Abstract
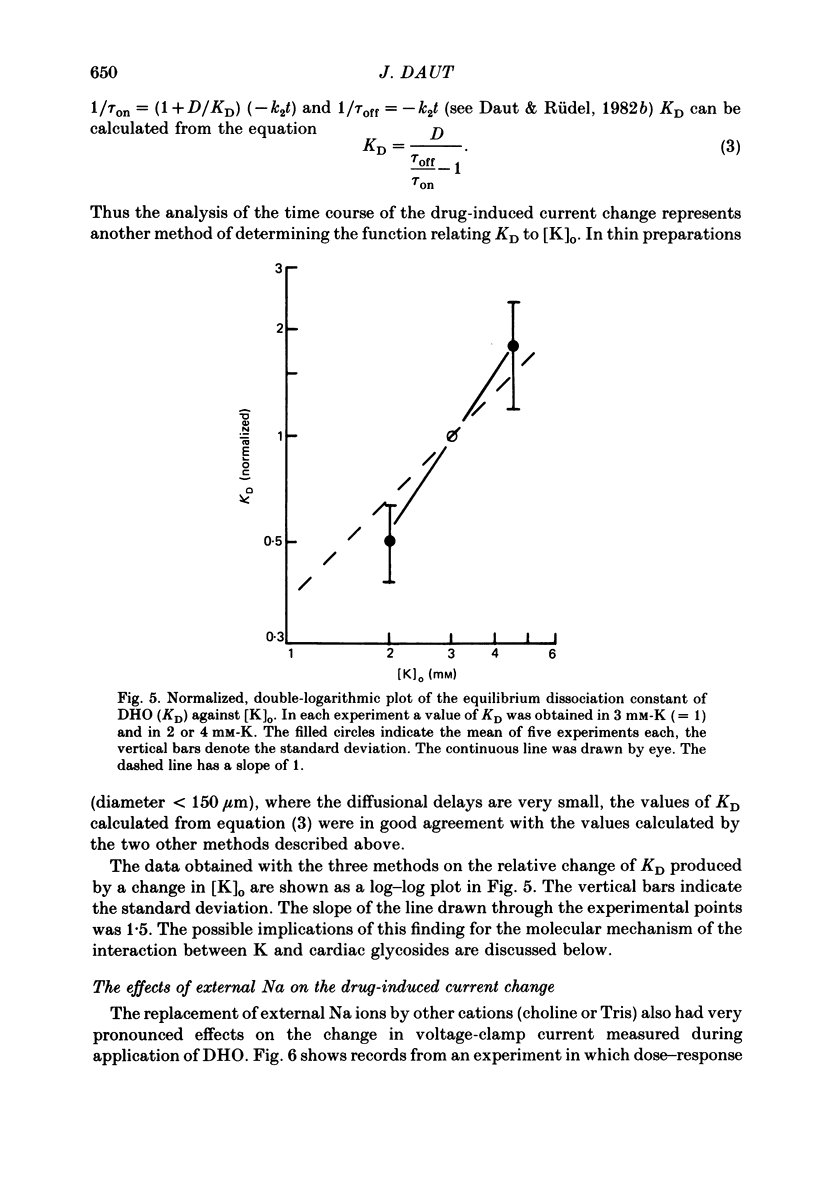
The inhibition of the electrogenic pump current in quiescent guinea-pig ventricular muscle by dihydro-ouabain (DHO) was studied with the three-micro-electrode voltage-clamp technique described previously (Daut, 1982c). From dose-response curves of the drug-induced current change (ID) the equilibrium dissociation constant of the binding of DHO to the Na-K pump (KD) and the electrogenic pump current flowing in the steady state (Ip) were inferred (Daut & Rüdel, 1982b). The external K concentration ([K]o) was varied between 2 and 4.5 mM (substituted by Na). KD was found to increase with increasing [K]o. A plot of log KD versus log [K]o gave a straight line with a slope of about 1.5. The time constants of the onset (tau on) and decay (tau off) of ID are supposed to represent the chemical kinetics of binding and unbinding of the drug (Daut & Rüdel, 1981, 1982b). Tau on was found to be inversely related to [K]o whereas tau off was found to be independent of [K]o. Ip was found to be independent of [K]o. This was interpreted to indicate that in the steady state Ip is mainly determined by the passive influx of Na into the cell, which may be relatively insensitive to small changes in [K]o. The effects of [K]o on the drug-induced current change are consistent with competitive inhibition of the binding of DHO to the Na-K pump. It is suggested that K ions and cardiac glycosides compete for extracellular binding sites on the same conformation of the Na-K pump. The external Na concentration ([Na]o) was varied between 147 and 49 mM (substituted by choline or Tris). Reduction of [Na]o produced a proportional decrease of Ip. This may be a consequence of the accompanying reduction of passive Na influx and the resulting decrease in intracellular Na activity (alpha iNa). Reduction of [Na]o markedly increased KD. This effect may be mediated by competition between Na and K at the K-loading sites of the pump and/or by separate modulatory Na-binding sites. It is concluded that the well known effects of external Na and K on the positive inotropic action of cardiac glycosides can be fully accounted for by the marked changes in the apparent binding affinity of the drug reported here.
Full text
PDF


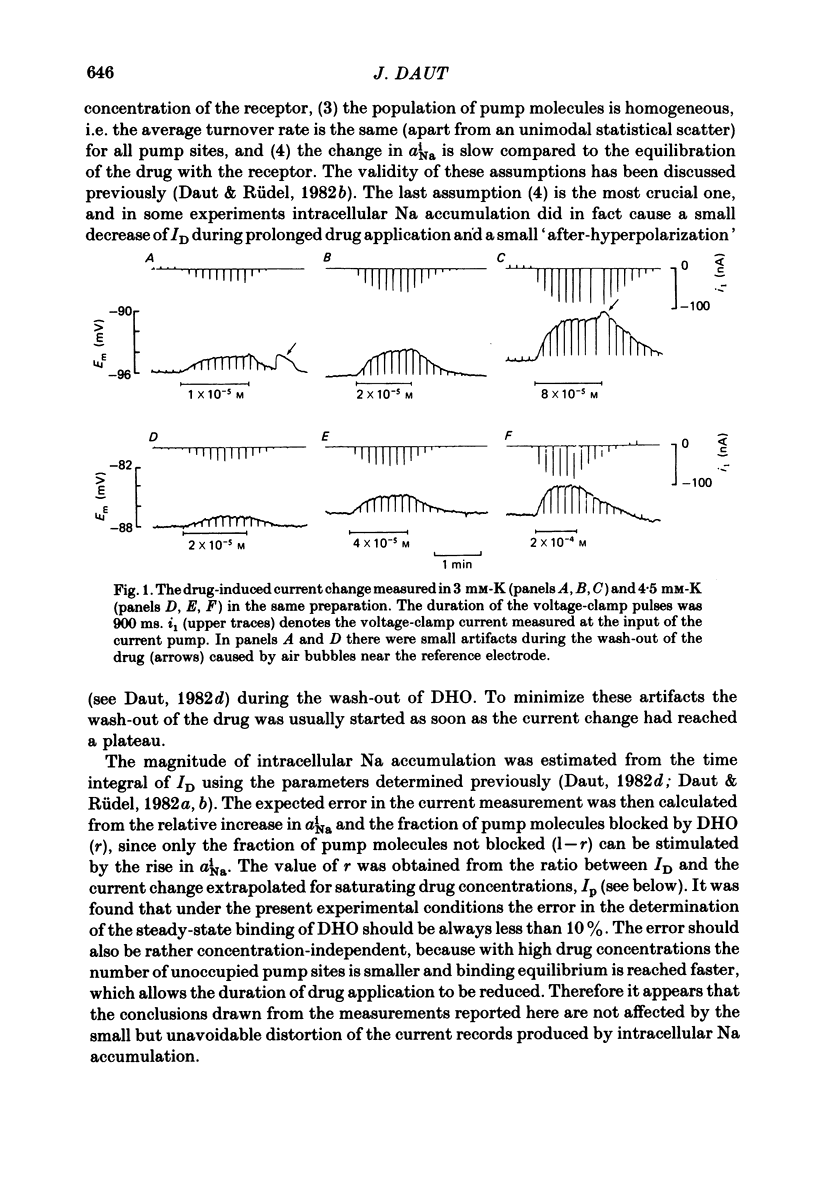
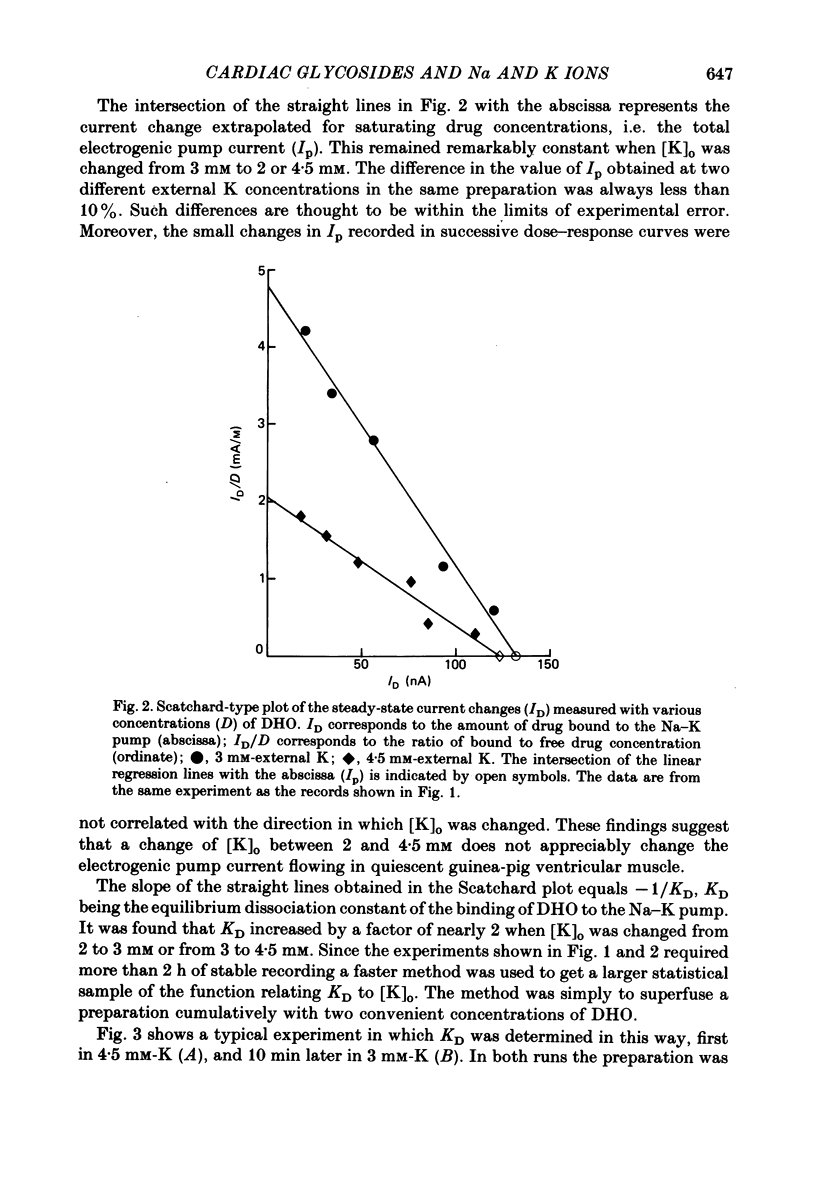
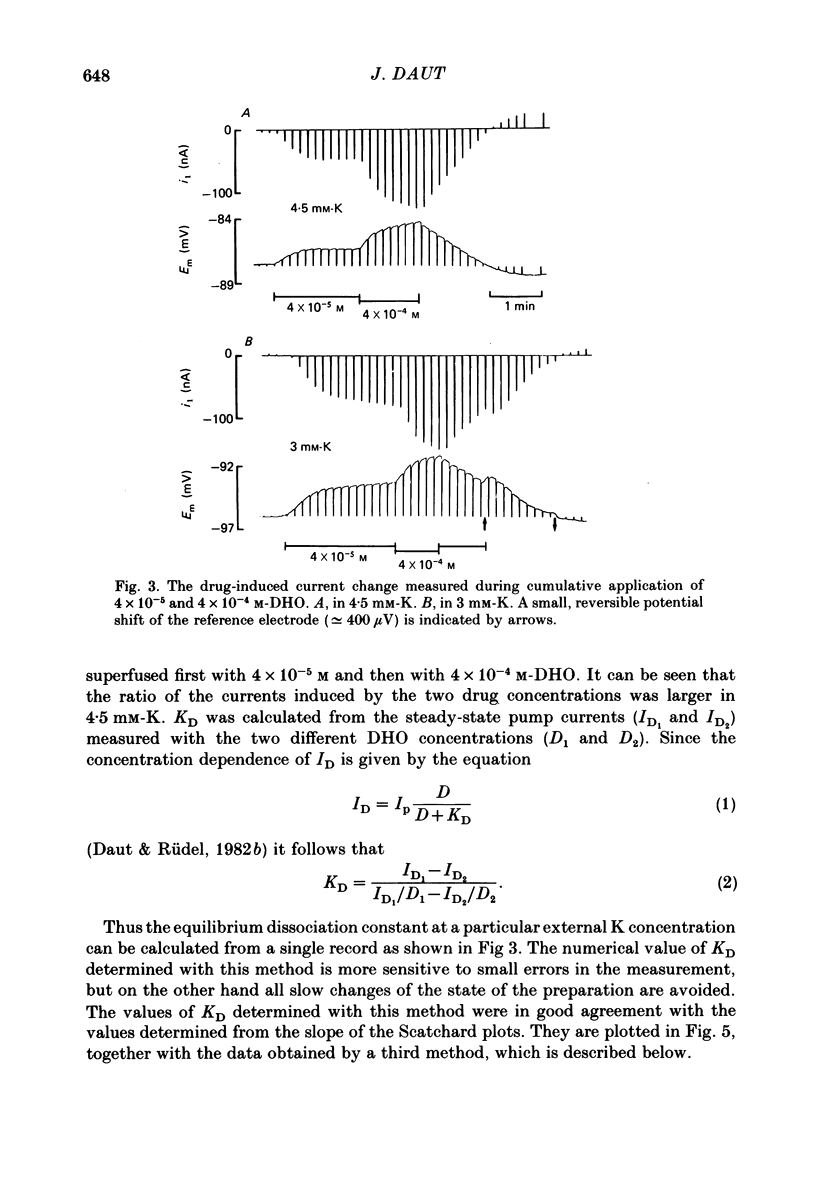
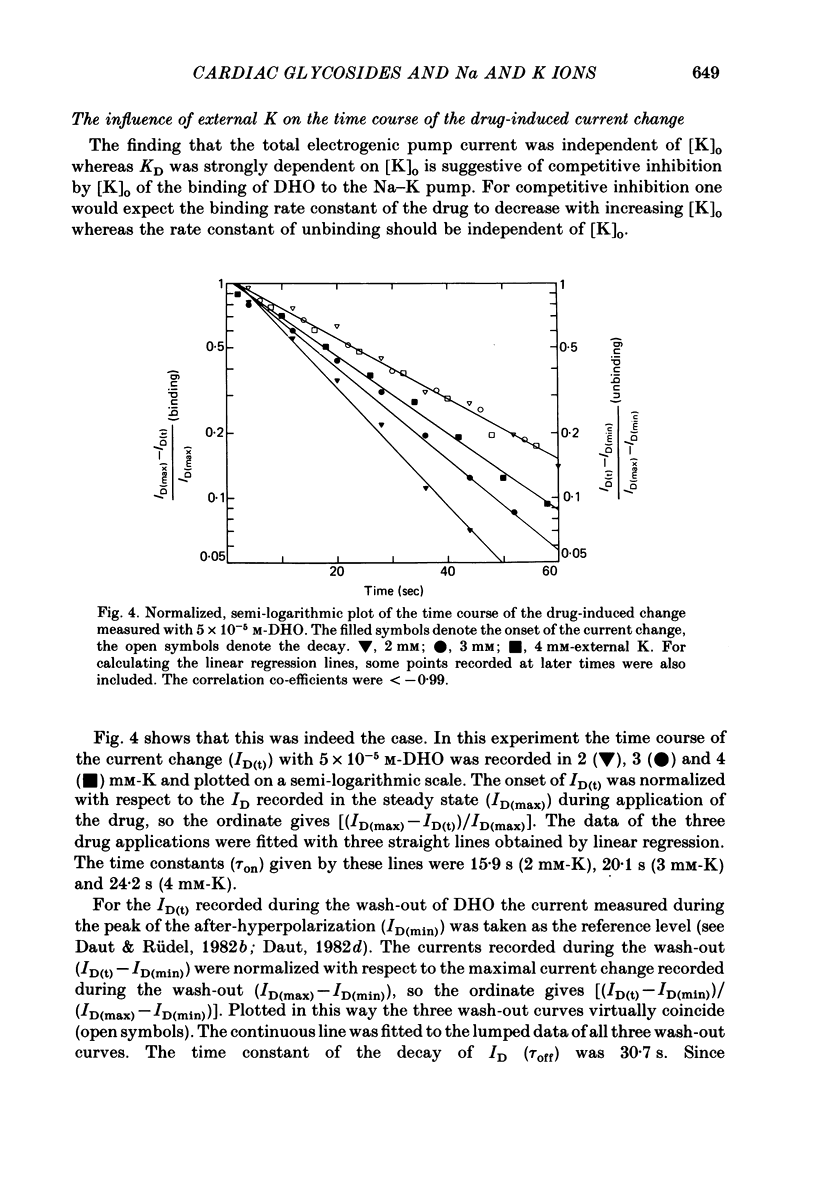
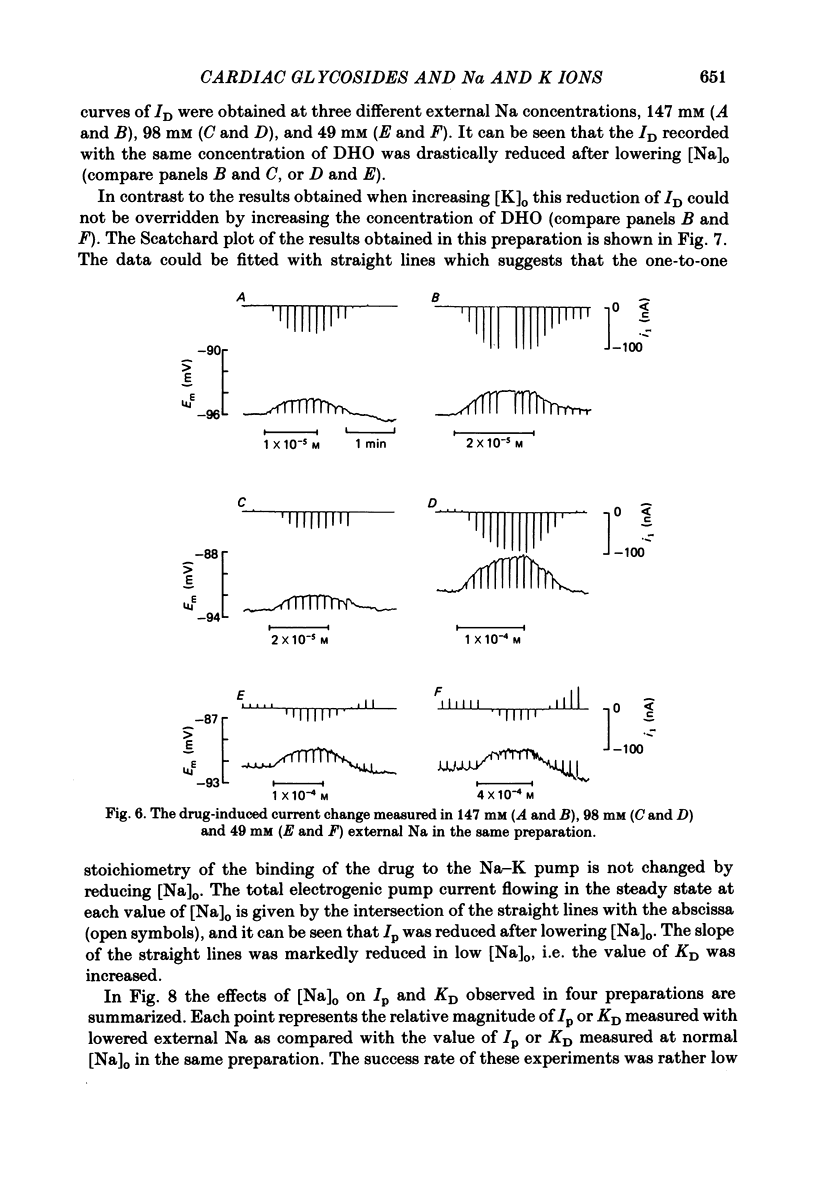









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Hirai M., Oka T. Sodium ions and the development of the inotropic action of ouabain in guinea-pig heart. Eur J Pharmacol. 1979 Dec 7;60(2-3):189–198. doi: 10.1016/0014-2999(79)90218-8. [DOI] [PubMed] [Google Scholar]
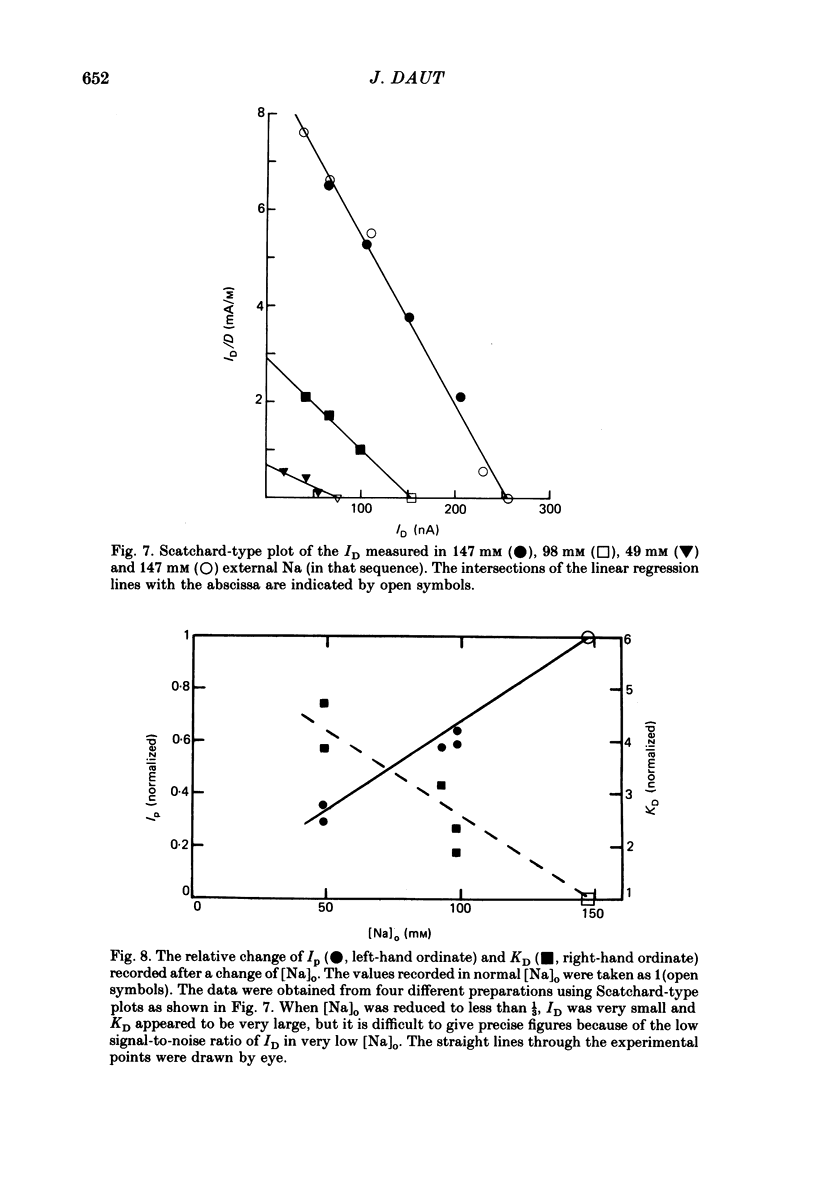
- Akera T., Olgaard M. K., Temma K., Brody T. M. Development of the positive inotropic action of ouabain: effects of transmembrane sodium movement. J Pharmacol Exp Ther. 1977 Dec;203(3):675–684. [PubMed] [Google Scholar]
- Akera T., Temma K., Wiest S. A., Brody T. M. Reduction of the equilibrium binding of cardiac glycosides and related compounds to Na+,K+-ATPase as a possible mechanism for the potassium-induced reversal of their toxicity. Naunyn Schmiedebergs Arch Pharmacol. 1978 Sep;304(2):157–165. doi: 10.1007/BF00495552. [DOI] [PubMed] [Google Scholar]
- Albers R. W., Koval G. J., Siegel Studies on the interaction of ouabain and other cardio-active steroids with sodium-potassium-activated adenosine triphosphatase. Mol Pharmacol. 1968 Jul;4(4):324–336. [PubMed] [Google Scholar]
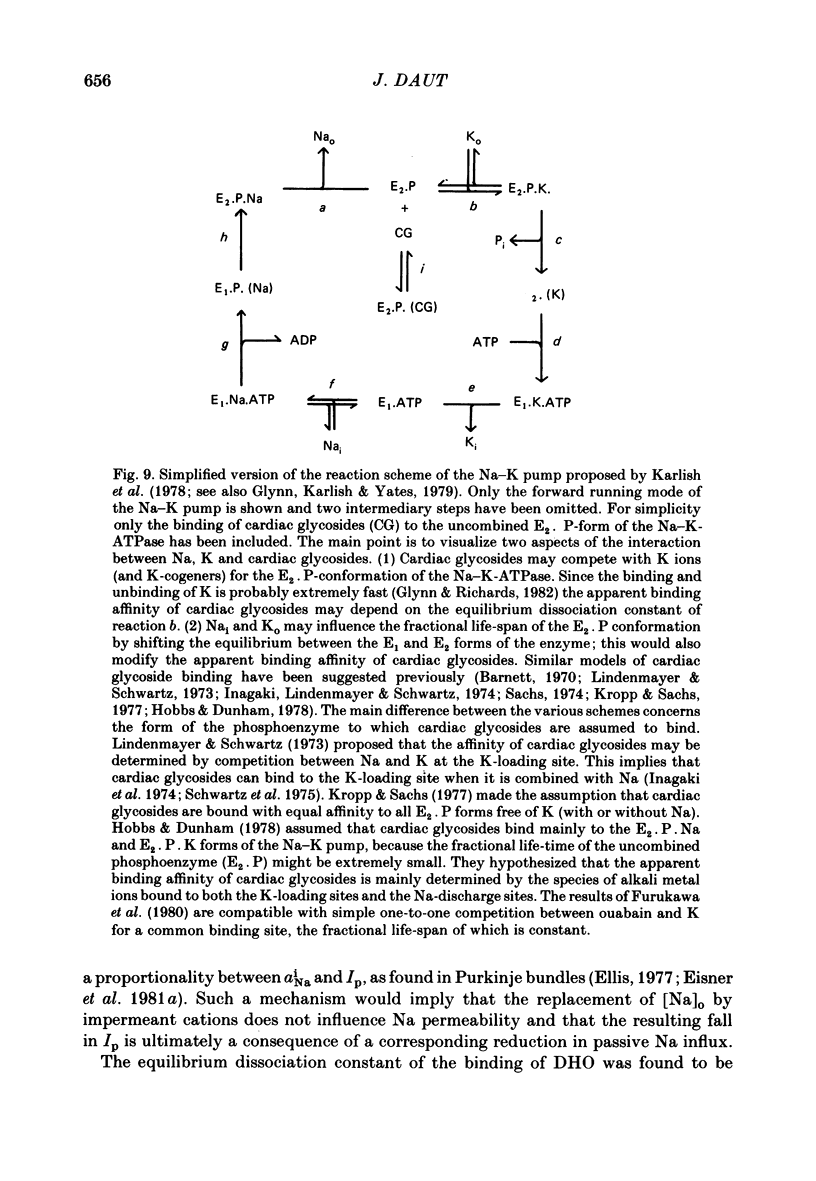
- Baker P. F., Manil J. The rates of action of K+ and ouabain on the sodium pump in squid axons. Biochim Biophys Acta. 1968 Mar 1;150(2):328–330. doi: 10.1016/0005-2736(68)90181-8. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Binding of the cardiac glycoside ouabain to intact cells. J Physiol. 1972 Jul;224(2):441–462. doi: 10.1113/jphysiol.1972.sp009904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Inhibition of the sodium pump in squid giant axons by cardiac glycosides: dependence on extracellular ions and metabolism. J Physiol. 1972 Jul;224(2):463–475. doi: 10.1113/jphysiol.1972.sp009905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Potassium ions and the binding of cardiac glycosides to mammalian cells. Nature. 1970 May 9;226(5245):521–523. doi: 10.1038/226521a0. [DOI] [PubMed] [Google Scholar]
- Barnett R. E. Effect of monovalent cations on the ouabain iniibition of the sodium and potassium ion activated adenosine triphosphatase. Biochemistry. 1970 Nov 24;9(24):4644–4648. doi: 10.1021/bi00826a004. [DOI] [PubMed] [Google Scholar]
- Beauge L. A., Adragna N. The kinetics of ouabain inhibition and the partition of rubidium influx in human red blood cells. J Gen Physiol. 1971 May;57(5):576–592. doi: 10.1085/jgp.57.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blostein R., Chu L. Sidedness of (sodium, potassium)-adenosine triphosphate of inside-out red cell membrane vesicles. Interactions with potassium. J Biol Chem. 1977 May 10;252(9):3035–3043. [PubMed] [Google Scholar]
- Bodemann H. H., Hoffman J. F. Side-dependent effects of internal versus external Na and K on ouabain binding to reconstituted human red blood cell ghosts. J Gen Physiol. 1976 May;67(5):497–525. doi: 10.1085/jgp.67.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosteels S., Carmeliet E. The components of the sodium efflux in cardiac Purkyne fibres. Pflugers Arch. 1972;336(1):48–59. doi: 10.1007/BF00589141. [DOI] [PubMed] [Google Scholar]
- Caprio A., Farah A. The effect of the ionic milieu on the response of rabbit cardiac muscle to ouabain. J Pharmacol Exp Ther. 1967 Mar;155(3):403–414. [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. An analysis of the actions of low concentrations of ouabain on membrane currents in Purkinje fibres. J Physiol. 1976 Aug;260(1):75–103. doi: 10.1113/jphysiol.1976.sp011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J., Rüdel R. Cardiac glycoside binding to the Na/K-ATPase in the intact myocardial cell: electrophysiological measurement of chemical kinetics. J Mol Cell Cardiol. 1981 Aug;13(8):777–782. doi: 10.1016/0022-2828(81)90260-1. [DOI] [PubMed] [Google Scholar]
- Daut J., Rüdel R. The electrogenic sodium pump in guinea-pig ventricular muscle: inhibition of pump current by cardiac glycosides. J Physiol. 1982 Sep;330:243–264. doi: 10.1113/jphysiol.1982.sp014339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. The passive electrical properties of guinea-pig ventricular muscle as examined with a voltage-clamp technique. J Physiol. 1982 Sep;330:221–242. doi: 10.1113/jphysiol.1982.sp014338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S., Marks B. H. Factors that regulate ouabain-H3 accumulation by the isolated guinea-pig heart. J Pharmacol Exp Ther. 1969 Dec;170(2):318–325. [PubMed] [Google Scholar]
- Ebner F., Reiter M. The dependence on contraction frequency of the positive inotropic effect of dihydro-ouabain. Naunyn Schmiedebergs Arch Pharmacol. 1977 Oct;300(1):1–9. doi: 10.1007/BF00505073. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. The role of the sodium pump in the effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:279–301. doi: 10.1113/jphysiol.1979.sp012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The dependence of sodium pumping and tension on intracellular sodium activity in voltage-clamped sheep Purkinje fibres. J Physiol. 1981 Aug;317:163–187. doi: 10.1113/jphysiol.1981.sp013819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The effects of rubidium ions and membrane potentials on the intracellular sodium activity of sheep Purkinje fibres. J Physiol. 1981 Aug;317:189–205. doi: 10.1113/jphysiol.1981.sp013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Deitmer J. W. The relationship between the intra- and extracellular sodium activity of sheep heart Purkinje fibres during inhibition of the Na-K pump. Pflugers Arch. 1978 Nov 30;377(3):209–215. doi: 10.1007/BF00584274. [DOI] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann E., Schoner W. Ouabain-receptor interactions in (Na+ + K+)-ATPase preparations. II. Effect of cations and nucleotides on rate constants and dissociation constants. Biochim Biophys Acta. 1973 Dec 22;330(3):302–315. doi: 10.1016/0005-2736(73)90235-6. [DOI] [PubMed] [Google Scholar]
- Furukawa H., Bilezikian J. P., Loeb J. N. Kinetics and thermodynamics of ouabain binding by intact turkey erythrocytes: effects of external sodium ion, potassium ion, and temperature. J Gen Physiol. 1980 Oct;76(4):499–516. doi: 10.1085/jgp.76.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. Activation of electrogenic Na+/K+ exchange by extracellular K+ in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4035–4039. doi: 10.1073/pnas.77.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P. The effects of sodium and potassium on ouabain binding by human erythrocytes. J Gen Physiol. 1972 Nov;60(5):609–629. doi: 10.1085/jgp.60.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The sensitivity of the sodium pump to external sodium. J Physiol. 1967 Sep;192(1):175–188. doi: 10.1113/jphysiol.1967.sp008295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Grabowski W., Thielen J. Activation of the electrogenic sodium pump in guinea-pig atria by external potassium ions. J Physiol. 1978 Mar;276:515–524. doi: 10.1113/jphysiol.1978.sp012250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Richards D. E. Occlusion of rubidium ions by the sodium-potassium pump: its implications for the mechanism of potassium transport. J Physiol. 1982 Sep;330:17–43. doi: 10.1113/jphysiol.1982.sp014326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. H., Coltart D. J., Schweizer E., Snidow G., Harrison D. C. Dose response in vivo to digoxin in normo-and hyperkalaemia: associated biochemical changes. Cardiovasc Res. 1975 Jul;9(4):515–523. doi: 10.1093/cvr/9.4.515. [DOI] [PubMed] [Google Scholar]
- Harrison C. E., Jr, Wakim K. G. Inhibition of binding of tritiated digoxin to myocardium by sodium depletion in dogs. Circ Res. 1969 Feb;24(2):263–268. doi: 10.1161/01.res.24.2.263. [DOI] [PubMed] [Google Scholar]
- Hegyvary C., Post R. L. Binding of adenosine triphosphate to sodium and potassium ion-stimulated adenosine triphosphatase. J Biol Chem. 1971 Sep 10;246(17):5234–5240. [PubMed] [Google Scholar]
- Hobbs A. S., Dunham P. B. Interaction of external alkali metal ions with the Na-K pump of human erythrocytes: a comparison of their effects on activation of the pump and on the rate of ouabain binding. J Gen Physiol. 1978 Sep;72(3):381–402. doi: 10.1085/jgp.72.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki C., Lindenmayer G. E., Schwartz A. Effects of sodium and potassium on binding of ouabain to the transport adenosine triphosphatase. J Biol Chem. 1974 Aug 25;249(16):5135–5140. [PubMed] [Google Scholar]
- Karlish S. J., Yates D. W., Glynn I. M. Conformational transitions between Na+-bound and K+-bound forms of (Na+ + K+)-ATPase, studied with formycin nucleotides. Biochim Biophys Acta. 1978 Jul 7;525(1):252–264. doi: 10.1016/0005-2744(78)90219-x. [DOI] [PubMed] [Google Scholar]
- Kropp D. L., Sachs J. R. Kinetics of the inhibition of the Na-K pump by tetrapropylammonium chloride. J Physiol. 1977 Jan;264(2):471–487. doi: 10.1113/jphysiol.1977.sp011678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y., Noma A., Irisawa H. Electrogenic sodium pump in rabbit atrio-ventricular node cell. Pflugers Arch. 1981 Oct;391(4):261–266. doi: 10.1007/BF00581504. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Ion fluxes in cardiac excitation and contraction and their relation to myocardial contractility. Physiol Rev. 1968 Oct;48(4):708–757. doi: 10.1152/physrev.1968.48.4.708. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Kang D. H., Sokol J. H., Lee K. S. Relation between intracellular Na ion activity and tension of sheep cardiac Purkinje fibers exposed to dihydro-ouabain. Biophys J. 1980 Feb;29(2):315–330. doi: 10.1016/S0006-3495(80)85135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Zelis R., Mason D. T. Linear dose response and quantitative attenuation by potassium of the inotropic action of acetylstrophanthidin. Clin Pharmacol Ther. 1977 Jul;22(1):34–41. doi: 10.1002/cpt197722134. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Klaus W. The subcellular basis for the mechanism of inotropic action of cardiac glycosides. Pharmacol Rev. 1971 Sep;23(3):193–261. [PubMed] [Google Scholar]
- Lin C. I., Vassalle M. Role of sodium in strophanthidin toxicity of Purkinje fibers. Am J Physiol. 1978 Apr;234(4):H477–H486. doi: 10.1152/ajpheart.1978.234.4.H477. [DOI] [PubMed] [Google Scholar]
- Linden J., Brooker G. Sodium requirement for effects of ouabain on contraction of isolated guinea pig atria. Circ Res. 1980 Apr;46(4):553–564. doi: 10.1161/01.res.46.4.553. [DOI] [PubMed] [Google Scholar]
- Lindenmayer G. E., Schwartz A. Nature of the transport adenosine triphosphatase digitalis complex. IV. Evidence that sodium-potassium competition modulates the rate of ouabain interaction iwth (Na + +K + ) adenosine triphosphatase during enzyme catalysis. J Biol Chem. 1973 Feb 25;248(4):1291–1300. [PubMed] [Google Scholar]
- Marban E., Tsien R. W. Enhancement of calcium current during digitalis inotropy in mammalian heart: positive feed-back regulation by intracellular calcium? J Physiol. 1982 Aug;329:589–614. doi: 10.1113/jphysiol.1982.sp014321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F. I., Nimmo L., Kapadia G. G., Goldsmith C. The effect of acute hypokalemia on the myocardial concentration and body distribution of tritiated digoxin in the dog. J Pharmacol Exp Ther. 1971 Aug;178(2):271–281. [PubMed] [Google Scholar]
- Post R. L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6530–6540. [PubMed] [Google Scholar]
- Prindle K. H., Jr, Skelton C. L., Epstein S. E., Marcus F. I. Influence of extracellular potassium concentration on myocardial uptake and inotropic effect of tritiated digoxin. Circ Res. 1971 Mar;28(3):337–345. doi: 10.1161/01.res.28.3.337. [DOI] [PubMed] [Google Scholar]
- REITER M. DIE BEZIEHUNG VON CALCIUM UND NATRIUM ZUR INOTROPEN GLYKOSIDWIRKUNG. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1963 Sep 2;245:487–499. [PubMed] [Google Scholar]
- REPKE K. UBER DEN BIOCHEMISCHEN WIRKUNGSMODUS VON DIGITALIS. Klin Wochenschr. 1964 Feb 15;42:157–165. doi: 10.1007/BF01482616. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter M. Die Wertbestimmung inotrop wirkender Arzneimittel am isolierten Papillarmuskel. Arzneimittelforschung. 1967 Oct;17(10):1249–1253. [PubMed] [Google Scholar]
- Reiter M., Stickel F. J., Weber S. The influence of the extracellular potassium concentration on the glycoside effects upon contractile force and action potential duration of the guinea- pig papillary muscle. Experientia. 1966 Oct 15;22(10):665–666. doi: 10.1007/BF01902431. [DOI] [PubMed] [Google Scholar]
- SCHATZMANN H. J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv Physiol Pharmacol Acta. 1953;11(4):346–354. [PubMed] [Google Scholar]
- SCHATZMANN H. J. THE ROLE OF NA+ AND K+ IN THE OUABAIN-INHIBITION OF THE NA+ + K+-ACTIVATED MEMBRANE ADENOSINE TRIPHOSPHATASE. Biochim Biophys Acta. 1965 Jan 25;94:89–96. doi: 10.1016/0926-6585(65)90011-7. [DOI] [PubMed] [Google Scholar]
- Sachs J. R. Interaction of external K, Na, and cardioactive steroids with the Na-K pump of the human red blood cell. J Gen Physiol. 1974 Feb;63(2):123–143. doi: 10.1085/jgp.63.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Kinetics of the inhibition of the Na-K pump by external sodium. J Physiol. 1977 Jan;264(2):449–470. doi: 10.1113/jphysiol.1977.sp011677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R., Welt L. G. The concentration dependence of active potassium transport in the human red blood cell. J Clin Invest. 1967 Jan;46(1):65–76. doi: 10.1172/JCI105512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev. 1975 Mar;27(01):3–134. [PubMed] [Google Scholar]
- Schwartz A., Matsui H., Laughter A. H. Tritiated digoxin binding to (Na+ + K+)-activated adenosine triphosphatase: possible allosteric site. Science. 1968 Apr 19;160(3825):323–325. doi: 10.1126/science.160.3825.323. [DOI] [PubMed] [Google Scholar]
- Schönfeld W., Schön R., Menke K. H., Repke K. R. Identification of conformational states of transport ATPase by kinetic analysis of ouabain binding. Acta Biol Med Ger. 1972;28(6):935–956. [PubMed] [Google Scholar]
- VASSALLE M., GREENSPAN K., HOFFMAN B. F. AN ANALYSIS OF ARRHYTHMIAS INDUCED BY OUABAIN IN INTACT DOGS. Circ Res. 1963 Aug;13:132–148. doi: 10.1161/01.res.13.2.132. [DOI] [PubMed] [Google Scholar]
- WILBRANDT W. Zur Frage der Beziehungen zwischen Digitalis-und Kalzium-wirkungen. Wien Med Wochenschr. 1958 Sep 27;108(38-39):809–814. [PubMed] [Google Scholar]
- Whittam R., Ager M. E. Vectorial aspects of adenosine-triphosphatase activity in erythrocyte membranes. Biochem J. 1964 Nov;93(2):337–348. doi: 10.1042/bj0930337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins J. R., Bentolila J. J. Sodium dependence of the positive inotropic effect of cardiac glycosides. J Pharmacol Exp Ther. 1980 Dec;215(3):569–574. [PubMed] [Google Scholar]


