processes. Production of APP by the liver is induced by proinflammatory cytokines, such as interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), and IL-6, which are released by host cells in response to microbial stimuli (2, 61). Localized cellular production of APP, such as serum amyloid A (SAA), at sites of tissue injury has also been documented (38, 74). Whereas substantial increases in APP levels occur during acute infection, systemic levels of these molecules appear to return to baseline or near-baseline levels during chronic processes in the host (2). Since may of these molecules are present at baseline at high levels, small fluctuations in their concentrations may appear inconsequential. However, recent data suggest that even a modest elevation in the levels of CRP may be associated with development of CHD (55). Deposition of APP, including CRP, SAA, and complement, has been documented in atheromatous lesions along vessel walls (23, 31, 52, 68, 70, 74). These data emphasize the necessity for examination of the relationship between APP levels, persistent periodontal infection, and atherogenesis.
Although ICAM and VCAM are expressed on the cell surface, it has been postulated that soluble forms of these molecules (sICAM and sVCAM) are thought to arise following enzymatic cleavage from the cell surface (67). The physiologic functions of sCAM molecules remain unclear, but it has been postulated that the molecules have an immunomodulatory role in chronic or subclinical inflammation (67). In vitro evidence suggests that soluble forms of CAM may interfere with the interaction of leukocytes and endothelial cells (7). Because altered levels of sICAM and sVCAM have been documented in the presence of several inflammation-associated conditions, these molecules have potential utility as biomarkers for inflammatory and immune disorders (18, 32, 40, 57). To date, there is little information available concerning the systemic levels of these molecules in patients with PD, and the association of increased levels of sCAM in circulation as a risk factor for coronary and vascular disease remains controversial (11, 27, 48, 56).
The purpose of the present study was to compare levels of acute-phase molecules in subjects with manifest CVD and/or PD or neither disease. CRP levels were measured because increases in CRP levels have been reported in the presence of PD or CVD alone; CRP colocalizes with complement (31) and may be involved in monocyte recruitment to the arterial wall (64). SAA levels were measured because of the ability of SAA to influence lipid metabolism (23, 75) and the lack of information regarding systemic levels of the molecule in the presence of PD. Ceruloplasmin levels were evaluated because of the ability of this molecule to function as a superoxide dismutase with the capacity to buffer cellular damage due to lipoprotein oxidation, which may contribute to atherogenic processes (60). α1-Acid-glycoprotein (AAG) levels were evaluated because of their immunomodulatory potential, especially with respect to lymphocytes and the levels of proinflammatory cytokines (65). Although local increases in levels of α1-antichymotrypsin (ACT) have been reported in PD relative to its function as a serine proteinase of cathepsin G of neutrophils (63), no information concerning its systemic levels in the presence of PD is available. ACT has no apparent role in development of CVD, and therefore no increase in this APP was expected in subjects with CHD. In addition, immunohistological examination of endarterectomy specimens of carotid arteries from subjects with atherosclerosis was undertaken to document the presence of SAA and CRP in association with atheromatous lesions.
This study was undertaken to determine the extent to which periodontitis influences systemic levels of inflammatory mediators suspected to potentiate atherogenic processes. The hypothesis underlying these studies is that infectious processes involved in PD stimulate both local and systemic inflammatory responses in the host. Further, inflammatory mediators have the inherent potential to promote atherogenic processes.
MATERIALS AND METHODS
Study design and sampling.
The levels of APP and CAM were examined in serum samples obtained from participants of the Erie County Periodontal Epidemiological Study (22). Blood was collected from subjects at the time of clinical evaluation. Sera were harvested within 2 h of blood collection, promptly divided into aliquots, and stored at −80°C until testing. The periodontal status of the subjects was established by trained and calibrated examiners, who performed standardized examinations, including gingival assessments (bleeding on probing), calculus index, and measurement of pocket depth and clinical attachment levels on six sites of all teeth present in the dentition. Subjects were classified as having PD if they exhibited a mean attachment loss of ≥4 mm, whereas periodontally healthy subjects exhibited a mean attachment loss of <2 mm. In addition, all subjects included in the study completed a detailed medical-dental questionnaire providing information on their history of systemic diseases, use of medications, history of habits (smoking, alcohol consumption, etc.), stress and distress levels, and coping mechanisms (22). Subjects who responded affirmatively in the questionnaire to a history of myocardial infarction, stroke, or angina were classified as positive for CVD. Accordingly, 80 subjects meeting these entry criteria were included in the study. The accuracy of the self-reported medical history data was assessed as described previously by Ho et al. (26). The overall agreement between two visits was substantial (κ = 0.80). The agreement for systemic diseases, including CVD and events, was κ = 0.90. After analysis of the levels of the APP and CAM in serum, data were classified into groups according to the subjects' clinical histories in order to facilitate comparative statistical analysis. Collection of blood and subject history was done with the approval and within the guidelines of the Internal Review Board for experimental studies involving human subjects of the State University of New York at Buffalo.
Statistical analysis.
Subjects were grouped by disease category as shown in Table 1. Descriptive statistics (mean and standard error [SE]) were calculated for each parameter measured for each subject group. Group comparisons were performed by analysis of covariance controlling for smoking. Post hoc testing was also used to identify groups exhibiting statistically significant differences. In addition, correlation between parameters was determined by linear regression analysis. The percentages of subjects from each group with levels outside the normal range reported in the literature and by manufacturers of the assay kits were also calculated in order to compare patterns of altered levels among the four groups.
TABLE 1.
Periodontal criteriaa
| Subject group | Mean ± SEb
|
||
|---|---|---|---|
| Bone loss (mm) | Attachment loss (mm) | Bleeding on probing | |
| PD− CVD− | 1.88 ± 0.15 | 1.61 ± 0.08 | 0.364 ± 0.053 |
| PD+ CVD− | 6.01 ± 0.43∗ | 5.40 ± 0.23∗∗ | 0.502 ± 0.071∗∗∗ |
| PD− CVD+ | 2.36 ± 0.21 | 1.72 ± 0.04 | 0.308 ± 0.048 |
| PD+ CVD+ | 5.55 ± 0.33∗ | 4.85 ± 0.22∗∗ | 0.485 ± 0.072∗∗∗ |
The periodontal criteria were determined as follows. CVD+, the medical history revealed past or present history of one or more of the following: angina, myocardial infarction, and/or stroke. Periodontal status was defined by attachment loss as follows: <2 mm, no PD (PD−); ≥4 mm, PD (PD+). Statistical analyses included analysis of variance (ANOVA) of mean values ± the SE for patients in each group and ANOVA to test the factors and interaction. The P values for the latter were derived by using the nonparametric Kruskal-Wallis test.
∗, the ANOVA results for bone loss show a highly significant PD effect (P < 0.0001), with no CVD effect (P = 0.97) and a nonsignificant interaction term (P = 0.134). ∗∗, the ANOVA results for attachment loss show a highly significant PD effect (P < 0.0001) and a trend indicating interaction (P = 0.053). ∗∗∗, in comparing bleeding-on-probing data among the groups, there is a significant difference by PD (P = 0.025); there is no difference by CVD or interaction of CVD with PD.
Inflammatory mediator quantitation: methodology.
Sera were coded and analyzed without prior knowledge of the clinical condition of the subjects. Coded sera were analyzed in duplicate for the following APP and CAM by using commercially available kits. For APP, CRP was analyzed by enzyme-linked immunosorbent assay (ELISA; Hemagen, Waltham, Mass.) at a working range of 1 to 50 μg/ml and by radial immunodiffusion (RID) assay (The Binding Site, San Diego, Calif.) by using Nanorid kits, SAA was evaluated by ELISA (Hemagen) at a working range of 1 to 100 μg/ml, and ceruloplasmin, AAG, and ACT were analyzed by RID assay using Nanorid Kits (The Binding Site). The soluble CAM sICAM-1 and sVCAM were evaluated by ELISA (R&D, Minneapolis, Minn.).
Immunohistochemical evaluation of carotid specimens.
Endarterectomy specimens from (i) 14 patients with atherosclerotic blockages of the carotid arteries obtained at surgery and (ii) two coronary arteries from patients with no history of atherosclerosis or CHD who died suddenly were collected in Michel's transport media (Beutner Laboratories, Buffalo, N.Y.). Arterial specimens were soaked in sterile phosphate-buffered saline (PBS) for 30 min before being processed for examination by immunofluorescence microscopy to document the deposition of SAA and/or CRP along vessel walls. Specimens were immersed in liquid nitrogen and consecutively cut with a cryocut microtome into 4-μm-thick sections. Cross-sections were taken where developing plaque lesions and proximal regions of macroscopically normal vessel intima were evident along vessel walls. Specimens were tested for the deposition of CRP and SAA by using the methodology for indirect immunofluorescence described by Beutner et al. (6). Briefly, to detect the presence of SAA, sections were incubated consecutively with antibodies to SAA raised in rats (Biosource International, Camarillo, Calif.) and phycoerythrin-labeled goat anti-rat immunoglobulin G (IgG, Jackson Laboratories, West Grove, Pa.) with washes in PBS (pH 7.4) between incubations. To detect CRP deposition, sections were incubated consecutively with antibodies to CRP raised in chickens (Accurate Antibodies, Westbury, N.Y.) and fluorescein isothiocyanate (FITC)-labeled rabbit anti-chicken IgG (Jackson Laboratories), with washes in PBS following the incubations. Specimens were overlaid with coverslips to which mounting medium containing 25% diazo-bicyclo-2,22-octane (DABCO) had been applied (The Binding Site). Coverslips were sealed before being viewed with an epi-illuminated fluorescence microscope (Zeiss) or with a Nikon Optiphot 2 and the Bio-Rad MRC 1024 Confocal Imaging system. Controls included replacement of the primary antibodies with PBS, normal serum, or unrelated specific antibody to soy trypsin inhibitor (R&D). The carotid specimens that were examined by immunofluorescence as described for the deposition of CRP and SAA were randomly chosen from the set of endarterectomy specimens also tested by Haraszthy et al. for the presence of periodontal pathogens by PCR (24).
RESULTS
Comparative studies of inflammatory mediators. (i) APP.
For each subject, the levels of APP were measured. After testing, subjects were grouped according to the presence of CVD and PD, and the mean level (±SE) of each APP was calculated. Results were analyzed by ANCOVA for the following cofactors: age, smoking, gender, sex, rheumatoid arthritis, and diabetic status. Only smoking affected the statistical significance in the present study, and thus all data presented here were adjusted for smoking. In addition, the percentages of subjects within each group with levels outside the established normal range for each parameter measured were calculated. Percentage calculations made it possible to compare patterns of altered levels among the four groups. The results of these analyses are summarized in Tables 2, 3, and 4 and Fig. 1. Significantly elevated levels of CRP, SAA, and AAG were noted in subjects from the combined disease group. These data suggested that there were two populations of subjects with PD: those with elevated levels of CRP, SAA, and AAG who presented with CVD and those whose systemic levels of these molecules fell within the normal to moderately elevated range who did not present with CVD.
TABLE 2.
Mean levels of CRP and SAA in serum as measured by ELISA
| Group no. | Subject group | No. of subjects | Mean level (μg/ml)a in serum ± SE
|
|
|---|---|---|---|---|
| CRP | SAA | |||
| 1 | PD− CVD− | 26 | 1.68 ± 1.42 | 15.92 ± 3.36 |
| 2 | PD+ CVD− | 20 | 2.40 ± 1.89 | 11.15 ± 4.58 |
| 3 | PD− CVD+ | 18 | 3.35 ± 1.56 | 19.44 ± 3.70 |
| 4 | PD+ CVD+ | 16 | 8.58 ± 1.74 | 27.75 ± 4.13 |
Overall P of <0.05 for CRP values (P = 0.0162) and SAA values (P = 0.389). Specific post hoc comparisons were as follows for CRP: P = 0.0029 (groups 1 and 4), 0.0192 (groups 2 and 4), and 0.0282 (groups 3 and 4). Specific post hoc comparisons were as follows for SAA: P = 0.0294 (groups 2 and 3) and 0.0090 (groups 2 and 4). A summary table of APP levels was determined by ELISA analysis. The data were analyzed using by ANCOVA and reported as the group mean ± SE, with an adjustment for smoking. Normal levels as reported by the kit manufacturers were as follows: CRP range, 0.6 to 1.9 μg/ml; and SAA range, 1.0 to 10.0 μg/ml (reported mean, 3.0 to 4.0 μg/ml).
TABLE 3.
Mean levels of AAG, ACT, and ceruloplasmin as measured by RID assay
| Group no. | Subject group | No. of subjects | Mean level (mg/liter)a in serum ± SE
|
||
|---|---|---|---|---|---|
| AAG | ACT | Ceruloplasmin | |||
| 1 | PD− CVD− | 26 | 943.7 ± 110.8 | 563.4 ± 44.2 | 308.7 ± 54.3 |
| 2 | PD+ CVD− | 20 | 854.6 ± 134.8 | 698.5 ± 51.3 | 311.7 ± 59.4 |
| 3 | PD− CVD+ | 18 | 872.5 ± 134.4 | 529.4 ± 56.8 | 731.0 ± 59.4 |
| 4 | PD+ CVD+ | 16 | 1365.8 ± 145.8 | 589.4 ± 56.7 | 338.1 ± 67.5 |
Overall P values were as follows: AAG, 0.0408 (statistically significant); ACT, 0.1273; and ceruloplasmin, 0.0001 (statistically significant). Specific P values were as follows for AAG: P = 0.0273 (groups 1 and 4), 0.0109 (groups 2 and 4), and 0.0158 (groups 3 and 4). Specific P values were as follows for ceruloplasmin: P = 0.0001 (groups 1 and 3, 2 and 3, and 3 and 4). The mean and SE of AAG, ACT, and ceruloplasmin levels of subjects in the four groups were determined by RID assay. The data were analyzed by using ANCOVA and reported as the group mean ± the SE, with an adjustment for smoking. Normal levels as reported by the kit manufacturers were as follows: AAG range, 512 to 869 mg/liter (mean, 683 mg/liter), ACT range, 356 to 596 mg/liter (mean, 476 mg/liter) and ceruloplasmin range, 230 to 370 mg/liter (mean, 292 mg/liter).
TABLE 4.
Mean levels of soluble CAM in serum as measured by ELISA
| Group no. | Subject group | No. of subjects | Mean level (ng/ml)a in serum ± SE
|
|
|---|---|---|---|---|
| sVCAM | sICAM | |||
| 1 | PD− CVD− | 26 | 896.5 ± 130.0 | 289.1 ± 24.8 |
| 2 | PD+ CVD− | 20 | 953.0 ± 188.9 | 315.9 ± 36.0 |
| 3 | PD− CVD+ | 18 | 1,538.1 ± 152.5 | 333.3 ± 29.1 |
| 4 | PD+ CVD+ | 16 | 1,334.9 ± 166.3 | 293.0 ± 31.7 |
Overall P values were as follows: sVCAM, 0.0054 (P < 0.05); and sICAM, 0.6495. For post hoc comparisons for sVCAM, P = 0.0015 (groups 1 and 3), 0.0399 (groups 1 and 4), and 0.0257 (groups 2 and 3). A summary table of sCAM levels was determined by ELISA analysis. The data were analyzed by using ANCOVA and reported as the group mean ± SE, with an adjustment for smoking. Normal levels as reported by the kit manufacturers were as follows: sVCAM range, 395 to 714 ng/ml (mean, 553 ng/ml); and sICAM range, 114.7 to 306.4 ng/ml (mean, 210.6 ng/ml).
FIG. 1.
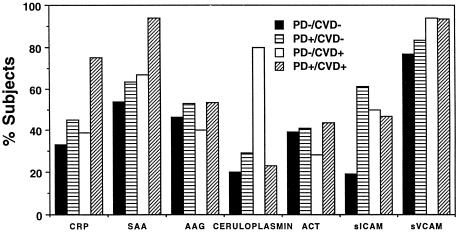
Percentage of subjects with elevated APP and soluble CAM. Solid bars represent PD− CVD− subjects, horizontal lines represent PD+ CVD− subjects, open bars represent PD− CVD+ subjects, and diagonal lines represent PD+ CVD+ subjects. Normal levels as reported by the kit manufacturers were as follows: CRP range, 0.6 to 1.9 μg/ml; SAA range, 1.0 to 10.0 μg/ml (reported mean, 3.0 to 4.0 μg/ml); AAG range, 512 to 869 mg/liter (mean, 683 mg/liter); ACT range, 356 to 596 mg/liter (mean, 476 mg/liter); ceruloplasmin range, 230 to 370 mg/liter (mean, 292 mg/liter); sVCAM range, 395 to 714 ng/ml (mean, 553 ng/ml); and sICAM range, 114.7 to 306.4 ng/ml (mean, 210.6 ng/ml).
(a) CRP.
Mean values (±SE) of CRP levels measured by ELISA in subjects with only PD (2.40 ± 1.89 μg/ml) or only CVD (3.35 ± 1.56 μg/ml) were increased compared to subjects with neither disease (1.68 ± 1.42 μg/ml). A statistically significant increase was noted in mean CRP levels in subjects with both conditions compared to all other groups (mean value of 8.58 ± 1.74 μg/ml, P ≤ 0.028) (Table 2). In addition to determination of CRP levels by ELISA, levels of CRP in serum were confirmed by RID assay. The levels of CRP in individual sera obtained by the two methods were comparable, with an overall correlation coefficient for the two assays of 0.88 as determined by regression analysis. The percentage of subjects with elevated levels of CRP compared to the reported normal range for CRP (0.6 to 1.9 μg/ml) was 33% in subjects with only PD, 45% in subjects with only CVD, and 39% in subjects with neither condition, whereas 75% of subjects with both diseases exhibited levels outside the normal range (P = 0.0565) (Fig. 1). These data suggest that PD subjects with CVD represent a subgroup of all subjects with CVD: i.e., subjects with both diseases exhibited higher mean levels of CRP in serum and percentages relative to the normal range compared to subjects with CVD and no PD.
(b) SAA.
Mean levels (±SE) of SAA were elevated above the reported normal range (1 to 10 μg/ml) in all subject groups. Mean levels of SAA in subjects with only PD (11.15 ± 4.13 μg/ml) were significantly lower compared to subjects with only heart disease (19.44 ± 3.7 μg/ml, P ≤ 0.029) and those with both conditions whose SAA levels were markedly elevated (27.75 ± 4.13 μg/ml; P = 0.0090) (Table 2), whereas the SAA levels were above the normal range in 54% of subjects with neither disease, 63% of subjects with only PD, and 67% of subjects with only CVD; 94% of PD+ CVD+ subjects exhibited elevated levels of SAA in serum (P = 0.0615) (Fig. 1). These data suggest that PD subjects with CVD represent a subgroup of all subjects with CVD: i.e., subjects with both conditions exhibit higher mean levels of SAA and percentages above the normal range compared to those with only CVD and no PD.
(c) AAG.
Mean levels (±SE) of AAG in subjects with both CVD and PD were significantly higher (1,365.8± 145.8 μg/ml) than in subjects with neither disease (943.7 ± 110.8 μg/ml), with only PD (854.6 ± 134.8 μg/ml), or with only CVD (872.5 ± 134.4 μg/ml) (P ≤ 0.0273) (Table 3). These data suggest that subjects with PD and CVD represent a subgroup of all subjects with CVD: i.e., subjects with both diseases exhibited higher mean levels of AAG compared to subjects with CVD and no PD (P = 0.0158). No differences in the percentages of subjects from each group with levels outside the reported normal range (512 to 869 μg/ml) were detected; between 40 and 53% of the subjects from each group exhibited levels above the normal range for this APP (Fig. 1).
(d) ACT.
No statistically significant differences in levels of ACT were detected among any of the groups (Table 3). Also, no differences among the four groups were seen in the percentage of subjects with levels elevated above the reported normal range (356 to 596 μg/ml) for AAG, with 39 to 44% of the subjects among the four groups exhibiting elevated levels (Fig. 1).
(e) ceruloplasmin.
Mean levels (±SE) of ceruloplasmin in subjects with only CVD were twofold higher than mean levels measured in all other subjects groups (P = 0.0001) (Table 3). Eighty percent of the subjects with only CVD had elevated levels of ceruloplasmin relative to its reported normal range (230 to 370 μg/ml), whereas 20% of the subjects with neither condition, 23% with both conditions, and 29% with only PD exhibited such elevations (P = 0.0012) (Fig. 1). These data indicate that the levels of ceruloplasmin are elevated in subjects with CVD. However, in subjects with both CVD and PD, elevated ceruloplasmin levels were not observed.
(ii) CAM. (a) sVCAM.
A statistically significant increase in the levels of sVCAM in serum was noted in subjects with CVD regardless of the periodontal status compared to subjects with PD only or with neither condition (P ≤ 0.0399). Subjects with only PD exhibited significantly lower mean values (±SE) for sVCAM levels than did subjects with CVD alone (P = 0.0257) (Table 4). Notably, all groups had high percentages of elevated sVCAM levels relative to the reported normal range (395 to 714 ng/ml). The highest percentages were noted in the presence of CVD (94% of subjects with CVD only and 93% of subjects with both diseases) compared to 83% of subjects with PD only and 77% of subjects with neither condition (Fig. 1).
(b) sICAM.
No statistically significant differences in the mean levels (±SE) of sICAM were measured among the four groups by ELISA (Table 4). However, whereas only 19% of the subjects with neither disease had levels higher than the reported normal range (115 to 306 ng/ml), subjects with only PD (61%), only CVD (50%), or both conditions (47%) had significantly higher levels of sICAM (P = 0.0319) (Fig. 1).
Immunofluorescence studies.
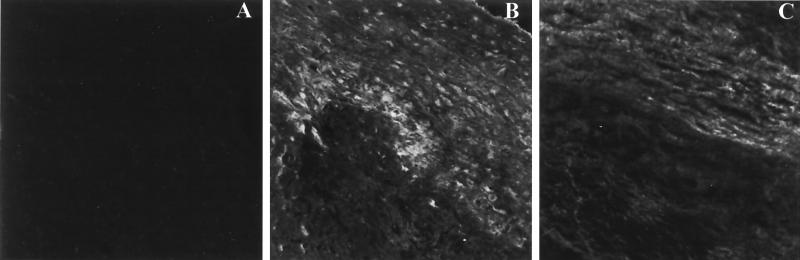
Of the 14 carotid specimens evaluated, 12 exhibited deposition of SAA and 13 exhibited CRP deposition in association with plaque lesions, although the extent varied among patients. CRP deposits were seen along the tunica intima and in association with cells located in the tunica media and adventitia. SAA deposits were visible mainly in the tunica adventitia along elastic fibers in an interrupted linear pattern. Photomicrographs obtained by confocal microscopy (Fig. 2) of carotid specimens from patients with atherosclerosis reveal deposits of SAA and CRP along the arterial walls.
FIG. 2.
Confocal images of immunofluorescence microscopy studies carried out on 4-μm-thick cross-sections of a carotid artery from a subject with atherosclerosis made with a cryocut microtome. The images document the deposition of APP along the vessel walls of carotid arteries obtained from patients who underwent endarterectomies. The vessels tested positive for the presence of DNA from periodontal pathogens (24). (A) Negative control. The vessel was incubated sequentially with normal rabbit serum and FITC-labeled anti-chicken IgG. Similarly, the vessels incubated sequentially with normal rabbit serum and FITC-labeled anti-rabbit IgG or incubations with secondary antibodies alone did not exhibit staining (data not shown). (B) CRP. The vessel was incubated sequentially with antibodies to CRP raised in chickens and fluorochrome-labeled anti-chicken IgG antibodies. (C) SAA. The vessel was incubated sequentially with antibodies to SAA raised in rabbits and fluorochrome-labeled anti-rabbit IgG antibodies. Deposition of SAA and CRP was observed along the intimal wall of the vessels. The extent of deposition varied among patients and was proportional to the severity of the atheromatous lesion formed along the vessel wall. The photomicrographs were obtained by using a Nikon epifluorescence microscope and the Bio-Rad MRC-024 three-channel laser scanning confocal imaging system equipped with a krypton argon laser operating at 514 nm at 100% power. The images were stored at 47 × 47 pixels (512 × 512 μ, full field) with a pixel size of 0.093 μ (final magnification, ×340).
The coronary arteries obtained from two 40-year-old subjects who died suddenly but exhibited no atherosclerotic lesions or heart disease were also assessed for the presence of CRP or SAA deposits. No deposition of CRP or SAA was noted along the arterial walls of these two subjects.
DISCUSSION
Periodontal pathogens have recently been identified in atherosclerotic lesions (10, 20, 24). In addition to perturbing inflammation locally, periodontal pathogens possess mechanisms which may impact the systemic balance of inflammatory mediators. It has been proposed that, in addition to localized inflammatory events which may influence systemic responses, low levels of bacteremia may contribute to systemic increases in inflammatory and immune responses (17, 36). Because of the proximity of the pathogens to vascular beds, bacterial and proinflammatory host molecules may also access the circulation through the epithelial layer whose integrity may be compromised by infectious and inflammatory processes (4). In addition, the invasive capacity of some strains of Fusobacterium nucleatum (Y. W. Han et al., J. Dent. Res. 77:666, 1998, abstr. 3197), A. actinomycetemcomitans (44), and P. gingivalis (13, 50) has recently been demonstrated in vitro. Theoretically, this capacity could potentiate both systemic entry and survival of the organism in the hostile host environment. Studies of carotid vessels obtained after endarterectomy by immunofluorescence microscopy recently demonstrated the presence of components along carotid arterial walls which reacted with periodontal pathogen-specific antisera (20). The deposition of APP shown in Fig. 2 may be a manifestation of host response to presence of bacterial components in the vessel wall and could influence inflammatory processes that exacerbate atherogenesis.
Studies reported here were designed to compare systemic levels of inflammation-associated molecules with intrinsic functional potential to interact with other atherogenic risk factors in subjects with or without PD and/or that manifest CVD. The most noteworthy observations emerging from the present study are summarized below.
Statistically significant elevations of mean levels of CRP were noted in subjects with both PD and CVD compared to all other groups. Clinical studies have previously shown that CRP levels were elevated in subjects with CVD (25, 30, 35, 45, 54, 55). Similarly, increases in systemic levels of CRP were noted in the presence of PD alone (14, 59, 72). In the present study CRP levels were increased threefold in the presence of both conditions compared to subjects exhibiting only PD, only CVD, or neither condition. These data indicate that subjects with both conditions may be a subset of all patients with CVD. Further, deposition of CRP and SAA were documented along carotid arterial walls in association with atheromatous plaque in several of the same endarterectomy carotid specimens that previously tested positive for the presence of DNA from periodontal pathogens by PCR (24).
Other reports in the literature provide evidence supporting a role for inflammation-associated atherogenesis. Several studies have demonstrated complement deposition in atheromatous lesions colocalizing with CRP (31, 52). Zhu et al. recently demonstrated that the total pathogen burden was a significant predictor of levels of CRP and postulated that increased levels of CRP contribute to the development of CVD through modulation of the inflammatory responses (77). Wiedermann et al also demonstrated increased levels of CRP in subjects with high levels of endotoxemia (associated with chronic or recurrent infection), and further demonstrated that these individuals were at increased risk for development of carotid atherosclerosis (69).
A trend toward elevated levels of SAA was seen in the presence of either PD or CVD alone and achieved significance when both conditions were present. Casl et al. reported that patients who experienced myocardial infarction following admission to the hospital exhibited mean levels of SAA of 45 mg/ml prior to the myocardial infarction (8). These levels are only slightly higher than the mean levels detected in the present study for subjects with both PD and CVD. Further, monitoring of SAA levels postinfarction proved to be the most accurate indicator for the incidence of complications and death in these patients (8). Thus, elevated SAA levels may be a clinically useful indicator of cardiovascular risk, especially in the presence of PD. The present results remain to be validated in a larger population base and merit further investigation. The deposition of SAA demonstrated in the atheromatous plaque of carotid specimens obtained by endarterectomy in the present study may promote atherogenic processes through several proposed mechanisms, including alteration of lipid transport (2, 23, 75) and lymphocyte chemotaxis and adhesion (62).
Significant elevations of ceruloplasmin levels were noted only in subjects with CVD alone compared to all other groups. Unexpectedly, elevations in levels of ceruloplasmin were not observed in the presence of both PD and CVD. Why ceruloplasmin levels would not be elevated in the presence of both diseases is presently unclear and requires further investigation. In a recent study of 1,000 subjects with confirmed atherosclerosis, Mayr et al. observed that the subset of subjects exhibiting statistically significant increases in the levels of antibodies to C. pneumoniae, H. pylori, Escherichia coli, and lipopolysaccharides and a higher frequency of chronic infections also exhibited statistically significant increases in levels of ceruloplasmin (43).
Marginally significant elevations of AAG levels were noted in subjects with both conditions. Measurements of total AAG were made in the present study. These data may benefit from a more comprehensive analysis through evaluation of levels of AAG glycoforms associated with myocardial infarction. Myocardial infarction-associated AAG isoforms exhibit the ability to modulate cytokines, including IL-1, IL-6, and TNF (29, 58). Immunomodulation appears to be mediated mainly through the binding of specific glycoforms to subpopulations of monocytes and lymphocytes, resulting in the elaboration of cytokines by the cells. The cytokines, in turn, modulate T-cell proliferation and function (29, 58). Because of the immunomodulatory potential associated with the carbohydrates of the AAG glycoforms, these molecules, or pseudo-glycoproteins mimicking their activity, represent molecules with pharmacological potential for modulation or prevention of atherogenesis-promoting processes (51, 58).
Data in the literature concerning tissue-associated and circulating levels of VCAM and ICAM in the presence of coronary and vascular disease continue to be controversial. In the present study, sVCAM levels showed statistically significant increases in subjects with CVD, regardless of the PD status. In contrast, the levels of sICAM showed no significant differences among the four groups. However, the levels of sICAM were significantly elevated in the presence of either disease compared to subjects with neither disease. Studies in larger populations will be necessary to validate the present findings. In addition, a comparative study of assay kits used in previous studies to measure the levels of these molecules in serum within the same patient population may be needed to assess whether the controversial findings are a function of the assay used in the study. If results obtained in the present study are reproducible in a larger population, monitoring sVCAM levels in patients with PD may be a potentially useful clinical parameter for identifying covert atherogenic processes in high-risk individuals.
Locally increased levels of ACT in the oral cavity have been demonstrated in subjects with PD in response to neutrophil-produced cathepsin G (63). In the present study, ACT levels were measured to determine whether systemic elevations in the molecule could be detected in the presence of PD. Although a trend toward higher levels of the molecule was observed in the presence of PD, differences in levels of the molecule among the four groups did not achieve statistical significance. Since neutrophils do not appear to play a major role in the development of atherosclerosis, the lack of increased levels in the presence of manifest CVD was not surprising.
Several limitations of the present study bear mention. Assessment for presence of CVD in the present retrospective study was based on self-reported manifest CVD. The possibility that some of the subjects among the four groups in the present study may have had silent underlying CVD cannot be ruled out. The presence of “silent” CVD may account for detection of levels of inflammation-associated molecules outside the normal range in a subgroup of subjects who reported no history of CVD. Despite this shortcoming, distinct differences in the levels of CRP, SAA, AGG, sVCAM, and ceruloplasmin were still observed among the four groups defined by clinical history in this retrospective study. Assessment of the covert CVD presence in prospective studies of a large population controlling for additional risk factors is warranted to corroborate these preliminary findings. A further shortcoming of the present study was the inability to obtain information from all subjects undergoing endartarectomy regarding a past history of PD. A prospective study of subjects with diagnosed PD involving monitoring the development of atherogenic processes is proposed to address this shortcoming. However, the presence of DNA from periodontal pathogens detected in carotid specimens obtained by endarterectomy (24) provides indirect evidence of exposure to PD-causing organisms. In the current study, ANCOVA analyses were undertaken, with adjustments for smoking. Attempts made to adjust for other underlying inflammatory diseases, including arthritis and diabetes, which might also influence levels of acute-phase reactants, did not impact the statistical outcomes. However, because of the relatively small sizes of the groups, the numbers of subjects with these underlying conditions may have been too small to affect intragroup statistical outcomes.
Currently, a larger prospective study that takes into account the shortcomings identified in this retrospective pilot study is under way to determine whether the preliminary observations made here can be validated in a larger population. If mechanistic studies confirm that inflammation-associated molecules in the host may contribute to atherogenic processes, intervention to regulate systemic levels of these molecules may represent an alternative approach to modulating atherogenic processes. In summary, results obtained by retrospective analysis in the present study further support the growing body of evidence indicating a role for infectious and inflammatory processes in the promotion of atherogenesis.
Acknowledgments
This work was supported by U.S. Public Health Service grants DEO 7926, DEO 4898, and DE12085 from the NIDCR.
We acknowledge Paul Bronson for excellent technical assistance. We also thank Robert Summers and Alan Stonebreaker for providing expertise and help in obtaining the confocal images.
REFERENCES
- 1.Arbes, S. J., Jr., G. D. Slade, and J. D. Beck. 1999. Association between extent of periodontal attachment loss and self reported history of heart attack: an analysis of NHANES III data. J. Dent. Res. 78:1777-1782. [DOI] [PubMed] [Google Scholar]
- 2.Baumann, H., and I. Gauldie. 1994. The acute-phase response. Immunol. Today 15:596.. [DOI] [PubMed] [Google Scholar]
- 3.Beck, J., R. Garcia, G. Heiss, P. S. Vokonas, and S. Offenbacher. 1996. Periodontal disease and cardiovascular disease. J. Periodontol. 67:1123-1137. [DOI] [PubMed] [Google Scholar]
- 4.Beck, J. D., J. Pankow, H. A. Tyroler, and S. Offenbacher. 1999. Dental infections and atherosclerosis. Am. Heart J. 138:S528-S533. [DOI] [PubMed] [Google Scholar]
- 5.Benson, R. L., K. G. Smith, and H. Semenov. 1931. Experimental arteritis and arteriosclerosis associated with streptococcal innoculations. Arch. Pathol. 12:924-940. [Google Scholar]
- 6.Beutner, E. H., R. J. Nisengard, and B. Albini. 1983. Defined immunofluorescence and related cytochemical methods. Ann. N. Y. Acad. Sci. 420:9-12. [PubMed]
- 7.Blann, A. D., and G. Y. Lip. 2000. Cell adhesion molecules in cardiovascular disease: what can soluble levels tell us? J. Clin. Endocrinol. Metab. 85:1745-1747. [DOI] [PubMed] [Google Scholar]
- 8.Casl, M. T., B. Surina, I. Glojnaric-Spasic, E. Pape, N. Jagarinec, and S. Kranjcevic. 1995. Serum amyloid A protein in patients with acute myocardial infarction. Ann. Clin. Biochem. 32:196-200. [DOI] [PubMed] [Google Scholar]
- 9.Chiu, B., E. Viira, W. Tucker, and I. W. Fong. 1997. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation 96:2144-2148. [DOI] [PubMed] [Google Scholar]
- 10.Chiu, B. 1999. Multiple infections in carotid atherosclerotic plaques. Am. Heart J. 138:S534-S536. [DOI] [PubMed] [Google Scholar]
- 11.De Caterina, R., G. Basta, G. Lazzerini, G. Dell'Omo, R. Petrucci, M. Morale, F. Carmassi, and R. Pedrinelli. 1997. Soluble vascular adhesion molecule I as a biohumoral correlate of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 17:2646-2654. [DOI] [PubMed] [Google Scholar]
- 12.DeStefano, F., R. F. Anda, H. S. Kahn, D. F. Williamson, and C. M. Russell. 1993. Dental disease and risk of coronary heart disease and mortality. BMJ 306:688-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshpande, R. G., M. B. Khan, and C. A. Genco. 1998. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect. Immun. 66:5337-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebersole, I. L., R. L. Machen, M. J. Steffen, and D. E. Willmann. 1997. Systemic acute-phase reactants, C-reactive protein and haptoglobin in adult periodontitis. Clin. Exp. Immunol. 107:347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fanghanel, J., T. Kocher, C. Schwahn, D. Gesch, O. Bernhardt, F. Mack, C. Kessler, and E. Hensel. 2000. Periodontal disease as a risk factor for myocardial infarction—study of health in Pomerania. J. Clin. Periodontol. 27(Suppl. 1):94. [Google Scholar]
- 16.Fong, I. W., B. Chiu, E. Viira, W. M. Fong, D. Jang, and J. Mahony. 1997. Rabbit model for Chlamydia pneumoniae infection. J. Clin. Microbiol. 35:48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredriksson, M. I., C. M. Figueredo, A. Gustafsson, K. G. Bergstrom, and B. E. Asman. 1999. Effect of periodontitis and smoking on blood leukocytes and acute-phase proteins. J. Periodontol. 70:1355-1360. [DOI] [PubMed] [Google Scholar]
- 18.Gearing, A. J., and W. Newman. 1993. Circulating adhesion molecules in disease. Immunol. Today 14:506-512. [DOI] [PubMed] [Google Scholar]
- 19.Genco, R., S. Chadda, and S. Rossi. 1997. Periodontal disease as a predictor of cardiovascular disease in a Native American population. J. Dent. Res. 76:408. [Google Scholar]
- 20.Glurich, I., H. Sojar, B. Albini, A. Ho, M. Zeid, R. Shah, G. Wick, R. J. Genco, and E. DeNardin. 1999. Does immune response to heat shock proteins in periodontal disease influence atherogenesis? Infect. Dis. Obstet. Gynecol. 7:101.
- 21.Grau, A. J., F. Buggle, C. Ziegler, W. Schwarz, J. Meuser, A. J. Tasman, A. Buhler, C. Benesch, H. Becher, and W. Hacke. 1997. Association between acute cerebrovascular ischemia and chronic recurrent infection. Stroke 28:1724-1729. [DOI] [PubMed] [Google Scholar]
- 22.Grossi, S. G., J. J. Zambon, A. W. Ho, G. Koch, R. G. Dunford, E. E. Machtei, O. M. Norderyd, and R. J. Genco. 1994. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J. Periodontol. 65:260-267. [DOI] [PubMed] [Google Scholar]
- 23.Hack, C. E., G. J. Wolbink, C. Schalkwijk, H. Speijer, W. T. Hermens, and H. van den Bosch. 1997. A role for secretory phospholipase A2 and C-reactive protein in the removal of injured cells. Immunol. Today 18:111-115. [DOI] [PubMed] [Google Scholar]
- 24.Haraszthy, V., J. J. Zambon, M. Trevisan, M. Zeid, and R. J. Genco. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 71:1554-1560. [DOI] [PubMed] [Google Scholar]
- 25.Haverkate, F., S. G. Thompson, S. D. Pyke, J. R. Gallimore, and M. B. Pepys. 1997. Production of C reactive protein and risk of coronary events in stable and unstable angina. European concerted action on thrombosis and disabilities angina pectoris study group. Lancet 349:462-466. [DOI] [PubMed] [Google Scholar]
- 26.Ho, A. W., S. G. Grossi, R. G. Dunford, and R. J. Genco. 1997. Reliability of a self-reported health questionnaire in a periodontal disease study. J. Periodontal Res. 32:646-650. [DOI] [PubMed] [Google Scholar]
- 27.Hwang, S. J., C. M. Ballantyne, A. R. Sharrett, L. C. Smith, C. E. Davis, A. M. Gotto, Jr., and E. Boerwinkle. 1997. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 96:4219-4225. [DOI] [PubMed] [Google Scholar]
- 28.Joshipura, K. J., E. B. Rimm, C. W. Douglass, D. Trichopoulos, A. Acsherio, and W. C. Willett. 1996. Poor oral health and coronary heart disease. J. Dent. Res. 75:1631-1636. [DOI] [PubMed] [Google Scholar]
- 29.Kazmierczak, M., M. Sobieska, K. Wiktorowicz, and H. Wysocki. 1995. Changes of the acute phase proteins glycosylation profile as a possible prognostic marker in myocardial infarction. Int. J. Cardiol. 49:201-207. [DOI] [PubMed] [Google Scholar]
- 30.Kuller, L. H., R. P. Tracy, J. Shaten, and E. N. Meilahn. 1996. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple risk factor intervention trial. Am. J. Epidemiol. 144:537-547. [DOI] [PubMed] [Google Scholar]
- 31.Lagrand, W. K., H. W. Niessen, G. J. Wolbink, L. H. Jaspars, C. A. Visser, F. W. Verheugt, C. J. Meijer, and C. E. Hack. 1997. C-reactive protein colocalizes with complement in human hearts during myocardial infarction. Circulation 95:97-103. [DOI] [PubMed] [Google Scholar]
- 32.Jander, S., F. Heidenreich, and G. Stoll. 1994. Serum and CSF levels of soluble intercellular adhesion molecule-1 (ICAM-1) in inflammatory neurologic diseases. Neurology 43:1809-1813. [DOI] [PubMed] [Google Scholar]
- 33.Laitinen, K., A. Laurila, L. Pyhala, M. Leinonen, and P. Saikku. 1997. Chlamidia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect. Immun. 65:4832-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, Y., S. Geva, C. M. E. Champagne, J. H. Southerland, P. N. Madianos, W. Smith, and S. Offenbacher. 2000. Effect of P. gingivalis dissemination on atherogenesis in ApoE(+/−) mice. J. Dent. Res. 79:313. [Google Scholar]
- 35.Liuzzo, G., L. M. Biasucci, J. R. Gallimore, R. L. Grillo, A. G. Rebuzzi, M. B. Pepys, and A. Maseri. 1994. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N. Engl. J. Med. 331:417-424. [DOI] [PubMed] [Google Scholar]
- 36.Loesche, W. J. 1994. Periodontal disease as a risk factor for heart disease. Compendium 15:976, 978-982, 985-986. [PubMed] [Google Scholar]
- 37.Loos, B. G., J. Craandijk, F. W. Hoek, P. M. Wertheim-van Dillen, and U. van der Velden. 2000. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J. Periodontol. 71:1528-1534. [DOI] [PubMed] [Google Scholar]
- 38.Malle, E., and F. C. De Beer. 1996. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur. J. Clin. Investig. 26:427-435 [DOI] [PubMed] [Google Scholar]
- 39.Maseri, A. 1997. Inflammation, atherosclerosis, and ischemic events-exploring the hidden side of the moon. N. Engl. J. Med. 336:1014-1016. [DOI] [PubMed] [Google Scholar]
- 40.Mason, J. C., P. Kapahi, and D. O. Haskard. 1993. Detection of increased levels of circulating intercellular adhesion molecule 1 in some patients with rheumatoid arthritis but not in patients with systemic lupus erythematosis. Lack of correlation of circulating vascular cell adhesion molecule 1. Arthritis Rheum. 36:519-527. [DOI] [PubMed] [Google Scholar]
- 41.Mattila, K. J., V. V. Valtonen, M. Nieminen, and J. K. Huttunen. 1995. Dental infection and the risk of new coronary events: prospective study of patients with documented coronary artery disease. Clin. Infect. Dis. 20:588-592. [DOI] [PubMed] [Google Scholar]
- 42.Mattila, K. J., V. V. Valtonen, M. S. Nieminen, and S. Asikainen. 1998. Role of infection as a risk factor for atherosclerosis, myocardial infarction, and stroke. Clin. Infect. Dis. 26:719-734. [DOI] [PubMed] [Google Scholar]
- 43.Mayr, M., S. Kiechl, J. Willeit,, G. Wick, and Q. Xu. 2000. Infections, immunity and atherosclerosis: associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation 102:833-839. [DOI] [PubMed] [Google Scholar]
- 44.Meyer, D. H., P. K. Sreenivasan, and P. M. Fives-Taylor. 1991. Evidence for invasion of human oral cell line by Actinobacillus actinomycetemcomitans. Infect. Immun. 59:2719-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrow, D. A., and P. M. Ridker. 2000. C-reactive protein, inflammation, and coronary risk. Med. Clin. N. Am. 84:149-161. [DOI] [PubMed] [Google Scholar]
- 46.Muhlstein, J. B., J. L. Anderson, E. H. Hammond, L. Zhao, S. Trehan, E. P. Schwobe, and J. F. Carlquist. 1998. Infection with Chlamydia pneumoniae accelerates development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation 97:633-636. [DOI] [PubMed] [Google Scholar]
- 47.Patel, P., M. A. Mendall, D. Carrington, D. P. Strachan, E. Leatham, N. Molineaux, J. Levy, C. Blakeston, C. A. Seymour, A. J. Camm, et al. 1995. Association of Helicobacter pyolori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factors. BMJ 311:711-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peter, K., P. Nawroth, C. Conradt, T. Nordt, T. Weiss, M. Boehme, A. Wunsch, J. Allenberg, W. Kubler, and C. Bode. 1997. Circulating vascular cell adhesion molecule I correlates with the extent of human atherosclerosis in contrast to circulating intercellular adhesion molecule I., E-selectin, P-selectin and thrombomodulin. Arterioscler. Thromb. Vasc. Biol. 17:505-512. [DOI] [PubMed] [Google Scholar]
- 49.Pieniazek, P., E. Karczewska, A. Duda, W. Tracz, M. Pasowicz, and S. J. Konturek. 1999. Association of Helicobacter pylori infection with coronary heart disease. J. Physiol. Pharmacol. 50:743-751. [PubMed] [Google Scholar]
- 50.Progulske-Fox, A., E. Kozarov, B. Dorn, W. Dunn, Jr., J. Burks, and Y. Wu. 1999. Porphyromonas gingivalis virulence factors and invasion of cells of the cardiovascular system. J. Periodontal Res. 34:393-399. [DOI] [PubMed] [Google Scholar]
- 51.Pukhal'skii, A. L., G. V. Shmarina, E. A. Kalashnikova, S. D. Shiyan, S. N. Kokarovtseva, D. A. Pukhal'skaya, and N. V. Bovin. 2000. Effect of semisynthetic analog of alpha(1)-acid glycoprotein on immunomodulatory and antiinflammatory activity of natural glycoprotein. Bull. Exp. Biol. Med. 29:567-570. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds, G. D., and R. P. Vance. 1987. C-reactive protein immunohistochemical localization in normal and atherosclerotic human aortas. Arch. Pathol. Lab. Med. 111:265-269. [PubMed] [Google Scholar]
- 53.Ridker, P. M. 1999. Inflammation, atherosclerosis, and cardiovascular risk: an epidemiologic view. Blood Coagul. Fibrinolysis 10(Suppl. 1):S9-S12. [PubMed] [Google Scholar]
- 54.Ridker, P. M., J. E. Buring, J. Shih, M. Matias, and C. H. Hennekens. 1998. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 98:731-733. [DOI] [PubMed] [Google Scholar]
- 55.Rohde, L. E., C. H. Hennekens, and P. M. Ridker. 1999. Survey of C-reactive protein and cardiovascular disease in apparently healthy men. Am. J. Cardiol. 84:1018-1022. [DOI] [PubMed] [Google Scholar]
- 56.Rohde, L. E., R. T. Lee, J. Rivero, M. Jamacochian, L. H. Arroya, W. Briggs, N. Rifai, P. Libby, M. A. Creager, and P. M. Ridker. 1998. Circulating cell adhesion molecules are correlated with ultrasound-based assessment of carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 18:1765-1770. [DOI] [PubMed] [Google Scholar]
- 57.Seth, R., F. D. Raymond, and M. W. Makgoba. 1991. Circulating ICAM-1 isoforms: diagnostic prospects for inflammatory and immune disorders. Lancet 338:83-84. [DOI] [PubMed] [Google Scholar]
- 58.Shiyan, S. D., and N. V. Bovin. 1997. Carbohydrate composition and immunomodulatory activity of different glycoforms of alpha1-acid glycoprotein. Glycoconj. J. 14:631-638. [DOI] [PubMed] [Google Scholar]
- 59.Slade, G. D., S. Offenbacher, J. D. Beck, G. Heiss, and J. S. Pankow. 2000. Acute-phase inflammatory response to periodontal disease in the US population. J. Dent. Res. 79:49-57. [DOI] [PubMed] [Google Scholar]
- 60.Stadnyk, A., and J. Gauldie. 1991. Acute-phase response during parasitic infection. Immunol. Today 12:A7-A12. [DOI] [PubMed] [Google Scholar]
- 61.Steel, D. M., and A. S. Whitehead. 1994. The major acute-phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol. Today 15:81-88. [DOI] [PubMed] [Google Scholar]
- 62.Suffredini, A. F., G. Fantuzzi, R. Badolato, J. J. Oppenheim, and N. P. O'Grady. 1999. New insights into the biology of the acute-phase response. J. Clin. Immunol. 19:203-214. [DOI] [PubMed] [Google Scholar]
- 63.Tervahartiala, T., Y. T. Konttinen, T. Ingman, R. Hayrinen-Immonen, Y. Ding, and T. Sorsa. 1996. Cathespin G in gingival crevicular fluid in adult periodontitis. J. Clin. Periodontol. 23:68-75. [DOI] [PubMed] [Google Scholar]
- 64.Torzewski, M., C. Rist, R. F. Mortensen, T. P. Zwaka, M. Bienek, J. Waltenberger, W. Koenig, G. Schmitz, V. Hombach, J. Torzewski, et al. 2000. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 20:2094-2099. [DOI] [PubMed] [Google Scholar]
- 65.van Dijk, W., E. C. Havenaar, and E. C. Brinkman-van der Linden. 1995. Alpha 1-acid glycoprotein (orosomucoid) pathophysiological changes in glycosylation in relation to its function. Glycoconj. J. 12:227-233. [DOI] [PubMed] [Google Scholar]
- 66.Virchow, R. 1971. Cellular pathology as based upon physiological and pathological histology, p. 396. J. B. Lippincott, Philadelphia, Pa. (Translation of second German edition.)
- 67.Wallen, N. H., C. Held, N. Rehnqvist, and P. Hjemdahl. 1999. Elevated serum intercellular molecule-1 and vascular adhesion molecule-1 among patients with stable angina pectoris who suffer cardiovascular death or non-fatal myocardial infarction. Eur. Heart J. 20:1039-1043. [DOI] [PubMed] [Google Scholar]
- 68.Walport, M. J., and K. A. Davies. 1996. Complement and immune complexes. Res. Immunol. 147:103-109. [DOI] [PubMed] [Google Scholar]
- 69.Wiedermann, C. J., S. Kiechl, S. Dunzendorfer, P. Schratzberger, G. Egger, F. Oberhollenzer, and J. Willeit. 1999. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results for the Brunek study. J. Am. Coll. Cardiol. 34:1975-1981. [DOI] [PubMed] [Google Scholar]
- 70.Wolbink, G. J., M. C. Brouwer, S. Buysmann, I. J. ten Berge, and C. E. Hack. 1996. CRP-mediated activation of complement in vivo: assessment by measuring circulating complement-C-reactive protein complexes. J. Immunol. 157:473-479. [PubMed] [Google Scholar]
- 71.Wu, T., M. Trevisan, R. J. Genco, J. P. Dorn, K. L. Falkner, and C. T. Sempos. 2000. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch. Intern. Med. 160:2749-2755. [DOI] [PubMed] [Google Scholar]
- 72.Wu, T., M. Trevisan, R. J. Genco, K. L. Falkner, J. P. Dorn, and C. T. Sempos. 2000. Examination of the relation between periodontal health status and cardiovascular risk factors: serum total and high density lipoprotein cholesterol, C-reactive protein and plasma fibrinogen. Am. J. Epidemiol. 151:273-282. [DOI] [PubMed] [Google Scholar]
- 73.Xu, Q., R. Kleindienst, G. Schett, W. Waitz, S. Jindal, R. S. Gupta, H. Dietrich, and G. Wick. 1996. Regression of arteriosclerotic lesions induced by immunization with heat shock protein 65-containing material in normocholesteremic, but not hypercholesteremic rabbits. Atherosclerosis 123:145-155. [DOI] [PubMed] [Google Scholar]
- 74.Yamada, T., T. Kakihara, T. Kamishima, T. Fukuda, and T. Kawai. 1996. Both acute-phase and constitutive serum amyloid A are present in atherosclerotic lesions. Pathol. Int. 46:797-800. [DOI] [PubMed] [Google Scholar]
- 75.Yamada, T., T. Miida, T. Yamaguchi, and Y. Itoh. 1997. Effect of serum amyloid A on cellular affinity of low density lipoprotein. Eur. J. Clin. Chem. Clin. Biochem. 35:421-426. [DOI] [PubMed] [Google Scholar]
- 76.Zeitler, H., Y. Ko, C. Zimmermann, G. Nickenig, K. Glanzer, P. Walger, A. Sachinidis, and H. Vetter. 1997. Elevated serum concentrations of soluble adhesion molecules in coronary artery disease and myocardial infarction. Eur. J. Med. Res. 2:389-394. [PubMed] [Google Scholar]
- 77.Zhu, J., A. A. Quyyumi, J. E. Norman, G. Csako, M. A. Waclawiw, G. M. Shearer, S. E. Epstein, et al. 2000. Effects of total pathogen burden on coronary artery disease and C-reactive protein levels. Am. J. Cardiol. 15:140-146. [DOI] [PubMed] [Google Scholar]