Abstract
The occurrence of venous diseases among adults is approximately 77% in females and 57% in males. These conditions are prevalent, progressive disorders that significantly affect individuals socially, physically, and psychologically, often resulting in various venous abnormalities that hinder effective blood circulation in the lower limbs. This review provides a comprehensive overview of venous diseases, focusing on their pathophysiology, symptoms, causes, risk factors, diagnosis, and complications. The symptoms associated with venous diseases are diverse and can include pain, heaviness, swelling, ulcers, and skin changes. Risk factors such as age, obesity, hormonal influences, and genetic predispositions are discussed in relation to their contribution to disease progression. The therapeutic modalities for managing venous diseases are explored, with a particular emphasis on natural products in alleviating symptoms and improving vascular health. Natural compounds, i.e., flavonoids, play a vital role in the circulatory system, supporting blood vessels and promoting healthy blood flow, in addition to their vasoprotective, antioxidant, anti-inflammatory, and anti-platelet properties. Overall, the ongoing research efforts on the efficacy of natural products will significantly enhance the management of several venous diseases in the coming years.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10787-025-01688-z.
Keywords: Venous diseases, Treatments, Phlebotropic, Venoactive, Varicose veins, Natural products, Risk factors
Introduction
Venous diseases are common progressive disorders which impose significant social, physical, and psychological impacts. Venous diseases are linked to significant pathological and hemodynamic changes in the lower limb veins that involve various venous anomalies significantly impairing blood return (Davies 2019). These venous anomalies result in a multiplicity of signs and symptoms which can range from mild clinical manifestations like varicose veins (VV), reticular veins, and telangiectasia to more severe ones like skin abnormalities and chronic venous leg ulcers (Costa et al. 2023).
Environmental and genetic factors inter play in the pathophysiology of venous disease leading to elevation of the ambulatory venous pressure; which significantly alters the entire structure and operation of the venous system (Youn and Lee 2019). Although any vein in the body may exhibit clinical symptoms, the lower limb venous system is frequently the most susceptible to venous diseases. The primary cause of this is the gravitational force’s greater resistance, which is noticeably greater than in other bodily parts (Alsaigh and Fukaya 2021).
Current statistics indicate that CVD affects a substantial percentage of the population, with severe forms leading to complications such as venous ulcers (Kelechi et al. 2015). Pharmacological treatments for venous diseases have evolved significantly over time, incorporating both natural and synthetic drugs, prominently featuring venoactive drugs, also known as venotonics. These medications are designed to address various pathophysiological mechanisms associated with CVD, including enhancing venous tone, reducing capillary permeability, and mitigating the release of inflammatory mediators. The efficacy of these drugs has been supported by numerous clinical trials, demonstrating their ability to improve symptoms such as pain, swelling, and heaviness in the legs (Bencsik et al. 2024). The aim of this review is to comprehensively document and analyze the various types of venous diseases, and its manifestations, including chronic venous insufficiency (CVI), varicose veins, and venous ulcers. This review will also explore the role of natural products in the management and treatment of these conditions.
Search strategy
This review seeks to bridge gaps in current understanding and practice regarding venous diseases and their management through both conventional pharmacological means and complementary natural therapies. Scientific data on this review were gathered from various literature sources, including reputable databases such as PubMed, Google Scholar, Springer, Scopus, and Science Direct and books focused on medicinal plants. A range of keyword combinations was utilized to facilitate this research, including terms like “Venous diseases/medicinal plants,” “Phytochemicals/anti-platelets,” “Plants as venoprotective,” and “Plant secondary metabolites/vasoactive.”
Anatomy, physiology and histology of the venous system in the lower limbs
Three major venous systems may be identified in the lower limbs: (1) superficial veins, which are mostly represented by the great and small saphenous veins, anterior accessory saphenous vein, and their branches. These veins transport blood from the skin and the subcutaneous tissues; (2) deep veins which are the key blood flow transporters; and (3) perforating veins which connect both systems (Ortega et al. 2021).
Histologically, three layers make up veins: (1) the inner layer (tunica intima) which is primarily made up of endothelial cells; (2) the middle layer (tunica media) which is made up of elastic fibers and vascular smooth muscle cells; and (3) the outer layer (tunica adventitia) which is made up of connective tissue to provide elasticity and support of the vessel (Jacobs et al. 2017). However, when venous diseases occurs, the vein’s typical structure is changed, with noticeable variations in the venous wall’s thickness and makeup (Taylor and Bordoni 2020).
Veins also contain bicuspid prolongations from the venous tissue called venous valves, which are necessary to keep blood flowing in the right direction and prevent venous reflux. In addition, to ensure unidirectional blood flow, various muscle pumps work in tandem with the venous valves. Often referred to as the “peripheral heart,” the calf muscle is particularly thought to be the most notable facilitator of venous return from the lower extremities to the heart (Tansey et al. 2019). Defects in both venous valves and calf muscle pump work lead to venous reflux and venous stasis thus causing evolution of venous diseases (Nicolaides et al. 2018).
Classification of venous diseases
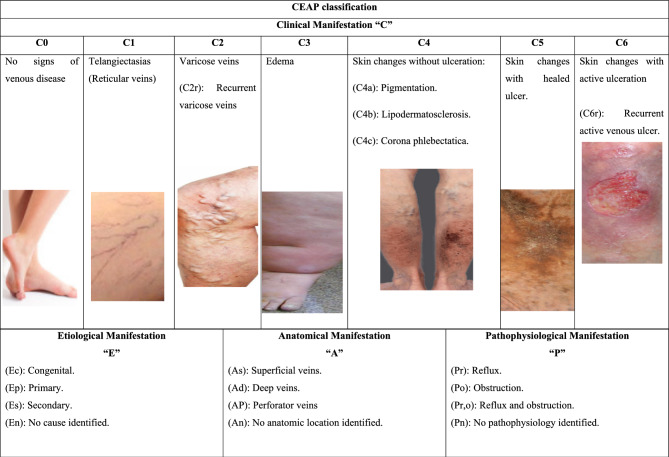
The most accurate and widely used approach for determining a precise venous disease diagnosis depend on the clinical, etiological, anatomical, and pathophysiological (CEAP) classification (Lurie et al. 2020). Table 1 summarizes the CEAP classification of venous diseases.
Table 1.
The clinical, etiological, anatomical, and pathophysiological (CEAP) classification of venous diseases
Clinical manifestations of venous diseases
Although the precise causes of venous diseases are not fully understood, it appears that genetics and a number of proteome variations interplay in the susceptibility, onset and progression of venous diseases (Raffetto 2018).
The clinical signs and symptoms in the early stages of venous diseases are infrequent but they tend to present and worsen over time. Although VV are the most characteristic manifestation, however, reticular veins also represent a crucial clinical manifestation. A superficial injury to a big VV may cause a hemorrhage, which in certain situations may be lethal (Castro-Ferreira, et al. 2018). The most noticeable presentation of advanced venous disease is pain in the lower limbs; typically described as discomfort, pressure and heaviness that worsen at the end of the day. When venous pressure rises and lymphatic drainage is compromised, patients often exhibit malleolar edema with fovea. Other causes of the edema, such as hypoalbuminemia, hypothyroidism, or heart failure, should be ruled out by differential diagnosis (Jarošíková, et al. 2023). It is noteworthy that most patients typically notice the esthetic alteration caused by the presence of dilated superficial veins. Patients suffering venous diseases are at an increased risk of developing deep venous thrombosis (DVT), primarily in the femoropopliteal region which might cause pulmonary thromboembolism (PE). Another possible clinical indicator of venous disease is superficial venous thrombosis (SVT) which is also strongly linked to an elevated risk of developing DVT and PE (Lutsey and Zakai 2023).
Early venous disease stages usually comprise eczema and skin pigmentation, primarily caused by deposition of hemosiderin. In addition, lipodermatosclerosis, and corona phlebectatica are also linked to venous disease. Corona phlebectatica is commonly recognized as anomalous visible cutaneous blood vessels at the ankle with venous cups, telangiectasias along with capillary stasis spots (Ortega et al. 2021).
Edema and inflammation impact skin and subcutaneous tissues during advanced stages of venous diseases, ultimately resulting in the rupture of the epidermis and cutaneous ulcer formation (Nicolaides 2020).
Etiology of venous diseases
The causes of venous diseases are multifactorial. The etiology of venous diseases is classified as per the CEAP classification into primary, secondary and congenital venous disease (Lurie et al. 2020).
Primary venous diseases
The primary venous disease is characterized by a progressive deterioration in the venous wall or venous valve causing improper vein dilatation and weakening; thus ultimately leading to pathological and evident venous reflux (Meissner et al. 2007a). The pathophysiology of primary venous disease includes sustained ambulatory venous hypertension that damages the vein wall and venous valves, thereby promoting the development and progression of venous disease. Primary venous disease is commonly characterized by truncal insufficiency, which occurs in the saphenous veins (Alsaigh and Fukaya 2021).
Primary venous disease is caused by a variety of genetic, environmental, occupational and life-style causes. The great majority of venous disease patients carry certain polymorphisms or genetic variations that contribute to the disease’s development. Actually, it's thought that around 17% of venous disease patients has a hereditary component (Baylis et al. 2021). The remaining percentage has been linked to a wide range of environmental elements that are typically linked to the risk factors for venous disease (Jawien 2003).
Genetic factors include familial predisposition and venous wall weakness. It is evident that there is a strong genetic component to venous diseases, particularly in conditions like VV and chronic venous insufficiency (CVI). Certain inherited disorders of the connective tissue (e.g., Ehlers-Danlos syndrome) can also predispose individuals to venous pathology. Genetic predisposition to weakened vein walls or faulty valve function increases the likelihood of venous dilation and valve incompetence (Bharath et al. 2014).
The age and gender of the patient also play important roles in the etiology and pathogenesis of venous diseases. Venous diseases are more common in older adults, as the veins lose elasticity, and the valve function deteriorates over time. Moreover, women are more likely than men to develop VV and other venous diseases, likely due to hormonal changes (e.g., pregnancy, menopause) and the higher likelihood of venous dilation in females (Robertson et al. 2008).
Environmental, occupational and life-style factors include prolonged standing or sitting as in case of occupations that involve long periods of standing or sitting which increase venous pressure, cause an inflammatory reaction in the venous wall and impair blood flow, leading to valve dysfunction and venous dilation (Elamrawy et al. 2021). Long-term standing causes a damaging and persistent venous stasis that gradually increases a person’s risk of developing venous reflux and venous insufficiency, especially when paired with other variables such aging (Bass 2007).
Obesity and increased body weight lead to higher pressure on the venous system, particularly in the lower extremities, contributing to valve failure and venous insufficiency (Davies et al. 2017). In addition, smoking is associated with a variety of venous diseases as it contributes to endothelial dysfunction and increases the risk of thrombosis (Vlajinac et al. 2012). Also, the hormonal changes during pregnancy, as well as the increased venous pressure from the growing uterus, can lead to venous dilation and VV. This is often a temporary condition but may become permanent in some cases (Ortega et al. 2022).
Secondary venous diseases
Secondary venous diseases are conditions that arise as a consequence of underlying or predisposing factors that alter the normal functioning of the venous system. Unlike primary venous diseases, which often occur without an identifiable cause (e.g., primary VV or CVI), secondary venous diseases result from external factors such as trauma, DVT, or external pressure on the venous system. Secondary venous disorders can cause similar symptoms to primary venous diseases, such as edema, pain, and skin changes, but their pathophysiology is driven by the underlying cause (Lurie et al. 2020). Secondary venous disease can be further classified into secondary intravenous disease, where the vein wall & valves are negatively impacted, and secondary extravenous disease, where there is no indication of vein damage but the venous hemodynamic is compromised (e.g., due to external factors like central venous hypertension, extrinsic compression from a tumor mass, previous DVT, post traumatic venous disease or in cases of limb muscle pump dysfunction) (Ortega et al. 2021).
DVT is one of the most common causes of secondary venous disease. When a blood clot forms in the deep veins, typically in the lower extremities, it causes partial or complete obstruction of venous return (Krüger-Genge et al. 2019). Over time, this can lead to post-thrombotic syndrome (PTS) or CVI, as the venous valves and vessel walls may be permanently damaged by the thrombus. If untreated, DVT can result in long-term venous stasis, valve incompetence, chronic pain, edema, skin pigmentation, and venous ulcers. The PTS is characterized by these chronic symptoms due to the damage inflicted on the veins by the clot leading to CVI with symptoms such as leg pain, swelling, heaviness, and skin changes (hyperpigmentation and lipodermatosclerosis) (Mazzolai et al. 2022).
External venous compression, either from an extrinsic mass or anatomical abnormality, can lead to secondary venous diseases. For example, tumors (such as pelvic cancers or abdominal masses), or compression due to obesity or pregnancy can obstruct venous return from the lower extremities or the pelvis (Eberhardt and Raffetto 2005).
Trauma or injury to the venous system such as those from penetrating trauma (resulting from knives ot bullets) or blunt trauma (fractures or dislocations) can lead to localized damage of the veins. This may result in venous thrombosis, venous insufficiency, or the formation of arterio-venous fistulas that alter normal venous flow. Consequently scarring or fibrosis of the veins occur resulting in CVI and long-term symptoms similar to those of primary venous diseases, such as swelling, pain, and skin changes (Stafforini and Singh 2023).
Structural abnormalities such as congenital venous malformations (CVMs) or vein walls weakened by prior surgery can result in secondary CVI. These structural defects often lead to impaired venous return and increased venous pressure, causing dilation of veins and valve incompetence (Youn and Lee 2018).
Iatrogenic causes such as surgical interventions, especially those involving the veins (e.g., vein stripping or VV surgeries), can result in secondary venous insufficiency. In some cases, iatrogenic damage to the venous system leads to scarring, stenosis, or valve dysfunction (Meissner et al. 2007b).
Obstruction of the Inferior Vena Cava can lead to secondary venous diseases, particularly in the lower extremities. Causes of Inferior Vena Cava obstruction include malignancy, thrombosis, or congenital anomalies. The result is venous congestion and impaired return of blood from the lower extremities. This condition can lead to severe edema, varicosities, and stasis dermatitis, and can result in the development of venous ulcers over time (Raju et al. 2006). It is noteworthy that secondary venous diseases generally progress faster than primary venous diseases (Labropoulos et al. 2009).
Congenital venous diseases
These conditions can result from abnormal development of the venous system during fetal development. The most common types of congenital venous diseases include CVMs which are clusters of abnormally formed veins that can cause a variety of symptoms depending on their size and location. These malformations result from the abnormal development of the venous system during fetal development. They are typically present at birth but may not be noticeable until later in life. Symptoms of CVMs include swelling, pain, and the formation of visible veins or a mass under the skin. Venous malformations can be located anywhere in the body but are commonly found in the legs, face, and abdomen. These malformations can grow over time, causing functional impairments or becoming prone to bleeding. In rare cases, they can cause DVT among other complications (Cooke-Barber et al. 2020).
Congenital VV, congenital CVI and congenital hemangiomas are among common congenital venous diseases. Hemangiomas are benign tumors made up of abnormal blood vessels. They are most commonly found in the skin but can also affect deeper tissues or organs. Symptoms include a rapidly growing red or purple mass under the skin, which may become painful or ulcerated. In rare cases, deep venous hemangiomas can cause more significant complications (Olsen et al. 2020).
Klippel-Trenaunay syndrome (KTS) is another rare congenital disorder caused by mutations in the PI3KCA gene. KTS is characterized by a combination of venous and capillary malformations accompanied by soft tissue or bony hypertrophy. The condition typically presents with one limb being larger than the other, with overgrowth of tissues (skin, fat, and bone), along with visible vascular malformations (Harnarayan and Harnanan 2022).
Moreover, Parkes Weber syndrome (PWS) is another congenital disease disturbing capillary, venous, arterio-venous and lymphatic systems. RASA1 gene is mutated in about 50% of PWS patients. However, about 10% of PWS patients show mutations in EPHB4 gene. It is noteworthy that PWS is commonly misdiagnosed with KTS where both cause limb overgrowth and vascular deformities (Banzic et al. 2017).
Bleb Nevus Syndrome (BRBNS) is a rare condition involving multiple venous malformations that affect the skin, gastrointestinal tract, and other internal organs. The “blue rubber blebs” are soft, blue, rubbery lumps often seen on the skin, and these venous malformations can also occur in internal organs (Agnese et al. 2010).
Other disorders associated with the development of congenital vascular diseases include cerebral autosomal-dominant arteriopathy with subcortical infarcts and lymphedema distichiasis syndrome (LDS; characterized by FOXC2 gene mutations), leukoencephalopathy (characterized by Notch3 gene mutations), Ehlers-Danlos syndrome (EDS; which is a severe congenital neutropenia characterized by changes in the G6PC3) and Chuvash Polycythemia (characterized by VHL mutations) (Fukaya et al. 2018).
Epidemiology of venous disease
Venous diseases are common and can significantly impact quality of life. The epidemiology of venous disease varies by region, age, sex, and other demographic factors, but several general patterns and statistics provide insight into its prevalence and impact.
Prevalence of venous disease
The prevalence of venous disease in the adult population is about 77% in women and 57% in men. The most common forms are VV & CVI, with DVT & venous ulcers being more severe manifestations (Salim, et al. 2021).
Approximately 20–25% of adults suffer from some form of VV, with a higher incidence in women. In fact, VV affect up to 40% of women and about 20–25% of men over the age of 40.
CVI affects approximately one in five adults globally. The prevalence of CVI increases with age, and it is more common in women, particularly those who have had multiple pregnancies or have a family history of venous disorders.
DVT affects 1 in 1000 individuals affected annually worldwide. However, the incidence is higher in hospitalized patients, those with cancer, and people with limited mobility or recent surgery. The overall lifetime risk of DVT is estimated at 1 in ten individuals, particularly among those over the age of 60 or those with other risk factors such as obesity, smoking, or a history of thrombosis.
It is estimated that 1–2% of people over 65 have a venous ulcer at any given time. In developed countries, venous ulcers account for about 70% of all lower extremity ulcers (Salim et al. 2021).
Geographic and demographic variations
The prevalence of venous disease can vary across different geographic regions. Studies suggest that venous disorders may be more common in developed countries due to lifestyle factors such as sedentary behavior, higher rates of obesity, and longer life expectancy. On the other hand, venous disease may be underdiagnosed or less commonly reported in developing countries.
While venous disease is common across all racial and ethnic groups, Caucasians have the highest incidence of VV and CVI. Black and Asian populations tend to have lower rates of VV (Salim et al. 2021).
Risk factors for venous disease
Risk factors for venous disease include age, female gender, obesity, prolonged standing or sitting, lack of physical activity, sedentary behavior, pregnancy, smoking, genetic factors and some chronic conditions like heart failure, diabetes, and inflammatory diseases (e.g., rheumatoid arthritis) (Chung and Heo 2023).
Diagnosis of venous diseases
The diagnosis of venous disease typically involves a combination of clinical assessment, imaging techniques, and laboratory tests. The common techniques include:
Clinical assessment: A detailed patient history should be taken to identify symptoms such as pain, swelling, heaviness, fatigue, or visible varicosities. Risk factors such as prolonged standing, obesity, pregnancy, or a family history of venous disease are also considered (Barstow and Kassop 2019). Physical examination includes signs such as visible VV, leg swelling or skin changes (e.g., pigmentation, ulcers, or atrophie blanche) (Ghosh et al. 2023).
Color Doppler ultrasound: Duplex Ultrasound is the gold standard for diagnosing venous disease since it is non-invasive and provides real-time images of the veins. It combines traditional ultrasound imaging with Doppler flow analysis. It allows clinicians to visualize blood flow in veins, assess vein size, and detect any abnormalities such as venous reflux, venous insufficiency or thrombosis (Martinelli et al. 2021).
Contrast venography, computed tomography venography and Magnetic Resonance Venography are currently less commonly used due to the prevalence of Duplex Ultrasound. However, venography can be performed in some cases where a detailed view of the venous system is required where ultrasound results are inconclusive (Chen et al. 2020; Min et al. 2010).
Plethysmographic techniques are also non-invasive techniques which assess the severity of CVI and evaluate the function of the venous system. They identify how effectively blood is moving through the veins and whether there is venous reflux (Sannikov et al. 2020).
Transcutaneous Oxygen Tension Measurement is used in assessing venous ulcers and CVI via measuring the oxygen level at the skin’s surface (Leenstra et al. 2020).
Trendelenburg tourniquet test involves elevating the leg and then having the patient stand to observe the veins. It can help assess the function of the venous valves (Hoffmann et al. 2004). Figure 1 summarizes the diagnosis of venous diseases.
Fig. 1.

Diagnostic techniques in venous diseases
Pathogenesis of venous diseases
The pathogenesis of venous diseases involves several interconnected processes, most notably venous hypertension, valve incompetence, and venous stasis.
Venous hypertension refers to elevated pressure in the veins, often due to a failure of the vein’s structural components or increased resistance to blood flow. This can result from incompetent venous valves; where the valves in the veins become damaged or dysfunctional and blood can flow backward (reflux) thus increasing venous pressure and promoting vein dilation Moreover, conditions like DVT can obstruct the venous flow, leading to increased pressure in the veins (Ortega et al. 2021).
Valve incompetence causes blood to flow backward, leading to blood pooling in the veins (venous reflux), which increases venous pressure and contributes to venous dilation and VV. Prolonged venous reflux leads to venous stasis, where blood pools in the lower extremities. This stasis can cause pain, swelling, and skin changes, and may eventually lead to venous ulcers. With increased pressure over time, the walls of the veins can become dilated and less elastic. This worsens the reflux and further exacerbates the venous hypertension. Over time, chronic venous hypertension can lead to structural changes in the vein walls, including thickening and fibrosis, further impeding venous return (Kumar et al. 2022).
Furthermore, CVI is often associated with a low-grade inflammatory response. The increased venous pressure can cause endothelial injury, leading to local inflammation. This may contribute to further venous wall changes, such as fibrosis and thickening. Chronic venous stasis can impair the function of the endothelial cells that line the blood vessels. This dysfunction promotes thrombosis, platelet aggregation, and the release of pro-inflammatory mediators, contributing to the worsening of venous disease (Navarrete et al. 2023).
The formation of thrombus in the deep veins can lead to venous obstruction and increased pressure in the venous system. This results in venous stasis and worsening of venous insufficiency. Over time, the clot can be resolved, but it often leaves behind damage to the vein walls and valves. PTS usually occurs as a result of long-term venous obstruction following DVT. It leads to chronic pain, swelling, skin changes, and ulcers, as the venous system is no longer capable of effectively draining blood from the lower extremities (Kumar et al. 2022).
Management and treatment of venous diseases
Treatment of venous diseases aims to alleviate symptoms, prevent complications, enhance prognosis and improve quality of life
The primary strategies for the treatment and management of various venous diseases include compression treatments that focus on venous hemodynamics, medications meant to manage venous insufficiency, pharmaceutical treatments meant to target certain disease pathophysiological pathways along with interventional treatments. It is noteworthy that older patients and patients with more comorbidities respond well to the various therapies they receive, even though they need more interventions than younger patients or patients who do not have any comorbidities (Pappas, et al. 2018).
Conservative management techniques include exercise, leg elevation, weight management and compression stockings. While interventional treatments are used in patients who are not responsive to conservative treatments. Interventional treatments include sclerotherapy, laser, radiofrequency ablation (RFA) and vein stripping or ligation (Orhurhu, et al. 2021).
Compression therapy raises the interstitial pressure and consequently diminishes the diameter of the superficial and deep veins causing a reduction in the venous pressure and edema while encouraging the calf muscles to contract. Most patients encounter significant improvements with minimal discomfort while using compression stockings, which are simple to use and often the first conservative strategy to take. Thigh length stockings offer the best results in terms of venous hemodynamics and volume reduction (Dissemond et al. 2018). In addition, compression stockings promote the healing of VV and CVI-related ulcers since they reduce the likelihood of recurrent ulcerations in venous disorders (Konschake et al. 2017). Nevertheless, it must be taken into consideration that patients with peripheral artery disease need to use this specific medication with caution because it may impair lower limb blood circulation and exacerbate the underlying condition. To rule out vascular involvement, which would make the use of compression stockings inappropriate for certain individuals, the ankle-brachial index should be computed (Rabe et al. 2020).
Similarly, a variety of venoactive medications can be used to pharmacologically treat venous diseases. These substances improve the inflammatory response by reducing vascular permeability. They may also, to some degree, raise vascular tone and affect platelets aggregation (Ortega et al. 2021). Acetylsalicylic acid, pentoxifylline, flavonoids, and saponins are among the pharmacological therapies. These pharmaceutical medicines are primarily used to treat mild symptoms like pain or early stages of edema. Furthermore, there is a moderate amount of data to support their use in treating specific clinical indications of CVI, such as trophic abnormalities, cramping, restless legs, edema, and paresthesia; nevertheless, there is conflicting information about their effectiveness in treating venous ulcerations (Perrin and Ramelet 2011).
However, some medications, including sulodexide or flavonoids as diosmin and hesperidin, seem to be of promise in treating venous ulcers and CVI, especially when used in conjunction with compression treatment (Carroll et al. 2019). Under certain restrictions, acetylsalicylic acid may be used in individuals with refractory ulcers. Further research is necessary to fully understand the role of these medications (Lichota et al. 2019). Anticoagulants have been suggested for use in additional venous disease situations, including venous ulcerations, SVT, DVT and PE (Lutsey and Zakai 2023).
Several surgical techniques and technological advancements have been approved as interventional therapies for the treatment of venous diseases. These techniques are used for the efficient control of the disease, avoidance of long-term consequences and targeting optimum cosmetic outcomes (Wittens et al. 2015).
VV treatment is the most common interventional procedure for patients with venous disease. One of the most widely used methods for managing VV in the lower limbs is ultrasound-guided sclerotherapy. This technique involves using various chemical agents, such as polidocanol, sodium tetradecyl sulfate, and glycerin, to treat veins and venules. Sclerotherapy is particularly beneficial for patients with existing health conditions where more invasive treatments may not be suitable. It can also serve as an alternative to other methods for those with advanced CVI and saphenous vein dysfunction who are not candidates for surgical intervention. The effectiveness of sclerotherapy in managing venous disease symptoms is comparable to other interventions, with a low incidence of adverse effects. In addition, it has shown notable benefits for patients with superficial venous reflux, aiding in ulcer healing and preventing recurrences at rates similar to other procedures (Wong et al. 2023).
Recent advancements have introduced sclerosant foam-based and catheter-based treatments, which offer comparable results to thermal ablation techniques (de-Abreu et al. 2017).
In contrast, open surgery (such as saphenous junction ligation and stripping) has shown mixed results regarding recurrence over the mid-to-long term. Some studies suggest that surgery is more effective than conservative treatments in addressing symptoms of VV. Before endovenous thermal or chemical ablation techniques emerged, surgery was considered the standard treatment for CVI. However, due to its invasiveness and higher complication rates, including hematomas, surgical wound infections, and nerve damage, surgery is now typically reserved for patients with large, tortuous superficial vein dilations or certain vascular malformations (De Maeseneer et al. 2022).
Vein stripping or ligation is a surgical procedure used to treat VV, particularly when more conservative or less invasive treatments are not effective. The procedure involves tying off (ligation) and removing (stripping) the affected veins. The goal is to eliminate the problematic veins, which may be causing symptoms like swelling, pain, and discomfort (Gao et al. 2022).
Today, ultrasound-guided endovenous ablation has largely replaced traditional surgical approaches in the treatment of venous disease. Two primary types of endovenous thermal ablation are used: endovenous laser ablation (EVLA) and RFA. Both procedures cause controlled thermal injury to the VV, leading to clot formation and eventual fibrosis of the saphenous vein. These methods provide similar outcomes to surgery but with less recovery time and a significantly lower risk of complications. Serious complications like DVT, SVT, skin pigmentation changes and nerve damage are rare (Cai et al. 2023).
More recently, cyanoacrylate-based treatments have emerged as an alternative, although their cost-effectiveness may not be ideal in many cases. For deep vein occlusions or obstructions, particularly in acute DVT or PTS, therapeutic options like endovenous percutaneous transluminal angioplasty (PTA) with stenting are available. Stents are primarily used in patients with chronic iliac vein occlusions that have not responded to medical therapy (Ilie-Ene et al. 2023).
Preventive measures and lifestyle changes
Prevention of venous diseases is key, especially in individuals at high risk (e.g., those with a family history, pregnant women, or those with prolonged immobility). Some preventive strategies include regular exercise, avoidance of prolonged standing or sitting, weight management and leg elevation to improve venous return (Todd 2012).
In summary, there is a variety of therapeutic options available for managing cardiovascular disease (CVD), each aimed at improving symptoms and alleviating the clinical impact of this chronic condition (Fig. 2).
Fig. 2.
Available treatment options in various venous diseases
Future directions for the management of venous diseases
The treatment of venous disease is continually evolving, with advancements focusing on improving patient outcomes, reducing recovery times, and minimizing complications. Future directions in the treatment of venous disease are likely to revolve around several key areas including:
Minimally invasive and non-surgical techniques
EVLA and RFA are already standard treatments, but future developments may enhance their effectiveness and precision. Newer technologies and improvements in catheter design may make these treatments even less invasive and more comfortable for patients (Cai et al. 2023).
Gene and cell-based therapies
In the future, gene therapies might be used to target the underlying causes of venous disease, such as venous wall weakness or valve dysfunction. Introducing specific genes that promote the repair of damaged veins or encourage the production of proteins that support vein structure could offer novel, long-term solutions. Stem cell therapy has the potential to regenerate damaged tissues, including the endothelial lining of veins. Stem cell injections could help restore vein function in patients with CVI or PTS, leading to improved circulation and reduced symptoms (Bashor et al. 2022).
Personalized treatment approaches
Genomic profiling may enable clinicians to tailor venous disease treatments based on an individual’s genetic makeup. This approach could identify the most effective therapies for each patient, improving outcomes and minimizing side effects.
Artificial intelligence (AI) and imaging could be used to enhance diagnostic imaging and predict patient responses to various treatments. Real-time imaging technologies, coupled with AI algorithms, may provide more precise mapping of venous structures and better guidance for interventional procedures (Li et al. 2023).
Innovative biomaterials and drug delivery systems
New materials for vein stents, catheters, and other devices may enhance the treatment of venous disease by promoting better integration with the vein wall, reducing complications and prevent vein re-occlusion or reduce inflammation. Targeted drug delivery involves the targeted delivery of drugs to the affected veins, which might help in reducing the need for systemic treatments and minimize side effects. For example, drugs that promote collagen formation or improve vein elasticity could be directly applied to diseased veins (Wang et al. 2022).
Advanced biological and pharmacological treatments
Research into drugs that improve venous tone, reduce venous reflux, and support venous wall integrity may play a key role in the treatment of venous disease. Agents that target the underlying pathophysiology, such as endothelial dysfunction, could become part of a multimodal approach alongside procedural treatments. Regenerative Medicine use growth factors, cytokines, and other biologic agents to regenerate vein tissues and restore valve function in VV (Aleksandrowicz et al. 2021).
6. Improved long-term outcomes and recurrence prevention.
Integrated multimodal approaches such as endovenous ablation followed by sclerotherapy or the use of pharmacological agents post-procedure. This multimodal approach could help reduce recurrence rates and improve long-term outcomes. Improved patient Monitoring via wearable technology could allow for continuous monitoring of venous disease, helping to track symptoms, such as swelling or pain, in real-time. This could enable early intervention to prevent complications and reduce the risk of recurrence (Jayaraj et al. 2021).
Regenerative medicine
Vein regeneration focus on regenerating damaged veins or improving the function of venous valves. Tissue engineering approaches could help rebuild or replace damaged veins, providing a long-term solution for patients with severe chronic venous insufficiency (Fernández-Colino and Jockenhoevel 2021).
In conclusion, the future of venous disease treatment is poised to be shaped by technological innovations, personalized approaches, and new therapies that address both the symptoms and underlying causes of the disease. These advancements hold the potential to improve patient care, reduce the invasiveness of treatments, and ultimately provide more effective, long-lasting solutions.
Key plants used for venous diseases
Blood clots, deep vein thrombosis (DVT), and superficial thrombophlebitis (SVT)
Blood clots, particularly DVT and SVT, represent significant health concerns due to their potential complications, including PE (Saha et al. 2016). DVT occurs when blood clots form in the deep veins, primarily in the legs, and is a major contributor to morbidity and mortality worldwide, with an estimated incidence of 1 in 1000 people annually (Wolberg et al. 2015). SVT involves inflammation of superficial veins, which can lead to DVT in some cases (Williams et al. 2020). Natural products have emerged as promising alternatives in the management of blood clots (Table 2). These conditions are often linked to cardiovascular diseases, which are a leading cause of mortality globally. Natural compounds, particularly those derived from plants, exhibit antithrombotic properties that can mitigate the risks associated with traditional therapies, which often come with significant side effects. Antiplatelet, anticoagulant, and fibrinolytic activities are the main mechanisms that reflect the efficacy and potential of these natural products in addressing thrombotic disorders (Mazumder et al. 2022; Fernández-Rojas et al. 2022; Islam et al. 2016). Unlike conventional antithrombotic drugs, many natural products exhibit minimal side effects, making them safer alternatives for long-term use (Mazumder et al. 2022). So, continued exploration of natural products is essential for identifying new antithrombotic agents that can complement or replace existing therapies.
Table 2.
Summary of the main natural used for blood clots, deep vein thrombosis (DVT), and superficial thrombophlebitis
| No | Plant name | Type of extract | Part used | Family | Methods | Results | Disease | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | Ginkgo biloba | Ethanol | Leaves | Ginkgoaceae | Clinical trials, lab studies | Improves circulation, decreases platelet aggregation, and blood clotting | Blood clots, DVT, thrombophlebitis | (Li et al. 2019a) |
| 2 | Aesculus hippocastanum | Water | Seeds | Sapindaceae | Clinical trials | Reduces swelling, strengthens veins and improves venous tone in CVI | Chronic venous insufficiency | (Bencsik et al. 2024) |
| 3 | Curcuma longa | Ethanol | Rhizomes | Zingiberaceae | Clinical trials, animal studies | Curcumin has anti-coagulant and anti-inflammatory effects | Blood clots, DVT | (McEwen 2015) |
| 4 | Centella Asiatica | Water | Leaves | Apiaceae | Clinical trials, lab studies | Improves blood vessel integrity and relieves symptoms of varicose veins | Chronic venous insufficiency | (Udombhornprabha 2018) |
| 5 | Capsicum annuum | Ethanol | Fruit | Solanaceae | Animal studies, clinical trials | Increases blood flow, dilates vascular structures, and may even prevent coagulation | Blood circulation, varicose veins | (Rhone et al. 2018) |
| 6 | Taraxacum officinale | Water | Root | Asteraceae | Animal studies, lab studies | Acts as a diuretic and improves circulation, thus relieving the symptoms of CVI | Venous circulation, inflammation | (López-Pérez et al. 2022) |
| 7 | Trifolium pratense | Methanol | Flower heads | Fabaceae | Clinical trials, lab studies | Promotes circulation and tones up blood vessels; good for varicose veins and venous insufficiency | Blood circulation, varicose veins | (Mokhtari et al. 2020) |
| 8 | Vaccinium myrtillus | Ethanol | Fruit, leaves | Ericaceae | Clinical trials, lab studies | Strengthens blood vessel walls and lessens swelling thus helping varicose veins | Venous insufficiency, clot prevention | (Sparreboom et al. 2004) |
| 9 | Pinus pinaster | Supercritical CO2 | Bark | Pinaceae | Clinical trials, animal studies | Pycnogenol increases circulation, defends veins, and minimizes swelling | Blood clots, DVT | (Gulati 2014) |
| 10 | Mangifera indica | Ethanol | Leaves | Anacardiaceae | Animal Studies | Blood flow maintained; prevents clotting through the strengthening of vein walls | Blood circulation, antioxidant support | (Ain 2022) |
| 11 | Vitis vinifera | Ethanol | Seeds | Vitaceae | Clinical trials, lab studies | Grape seed extract has antioxidants and may prevent clot formation | Blood clots, thrombophlebitis | (Bencsik et al. 2024) |
| 12 | Ruscus aculeatus | Methanol | Root | Ruscaceae | Clinical trials | Reduces symptoms of chronic venous insufficiency, including swelling and leg heaviness | Blood circulation, CVI | (Bihari, et al. 2022) |
| 13 | Fagus sylvatica | Ethanol | Leaves | Fagaceae | Clinical studies | Improves microcirculation and strengthens veins. Used in venous insufficiency symptoms | Venous insufficiency | (Sánchez-Ferrer et al. 2023) |
| 14 | Achillea millefolium | Ethanol | Flower, leaves | Asteraceae | Clinical trials, lab studies | Yarrow is known to enhance circulation and reduce the risk of clotting because of its anti-inflammatory effects | Blood clots, circulation | (Bijak et al. 2016) |
| 15 | Cinnamomum verum | Ethanol | Bark | Lauraceae | Animal studies, clinical trials | Improves circulation and blood flow and reduces blood viscosity | Venous health, blood circulation | (Raja et al. 2020) |
| 16 | Crocus sativus | Ethanol | Stigma | Iridaceae | Clinical trials | Saffron has anti-coagulant properties, which reduce blood clotting | DVT, blood clots | (Chen et al. 2023) |
| 17 | Commiphora wightii | Ethanol | Resin | Burseraceae | Clinical trials, animal studies | Known for its anti-inflammatory properties and improving blood flow | Blood circulation, varicose veins | (Rastogi et al. 2016) |
| 18 | Withania somnifera | Ethanol | Root | Solanaceae | Clinical studies | Enhances blood flow and exhibits slight anticoagulant properties, thereby supporting overall cardiovascular well-being | DVT, blood clots | (Basudkar et al. 2024) |
| 19 | Eleutherococcus senticosus | Ethanol | Root | Araliaceae | Clinical studies | Improves circulation and strengthens vein walls—helpful in cases of varicose veins and DVT | CVI, blood circulation | (Han et al. 2021) |
| 20 | Allium sativum | Ethanol | Bulb | Amaryllidaceae | Clinical trials, lab studies | Garlic improves circulation and has anti-clotting activity | Blood clots, varicose veins | (Alaraky 2018) |
| 21 | Boswellia serrata | Chloroform | Resin | Burseraceae | Clinical studies | Anti-inflammatory and blood circulation-enhancing effects | Blood clots, thrombophlebitis | (Valente et al. 2024) |
| 22 | Crataegus oxyacantha | Ethanol | Berries, leaves | Rosaceae | Clinical trials, lab studies | Hawthorn increases circulation and stabilizes blood vessels, thereby minimizing varicose veins and formation of clots | Blood circulation, venous health | (Nabavi et al. 2015) |
| 23 | Cinnamomum cassia | Ethanol | Bark | Lauraceae | Clinical studies | It improves circulation and possesses some anticoagulant properties | DVT, blood clots | (Chase et al. 2022) |
| 24 | Piper nigrum | Ethanol | Fruit | Piperaceae | Clinical studies | Improves circulation and may reduce the risk of clotting | Venous circulation | (Jain et al. 2023) |
| 25 | Echinacea purpurea | Ethanol | Root | Asteraceae | Clinical studies | Stimulates circulation and improves immune function, reduces swelling of veins | Varicose veins, circulation | (Lamponi 2021) |
| 26 | Zingiber officinale | Ethanol | Rhizomes | Zingiberaceae | Clinical trials, lab studies | It has good properties in improving circulation, ant-inflammatory, and blood-thinning | Blood circulation, venous health | (McEwen 2015) |
| 27 | Salvia miltiorrhiza | Methanol | Root | Lamiaceae | Clinical studies | Known to enhance circulatory dynamics and relieve venous edema and pain | CVI, varicose veins | (Cao et al. 2015) |
| 28 | Mentha piperita | Ethanol | Leaves | Lamiaceae | Clinical Studies | Peppermint can also help improve circulation and might reduce swelling and pain in the veins | Varicose Veins, Blood Circulation | (Leite et al. 2022) |
| 29 | Alchemilla vulgaris | Ethanol | Leaves | Rosaceae | Clinical Trials, Lab Studies | Known for its astringent and anti-inflammatory properties, it is beneficial for venous issues like thrombophlebitis | DVT, thrombophlebitis | (Radović et al. 2022) |
| 30 | Carica papaya | Ethanol | Leaves | Caricaceae | Clinical Studies | Improves circulation and reduces swelling associated with venous disease | Blood circulation, CVI | (Koehler, et al. 2022) |
| 31 | Citrus sinensis | Ethanol | Peel, fruit | Rutaceae | Clinical Trials, Animal Studies | Orange peel, which is high in flavonoids and antioxidants, enhances blood flow and decreases venous swelling | Blood circulation, varicose veins | (Leite et al. 2022) |
| 32 | Carthamus tinctorius | Ethanol | Flowers | Asteraceae | Clinical Studies | Reduces inflammation and supports proper blood circulation; used commonly for venous insufficiency | Venous health, circulation | (Ding et al. 2015) |
| 33 | Hypericum perforatum | Ethanol | Flowers | Hypericaceae | Clinical trials, animal studies | Known for its anti-inflammatory and mild anticoagulant properties, which help in blood flow and prevent clots | Venous circulation, blood clots | (Scholz et al. 2021) |
| 34 | Silybum marianum | Ethanol | Seed | Asteraceae | Clinical studies | Improves liver health and circulation, reducing symptoms of venous insufficiency | Venous health, circulation | (Lichota et al. 2020) |
| 35 | Zea mays | Ethanol | Stigma | Poaceae | Clinical trials, animal studies | Corn silk is claimed to reduce swelling and promote circulation in conditions such as DVT and varicose veins | Blood circulation, inflammation | (Raina, et al. 1997) |
| 36 | Tilia cordata | Ethanol | Flowers, Leaves | Malvaceae | Clinical studies | Linden has mild anticoagulant effects, therefore improves blood flow and lowers blood pressure | Venous circulation | (Risto et al. 2024) |
| 37 | Carya ovata | Hexane | Bark | Juglandaceae | Clinical trials, animal studies | Indicated to improve venous circulation and relieve symptoms related to venous insufficiency | Blood circulation, CVI | (Khidyrova and Shakhidoyatov 2002) |
| 38 | Plumbago zeylanica | Ethanol | Root, leaf | Plumbaginaceae | Animal studies | Historically used to improve blood circulation, it has mild anticoagulant properties | DVT, blood circulation | (Guguloth et al. 2023) |
| 39 | Mentha spicata | Ethanol | Leaves | Lamiaceae | Clinical studies | Known for its property to cause vasodilation that increases blood flow and reduces swelling in the lower limbs | Varicose veins, circulation | (Leite et al. 2022) |
| 40 | Citrus aurantium | Ethanol | Peel, fruit | Rutaceae | Animal studies, clinical trials | Bitter orange improves circulation and may prevent the formation of blood clots due to its flavonoid content | Blood clots, DVT | (Shahriar et al. 2018) |
| 41 | Eucalyptus globulus | Supercritical CO2 | Leaves, oil | Myrtaceae | Animal studies | Known to improve blood flow and reduce swelling due to its anti-inflammatory properties | Blood circulation, DVT | (DiGiorgio et al. 2017) |
| 42 | Rheum palmatum | Ethanol | Root | Polygonaceae | Clinical studies | Reduces inflammation, improves healthy blood circulation, and may be beneficial in preventing the formation of venous thrombus | Venous health, circulation | (Yang et al. 2017) |
| 43 | Glycyrrhiza glabra | Ethanol | Root | Fabaceae | Clinical trials, animal studies | Licorice has an anti-inflammatory action that reduces swelling and enhances venous circulation | DVT, thrombophlebitis | (Shi et al. 2020) |
| 44 | Cichorium intybus | Ethanol | Root | Asteraceae | Clinical trials, animal studies | Promotes blood flow, reduces swelling, and is good for the treatment of varicose veins | Varicose veins, blood circulation | (Khosropanah et al. 2023) |
| 45 | Cucurbita pepo | Ethanol | Seed, fruit | Cucurbitaceae | Animal studies, clinical trials | Rich in antioxidants, pumpkin improves circulation and may help reduce venous inflammation | Blood circulation, DVT | (Harenberg 2008) |
| 46 | Aloe vera | Water | Leaf gel | Asphodelaceae | Clinical studies | Aloe vera is used for its anti-inflammatory properties, which reduce swelling in the legs and improve circulation | Blood circulation, varicose veins | (Udombhornprabha 2018) |
| 47 | Rosmarinus officinalis | Ethanol | Leaves, Oil | Lamiaceae | Clinical trials, animal studies | Rosemary “Improves blood circulation and can lower clotting,” thanks to its mild anti-coagulant properties | Blood clots, DVT | (Bencsik et al. 2024) |
| 48 | Vitex agnus-castus | Ethanol | Berries, Leaves | Verbenaceae | Clinical studies | Supports venous health by promoting greater blood flow and reducing inflammation in the vascular system | Venous health, circulation | (Shukhman and Bernstein 2024) |
| 49 | Fucus vesiculosus | Ethanol | Seaweed | Fucaceae | Clinical trials, animal studies | May help improve blood circulation and support venous health due to its iodine and antioxidant properties | Blood circulation, varicose veins | (Chandika et al. 2022) |
| 50 | Butea monosperma | Ethanol | Bark, leaves | Fabaceae | Animal studies, laboratory research | To increase the circulation of blood, minimize edema, and thrombi formation | Blood clots, DVT | (Jarald et al. 2009) |
Chronic venous insufficiency (CVI)
CVI is a prevalent global pathology affecting 20–80% of the population worldwide and various factors influence it including hereditary and risk factors (i.e. obesity with BMI > 30 kg m−2, prolonged sitting or standing, physical inactivity, smoking, pregnancy and hormone therapy) (Krizanova et al. 2024). CVD affects all veins in the body, but lower extremity veins are most susceptible due to higher gravitational pressure compared to other regions (Kienzl et al. 2024).
Venous dysfunction, originating from venous hypertension, causes venous reflux, blood pooling, hypoxia, inflammation, and venous ulcers. CVI is also linked to severe complications like venous leg ulcers, which are more common in older patients and recur within three months (Krizanova et al. 2024).
The three fundamental modalities of conservative management for CVD are exercise and lifestyle (focusing on enhancing lower limb muscle strength, ankle flexibility, and physiotherapy, quitting smoking and reducing excessive alcohol consumption), compression therapy (utilizing elastic stockings or wraps on the legs, ankles, and feet to avert blood pooling and fluid accumulation), and pharmacological intervention (administering venoactive agents, including both natural and synthetic compounds such as butcher’s broom (Ruscus spp.) extract, micronized purified flavonoid fraction (MPFF), calcium dobesilate, horse chestnut (Aesculus hippocastanum L.) extract, hydroxyethyl rutosides (troxerutin, troxerutin), common grape (Vitis vinifera L.) extract, and sulodexide) (Bencsik et al. 2024). The pharmacist recommends conservative CVD therapy based on the individual patient’s symptoms and severity, following steps 1–4 (Fig. 3). In severe cases, Step 4; surgical invention i.e., removal of veins or vein sections that have lost their function, is the responsibility of a physician.
Fig. 3.

Steps for CVD therapy as based on the individual patient’s symptoms and severity
Certain medicinal plants have been found to alleviate symptoms of CVD and delay the progression of vessel wall impairment or destruction (Table 3) (Bencsik et al. 2024). Flavonoids, classified as medical food, are found to be the most effective treatment methods for CVI alongside compression treatment and exercise, despite various research methods being explored (Krizanova et al. 2024). Phlebotonics, a group of plant flavonoids, are considered venoactive and vasoprotective agents. They have varied opinions, with some studies showing benefits like diosmin (MPFF) for reducing chronic venous disease symptoms and slightly reducing edema and ankle circumference (Krizanova et al. 2024).
Table 3.
Summary of the main natural venoactive drugs for chronic venous disease; constituents, treatment duration, effects on symptoms, mechanism of action, possible side effects (Bencsik et al. 2024)
| Plant name | Common name | Family | Geographical origin | Part used | Active constituents | Duration for treatment | Relieve symptoms of | Mechanism of action | Possible side effects | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Aesculus hippocastanum L | Horse chestnut | Sapindaceae | Asia | Seeds | Acylated triterpene saponins: aescin 3–10% (= protoaescigenin + barringtogenol C) | At least 4 weeks of treatment | Discomfort and heaviness of legs related to minor venous circulatory disturbances and signs of bruises, such as local edema and haematoma | Decrease the release of inflammatory mediators (e.g. cytokines, arachidonic acid metabolites such as prostaglandins and leukotrienes) and block elastase and hyaluronidase activities in the process of degradation of connective tissue | Skin inflammation, thrombophlebitis, subcutaneous induration, severe pain, ulcers, sudden swelling of one or both legs, and cardiac or renal insufficiency | (Bencsik et al. 2024) |
| Vitis vinifera L | Red grape vine | Vitaceae | Leaves and seeds | polyphenols (different flavonoids such as flavonol-glycosides and glucuronides, catechins, epicatechins (both monomers and dimers), and anthocyanidins) | 12 weeks | Relieve symptoms of discomfort and heaviness of legs related to minor venous circulatory disturbances, (ii) symptomatic relief of itching and burning associated with hemorrhoids, and (iii) symptomatic treatment of cutaneous capillary fragility | Improve microcirculation, and oxygen supply, and significantly reduce edema, leg pain, heaviness, and tension | Hypersensitivity reactions of the skin (itching, erythema, urticaria), nausea, gastrointestinal complaints, and headache | ||
| Ginkgo biloba L | Maidenhair tree | Ginkgoaceae | China | leaves | Flavonols (quercetin, kaempferol, isorhamnetin, and their mono-, di-, and triglycosides), Biflavonoids, (amentoflavone, ginkgetin, bilobetin, sciadopitysin) and Terpene lactones (diterpenes, e.g. ginkgolides A, B, C, D, K, L, M, N, P, Q and bilobalide) | Used in combination with troxerutine and heptaminol, to protect against endothelial injury occurring during ischemic or hypoxic periods of chronic venous insufficiency | Gastrointestinal disorders (diarrhea, abdominal pain, nausea, vomiting), headaches, dizziness, and allergic reactions | |||
| Melilotus officinalis (L.) | Melilot or yellow sweet clover | Fabaceae | USA and Canada | Aerial parts | Coumarins (scopoletin, umbelliferone, melilotin). o-hydroxycinnamic acid derivative (melilotoside), Dicoumarols, Flavonoids (flavonols, e.g. quercetin, kaempferol), and triterpene saponins (melilotoside A, B, and C) | 1 or 2 weeks | Relieve symptoms of discomfort and heaviness of legs related to minor venous circulatory disturbances”, and “for the treatment of minor skin inflammations. Diminution of the sovrafascial edema |
Dicoumarol inhibits the reduction of vitamin K and thus prevents the gamma-carboxylation of the glutamate residues in clotting factors II, VII, IX, and X Coumarins stimulate proteolysis in the tissues affected by chronic lymphedema |
Allergic skin reactions | |
| Rosmarinus officinalis L | Lamiaceae | Leaves | Monoterpenes such as 1,8-cineol, α-pinene, camphor, bornyl acetate, borneol, camphene, α-terpineol and ( +)-verbenone. Also, abietane diterpenes, triterpenes, flavonoids and rosmarinic acid | Relief of dyspepsia and mild spasmodic disorders of the gastrointestinal tract. Relief of minor muscular and articular pain and in minor peripheral circulatory disorders | Treat phlebitis related to intravenous therapy and increase local circulation and cause heat and relief of pain and inflammation | Lack of adequate data | ||||
| Ruscus aculeatus L | Butcher’s broom | Asparagaceae | Europe | Leaves | Steroidal saponins such as ruscoside and ruscin | 2 weeks | Relieve the symptoms of discomfort and heaviness of legs related to minor venous circulatory disturbances. Also, relief of itching and burning associated with hemorrhoids |
Decreases capillary filtration rate in healthy volunteers and patients with CVD. Also, it reduces vascular permeability Ruscogenins has antielastase activity. Ruscus extract could significantly decrease the venous diameter in deep veins and at the same time led to an increase in flow parameters |
Inflammation of the skin or subcutaneous induration, ulcers, sudden swelling of one or both legs, or cardiac or renal insufficiencies In case of hemorrhoids, rectal bleeding occurs |
|
| Vaccinium species |
Bilberry Cranberry Blueberry |
Ericaceae | Eurasia | Fruit |
Anthocyanins, expressed as cyanidin 3-O glucoside Proanthocyanidins, apigenin, luteolin, chrysoeryiol, kaempferol, quercetin, and their glycosides, triterpenic and phenolic acids, and vitamins B 1, B 3, B 5, and C |
8 weeks | Relieve symptoms of discomfort and heaviness of legs, symptoms of cutaneous capillary fragility related to minor venous circulatory disturbances | nausea, vomiting, diarrhea, constipation, and dyspepsia | ||
| Centella asiatica (L.) | (gotu kola | Umbelli ferae (Apiaceae) | India | Aerial parts | Triterpene saponins, asiaticosides, madecasos side (brahminoside), asiatic acid, and madecassic acid (brahmic acid) | Three months |
Prevention and treatment of CVD, including post-thrombotic syndrome aid in the healing of minor wounds, but it is exclusively based upon long-standing use |
Strengthening of the weakened veins. Moreover, it can stimulate the formation of hyaluronidase and chondroitin sulfate and exert a balancing effect on the connective tissue. Its final beneficial effect in CVD seems to be mostly the improvement in microcirculation [122]. Besides that, it can decrease the capillary filtration rate by improving microcirculatory parameters | Hypersensitivity may occur to the active substance, or excessive oral intake of C. asiatica can cause headache and transient unconsciousness |
In addition, some secondary metabolites, such as triterpene saponins, exhibit beneficial effects on vascular walls, but their mechanism differs, involving inhibition of enzymes responsible for vein wall structure breakdown (Bencsik et al. 2024).
Other medicinal plants (Ruscus aculeatus L., Aesculus hippocastanum L., Centella asiatica (L.) Urb.) and their active compounds (ruscoside, aescin, asiaticoside) besides Ginkgonis folium, Meliloti herba, Rosmarini aetheroleum, Rusci rhizoma, and Myrtilli fructus recens also have important places (Bencsik et al. 2024).
Varicose and spider veins
VV, also known as varicosities, are enlarged, and twisted, causing backward flow and turbulence in blood circulation, damaging legs (Khan et al. 2024). VV can be classified as spider veins (angioectasis; telangiectases), thread veins, or matted veins (Gawande et al. 2024). They can cause blood reflux, venous hypertension, and swelling. Risk factors include gender (more occurrence in women), family history, prolonged standing, older age, hormonal Changes (i.e. puberty, pregnancy, multiparous and menopause, post-menopausal, hormone replacement, and other medicines containing estrogen and progesterone), lack of physical activity, obesity, alcohol, and smoking (Khan et al. 2024).
Some of the considerations that may guide the choice of treatment include 1) physical therapy: exercise, yoga and massage, 2) compression therapy: using the special type of compression stockings, 3) non-surgical treatment: including sclerotherapy (via injecting sclerosing agents such as sodium salicylate, polidacanol, chroamted glycine using small needles, and this treatment is accompanied by compression stockings to be worn after the sclerotherapy, laser treatment (sending strong bursts of light onto the vein), ultrasound guided foam sclerotherapy (damaging of the endothelial layer of the vein to create a blockage and scar formation in the dilated veins), and endothermal ablation (using energy from radiofrequency and lasers to fasten the affected veins), 4) surgical treatment: including vein stripping (inserting Of special wires onto the affected vein) and ambulatory phlebectomy (superficial veins are removed by performing incisions in the skin), 5) natural treatment via several plants (Gawande et al. 2024).
Herbal medicines (Table 4) are commonly used for VV treatment, with formulations varying based on an individual’s constitution and symptoms.
Table 4.
Herbal medicines as used for treating varicose veins
| Herb | Description | Role | Reference |
|---|---|---|---|
| Habb-e-Asgand | Referred to Ashwagandha or Withania somnifera |
Possess strengthening qualities that could promote circulation and vascular health |
(Khan et al. 2024) |
| Qurs-e-Zeequn Nisa | Unani preparation with various botanical constituents, including Zingiber officinale (ginger) and Cyperus scariosus (nagarmotha) | Have anti-inflammatory and circulatory properties | |
| Majoon Ushba | Unani herbal paste | Increase blood flow and lessen swelling. It frequently contains components like Terminalia chebula (Halela Siyah) and Colchicum luteum (Ushba) | |
| Horse chestnut seed extract |
(Asculus hippocastanum; Family hippocastanaceae) contain aescin, tannins, flavonoids, quinines, sterols and some fatty acids, coumarins and scopolin Aescin is the most active constituent of the horse chestnut seeds and comprises about 16–20% |
Help in toning the veins, reduce the vascular permeabilityand enhance the venous return | (Gawande et al. 2024) |
| Gotu kola |
Centella asiatica; Family umbelliferae contains a chemical called triterpenic fraction of Centella asiatica |
Reducing swelling and improving blood flow | |
| Apple cider vinegar | Raw grated apple extracted from fruits of Malus pumila, Family Rosaceae | Helped in providing relief from the pain irritation, ulceration, pigmentation, edema, cramps and itching | |
| Butcher broom |
Root of Ruscus aculeatus; Family Liliaceae contain active constituent steroidal saponins, neoruscogenin and ruscogenin |
Anti-inflammatory, vasoconstriction, antihemorrhagic | |
| Garlic |
Ripe bulbs of Allium Sativum; Family Liliaceae Contains allicin (volatile oil), carbohydrates, proteins, fats and mucilage |
Reducing inflammation and the symptoms of varicose veins | |
| Amla |
Fresh fruit pericarp of Emblica officianalis Gareth or Phyllanthus emblica linn. Family Euphobiaceae contains emblicanin A and B, punigluconin and pedunculagin vitamin, iron and calcium |
Help improve blood circulation and reduce inflammation | |
| Tomato | Fruit of Solanum lycopersicum derived from two wild ancestor species, Solanum pimpinellifolium and Solanum cerasiforme | Cure varicose veins | |
| Grapes seeds extract |
Seeds of Vitis vinifera; family Vitaceae oligomeric proanthocyanidins (OPCs) |
OPCs make blood vessels more elastic and also less likely to leak fluids that cause the leg swelling often associated with varicose veins Reducing the swelling itching and pains caused due to varicose veins |
|
| Ginger | (Rhizome (underground stem) of Zingiber officinale; Zingiberaceae) | Has the super ability to dissolve fibrin and reinstate blood circulation in vessels. Remember it is not an easy task to break down fibrins and it requires powerful food such as ginger to accomplish | |
| Terminalia arjuna | Bark extract of Terminalia arjuna | Reducing the symptoms like pain, edema, inflammation, pigmentation, induration and also expediting ulcer healing | (Gawande et al. 2024) |
Leech therapy is an effective method of bloodletting for treating VV. The leech discharges its saliva, which contains bioactive chemicals, which can extract stagnant or clotted blood from the veins. This therapy helps cleanse the blood, reduce vein pressure, and congestion. The Unani system of medicine uses Irsal-e Alaq (leech therapy) and Tanqiya-e Sauda (evacuation of black bile from the body) for managing varicose veins. The treatment regimen includes Itrifal Sagheer with Zanjabeel and Joshanda Aftimoon, which effectively reduces symptoms and signs of varicose veins (Khan et al. 2024).
Venous ulcers
Venous ulcers, specifically venous leg ulcers, represent a significant clinical challenge as they are the most prevalent type of chronic wound, accounting for 60–80% of all leg ulcers (Franks et al. 2016). These ulcers typically arise from CVI and venous hypertension, conditions characterized by impaired venous return and elevated venous pressure (Kumar et al. 2022). Chronic inflammation plays a pivotal role in the ulcer formation process; inflammatory cells become trapped in the fibrin cuff surrounding the ulcer, leading to further tissue damage and impaired healing (Yang et al. 2024). Traditional treatment modalities include compression therapy, wound care, and surgical interventions; however, these approaches may not always yield satisfactory results. Consequently, there is growing interest in the role of natural products as adjunctive therapies in the management of venous ulcers (Table 5). Natural products have been recognized for their potential therapeutic benefits in enhancing wound-healing processes. Various phytotherapeutic agents possess anti-inflammatory, antioxidant, and antimicrobial properties that can promote tissue regeneration and improve vascular health. By integrating insights from current literature on both conventional treatments and emerging natural therapies, this document seeks to provide a comprehensive overview that may inform clinical practice and guide future research efforts in optimizing care for patients suffering from venous ulcers.
Table 5.
Phytochemicals concerning the treatment of venous ulcer diseases
| Plant | Part used | Active constituents | Method | Results | References |
|---|---|---|---|---|---|
| Acacia arabica (Lam.) | Gum | Arabinogalactan-proteins | In-vitro antimicrobial assay for the microbes that are associated with ulcer; Bacillus licheniformis (ATCC 14580), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) and Staphylococcus aureus (ATCC 25923) using hydrogel wound dressing | Gum acacia showed MIC 500 to 600 µg mL−1 against species known to be implicated in wound infections | (Bhatnagar et al. 2013) |
| Acalypha indica | Aerial parts | Alkaloid; Acalyphine, flavonoids; luteolin, quercetrin and kaempferol, saponins and tannins | To estimate several biochemical and biophysical studies and to examine histological modifications both with and without extract therapy, the wound tissue was excised using in-vivo model | Treatment reduced lipid peroxidation and oxidative stress while simultaneously raising ascorbic acid levels. In addition, it enhanced collagen synthesis | (Ganeshkumar et al. 2012) |
| Achillea biebersteinii Afan | Root | 1,8-cineole, α-terpinene, camphor, borneol, piperitone, and β-Eudesmol | Different solvent extracts of studied plant evaluated against excision and incision wound in rat and mice | All extracts showed remarkable healing activity against the two types of wounds | (Akkol et al. 2011) |
| Achyranthes aspera L | Leaf | Alkaloids; Achyranthine, saponin, flavonoids; quercetin, tannins and sterols | Simple ointment from plants applied with different concentrations (2.5%, 5% and 10% (w/w)) to wounded albino rats and compared with reference drug 1% silver sulphadiazine | Results showed elevation of fibrocyte count, notable level of neovascularization, and epithelization especially in plant ointment with concentration of 5%&10% | (Fikru et al. 2012) |
|
Actinidia deliciosa (Kiwi) |
Peel |
Vitamins (C and E), potassium, fiber and folic acid. Flavonoids; rutin, epicatechin and quercetin glycosides phenolic acids; ferulic acid and caffeic acid. Acids that are organic either citric, malic, or quinic |
In-vivo study was designed to assess the effect of kiwi peel gel and its nanoformulated gel | Reduction in wound area reached 69.12% on day 12 | (Bassam et al. 2024) |
| Ageratina pichinchensis | Aerial part |
Bioflavonoids; Taxifolin, oligomeric procyanidins, epicatechin and catechin Phenolic acids; ferulic and caffeic acids |
Thirty-four patients were divided into two groups and received A. pichinchensis extract and 7% propylene glycol alginate, respectively | Within 10 months of treatment, patients who received the extract showed 100% healing while the control group showed healing by 81.8% | (Romero-Cerecero et al. 2012) |
| Allii bulbus, Hypericum perforatum and Calendula officinalis | Bulb, aerial part and oil extract, respectively |
Organosulfur compounds; allicin, diallyl disulfide, trisulfide and S-allyl cysteine (A. bulbus) Naphthodianthrones; hypericin and pseudohypericin Phloroglucinols; hyperforin Flavonoids; Quercetin, kaempferol, and rutin (H. perforatum) α-cadinol, γ-cadinene (C.officinalis) |
Twenty five patients treated with herbadermal® (A. bulbus, H. perforatum and C. officinalis) for 7 weeks | After the treatment, the percentage of treatment was 99.1% | (Kundaković et al. 2012) |
|
Aloe barbadensis (Aloe vera) |
Leaf, gel |
Anthraquinones; Aloin and Emodin, vitamins; B12, A, E and C, salicylic acid, amino acids and minerals |
The study designed to compare the antibacterial activity of A. vera leaf extracts and gel with common antibiotics (methicillin, erythromycin, bacitracin, novobiocin and vancomycin) against Gram-positive (S. aureus, Staphylococcus epidermidis, and Streptococcus pyogenes) and Gram-negative (P. aeruginosa) bacteria isolated from human skin wounds, burns, and acne | Vancomycin, the most effective antibiotic, had 80.5% and 72.2% efficacy against gram positive and Gram-negative isolates, respectively, whereas A. vera exhibited 100% activity against gram negative bacteria and 75.3% against all tested Gram-positive isolates. In addition, A. vera gel shown antibacterial efficacy with MIC < 400 μg/mL against multidrug-resistant P. aeruginosa that isolated from burn wound infection patients | (Bashir et al. 2011) |
| Gel | Clinical study, 50 patients with partial and superficial wounds divided into 2 groups (group 1 treated with Aloe vera gel and the other treated with 1% silversulphadiazine cream) | Significant pain relief and increase of epithelialization in case of aloe vera treated group | (Shahzad and Ahmed 2013) | ||
| Camellia sinensis | Leaf | Epigallocatechin-3-gallate | In- vitro study based on using chitosan green tea polyphenols complex (CGP) | (CGP) enhance granulation and epithelialization. These outcomes could be attributed to its antioxidant qualities and transglutaminase (TGM) expression activation | (Qin et al. 2013) |
| Centella asiatica | Herb | Triterpenoid saponin; Asiaticoside | In-vivo model on burn wound area of mice | Treatment enhance angiogenesis during skin wound healing due to the stimulation of VEGF synthesis brought on by the co-induction of MCP-1 expression in keratinocytes and IL-1β expression in macrophages by asiaticoside and MCP-1 | (Kimura et al. 2008) |
|
Chamomilla recutita Matricaria chamomilla |
Flower | Isolated flavonoid; apigenin | In-vitro and in-vivo model used to assess the effect of apigenin on collagen and skin thickness | Apigenin activates type-I and type-III collagen synthesis by promotion of smad2/3 signaling pathway and this was approved by different techniques as PCR and western blot | (Zhang et al. 2015) |
| Curcuma longa | Rhizome | Curcumin | In-vivo model on wound -excised rat | Curcumin enhance re-epithelialization, collagen synthesis and decrease ROS | (Dai et al. 2009) |
| Ficus racemosa | Root | Flavonoids, alkaloids and tannin | Incision and excision models using rats were used to assess the effect of plant extract | In incision model, aqueous plant extract showed greater breaking strength, the percentage of wound contraction, and the duration of epithelialization than excision model | (Murti and Kumar 2012) |
|
Echinacea pallida Nutt Or E. purpurea |
Root | Echinacoside and echinacin | In-vivo study was designed to assess the effect of a gel (1% ethylcellulose) with 100 mg of E. pallida dried extract that applied only once, on abraded skin of rats and then the wounded area was covered with a patch. Otherwise, vehicles are used only with control group | Rats given after 48 and after 48 and 72 h, rat treated by E. pallida did not exhibit any symptoms of inflammation, and it was approved histologically | (Speroni et al. 2002) |
| Garcinia mangostana | Fruit | Xanthone, mangostins, garcinone E, tannins and flavonoid | Nanoformulation of fruit extract | This formulation showed significant acceleration of healing compared with control with anti-inflammatory and antibacterial effect | (Charernsriwilaiwat et al. 2013) |
| Ginkgo biloba | Leaf |
Flavonoids; isorhamnetin, Quercetin, and kaempferol, Ginkgolides and organic acids |
Excision and dead space. Wound healing effect was studied on rats using dose 50 mg/kg | Ginkgo biloba has a significant pro-healing effect, which may be due to its influence on the collagenation stage of wound healing | (Bairy and Rao 2001) |
|
Ganoderma praelongum Glycyrrhiza glabra |
Fruit Root |
Terpenoids, polysaccharides, sterols and proteins Glycyrrhizin |
The effects of gel formulations containing 0.3% G. praelongum, 2.5% G. glabra, and a combination of the two extracts on the stages of wound infection, wound contraction, and epithelization were evaluated on wounded mice | Combination gel had promising effect against methicillin resistant microbes (in-vitro). Moreover, different formulations showed considerable contraction and epithelization (in-vivo) | (Ameri et al. 2013) |
| Hevea brasiliensis | Rubber tree | Latex that consists of 35% cis-1,4-polyisoprene, 5% non-isoprene molecules, and 60% water | Fourteen patients were chosen to receive Hevea brasiliensis biomembrane treatment, and seven received Fibrase® as control drug. Over the duration of 120 days, the wound-healing index was used to track the patients’ clinical and photographic progress | After the treatment duration 42% of ulcer healing was observed | (Frade et al. 2012) |
| Hibiscus rosa | Flower | Anthocyanin, flavonoids, phenolics and polysaccharides | (5 and 10% w/w) plant extract were used to treat different types of wounds in rats (incision, dead-space and excision) | The results showed decreasing in the epithelization period and a rise in the dry and wet granuloma weights, tensile strength, and wound closure rate | (Bhaskar and Nithya 2012) |
| Hypercium perforatum | Oil of aerial part |
Hypericin Hyperphorin |
In-vivo model and X-ray was used to evaluate healing | Oil plant emulsion showed significant wound healing compared with mupirocin ointment that used as standard dry by percentage 97% and 68% | (Nayak et al. 2017) |
| Kigelia pinnata | Bark | Napthoquinone; Lapachol, Phenolic compounds, alkaloids, coumarins and iridoids | Rat with incision, dead-space and excision treated with oral aqueous extract (250 mg/kg and 500 mg/kg) | The bark extract had the ability to enhance hydroxyproline levels, improved granuloma breaking strength, and skin tensile strength | (Sharma et al. 2010) |
|
Lavandula angustifolia |
Flower | Essential oil; linalool and linalyl acetate | Prepared alginate nanofibers were evaluated against microorganisms and inflammatory markers that are associated with wound | Pro-inflammatory cytokines are significantly reduced when alginate-based nanofibers are applied to fibroblasts or animals. The suppression of the production of cytokines was also noticed | (Hajiali et al. 2016) |
| Lawsonia inermis (henna) | Leaf | Quinones (arbutin, lawsone and anthraquinone), flavonoids (kaempferol, apigenin, luteolin, quercetin, and catechin), terpenoids and xanthones | In-vivo study that designed using Wound dressings was made from oxidized starch nanofibers and henna-gelatin and assessed using immunohistochemistry | Reduction of inflammation and speed up the healing of wounds | (Hadisi et al. 2018) |
| Lygodium flexuosum | Leaf | Tannins, alkaloids, glycosides and flavonoids | Excision wound model using albino rats was used to assess different concentrations of plant extract (4 &5%) with different doses | The extract ointment had the ability to reduce the time needed for epithelization and raised the percentage of wound contraction | (Chandra et al. 2015) |
| Mimosa tenuiflora (Willd.) | Bark | Polyphenols, triterpenoidal and steroidal saponins and low quantity of alkaloids | Forty patients having a clinical diagnosis of VLU were part of the trial; twenty were randomly allocated to the experimental group that treated with extract hydrogel for 12 weeks and twenty to the control group. The study population was 61 years old on average | After the treatment, a significant decrease in ulceration areas was observed; by the eighth week, the ulcer-size reduction had reached 93% as the mean value for the group. Finally, all patients were cured at the end of the trial | (Rivera-Arce et al. 2007) |
| Nineteen women and 13 men suffered from VLU were subjected to treatment with extract hydrogel | After the treatment with hydrogel for 8 weeks the healing percentage was 22% | (Lammoglia‐Ordiales et al. 2012) | |||
| Moringa oleifera | Seed | α-tocopherol, β-Sitosterol, flavonoids mainly (Apigenin and Naringenin), phenolic acids such as (gallic, ferrulic caffeic and vanillic) and proteins | Hydrogel wound dressing is used in an in-vitro antimicrobial assay for the bacteria that cause ulcers, including Bacillus licheniformis (ATCC 14580), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and Staphylococcus aureus (ATCC 25923) | M. oleifera demonstrated MICs of 300–700 µg mL−1 against species that are known to cause wound infections | (Bhatnagar et al. 2013) |
| Nigella sativa | Seed oil | Nimbidin and thymoquinone | Wound healing was assessed using carbopol emulgel prepared from studied seed oil | Unsaturated fatty acids stimulate production of vascular endothelial growth factors that have evidenced a role in wound healing | (Thomas et al. 2024) |
| Ocimum basilicum (Basil) | Seed oil | Mucilage mainly Glucomannan and xylan | Dressing of seed mucilage with ZnO nanoparticles that crosslinked with borax was examined against bacteria associated with wound | Borax crosslinking enhanced the antibacterial dressings made from Basil Seed Mucilage (BSM) with ZnO-NP against S. aureus and E. coli | (Tantiwatcharothai and Prachayawarakorn 2020) |
| Olea europaea | Leaf | Secoiridoid oleuropein | Hexane and aqueous extract of the plant leaf was compared with reference ointment Madecassol® to heal linear incision and circular excision using in-vivo model | In comparison to the other groups, the animals treated with the aqueous extract showed an impressive rise in wound tensile strength (34.8%) on incision models and greater contraction (87.1%) on excision | (Koca et al. 2011) |
| Oreochromis niloticus L | Skin collagen | Gel from collagen extract | Cutaneous wound-healing process in rats was treated using isolated collagen extract for 15 days | Histochemical analysis revealed increasing of VEGF, TGF-β1 of the treated group | (Elbialy et al. 2020) |
| Pinus brutia | Bark |
Proanthicin, catechin, epicatechin, and taxifolin |
In-vivo model uses plant extract that is embedded in alginate gel dressing | The studied dressing showed a healing rate of 75.7% compared with control group with healing rate of 48.6% | (Karakaya et al. 2021) |
| Pinus.pinaster | Bark | Procyanidins, phenolic acids (ferulic and caffeic acids) and flavonoids (catechin, Taxifolin, quercetin and epicatechin) |
Clinical trial was achieved with three groups of patients who suffered from venous ulcer and each group consists of six patients Group 1: received 50 mg of Pycnogenol ® (P. pinaster) orally three times daily Group 2: 50 mg of Pycnogenol ® (P. pinaster) orally three times daily + oral application of the drug on the ulcer Group 3: acted as control |
Within 6 weeks, the oral + local treatment group showed faster healing, which was attributed to a significant increase in skin partial pressure of oxygen (PO2) and a decrease in skin partial pressure of carbondioxide (PCO2). Furthermore, there was a significant reduction in the ulcerated area | (Belcaro et al. 2005) |
| Polygonum cuspidatum | Aerial Part | Anthraquinone emodin, stilbene resveratrol, resveratrol glycoside, emodin methyl ether, and a few tannins and polysaccharides | In-vivo wound-healing model was used to assess the activity of extract and healing rates was reported at 3, 7, 14, and 21 days by immutohistochemical analysis | Treated group showed elevation of TGF-β1 that estimated by immutohistochemical analysis and increasing of fibroblasts accompanied with reduction of inflammatory cells | (Wu et al. 2012) |
| Punica granatum | Peel | Ellagitannins; punicalagin, ellagic acid, punicalin | Punica granatum peel gel was applied for 21 days on the wound that induced in diabetic rats | P. granatum had ability to regenerate collagen and enhance fibroblast infiltration that associated with elevation of TGF, VEGF and EGF | (Huan et al. 2013) |
| Rheum officinale | Root | Anthraquinone derivatives; emodin | Emodin Hydrogel 100, 200 and 400 μg/ml was evaluated against cutaneous wound of rats for 14 days | Emodin Hydrogel 400 μg/ml showed promising wound healing and tissue regeneration through TGF-β1 signaling pathway | (Tang et al. 2007) |
| Sesamum indicum | Seed oil | Sesamol | Sesamol was examined to know how it affects wound healing in albino rats, both in the normal and dexamethasone -delayed healing processes | These findings suggest that sesamol may be a useful medication for both normal and delayed wound healing | (Shenoy et al. 2011) |
| Sambucus ebulus | Leaf | Quercetin 3-O-glucoside | Circular excision and linear incision induced in rat and mice. Different plant fractions and isolated compound were formulated as ointments to heal different types of wounds | Methanolic fraction and isolated compound showed significant healing effect against different types of wounds | (Süntar et al. 2010) |
| Sida rhombifolia Linn | leaf | Alkaloids, saponin, tannin and sterols | Different concentration (25, 33, 50 and 80%) of plant extract ointment was used to heal Excision and incision wound in rats | The 50% ointment showed impressive fibrosis and collagenization | (Francis et al. 2018) |
| Sphaeranthus indicus | Aerial Part |
Essential oil; Caryophyllene, flavonoids; Quercetin, Kaempferol, Sesquiterpenes; Sphaeranthine |
Healing effect of plant cream extract was assessed on excised wounded model in Guinea pigs and compared with neomycin as control group | Plant cream enhanced the time of wound closure and suppressed the inflammatory markers | (Sadaf et al. 2006) |
| Spirulina platensis | Blue- green algae | Carbohydrates, protein, lipids and minerals | Algae were evaluated to treat wounds in rats for 21 days | Exerting antioxidant effects plays a great role in skin regeneration. Histological examination revealed that upregulating angiogenic bFGF and VEGF genes and occupying genes linked to scar formation, TGF-β and α-SMA | (Elbialy et al. 2021) |
| Syzygium aromaticum | Flower bud | Essential oil; eugenol, caryophyllene | In-vivo assay by applying nanoformulation of clove oil on wounded rats and compared with control group that treated with gentamycin | The formulation enhances the formation of leucine content, and the histological examination confirmed no inflammatory cell signals | (Alam et al. 2017) |
| Thymus vulgaris | Aerial part | Essential oil; thymol and carvacrol | Thyme oil with chitosan film dressing had assessed against different bacterial strains that affected wound | Significant Wound excision associated with antimicrobial activity against Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, | (Altiok et al. 2010) |
| Tridax procumbens | Leaves | Flavonoids; (quercetin, kaempferol, and apigenin), Alkaloids; tridanine Triterpenoids; β-amyrin, Phenolic compounds; chlorogenic acid | In-vitro assay of Electrospun PCL Nanofibers of plant extract to analyze its microbial activity against bacterial infection that is associated with wounds | Nanofibers improve wound healing and repair surfaces contaminated by harmful germs | (Suryamathi et al. 2019) |
| Vaccinium macrocarpon (Cranberry) | Fruit | Anthocyanins | In-vitro models are based on using smart hydrogel film on wound and monitoring bacterial infection | Suppression of bacterial infection (Staphylococcus aureus, Pseudomonas aeruginosa) | (Zepon et al. 2019) |
| Vernonia scorpioides | Leaves | sesquiterpene lactones; scorpiolide, Scorpioidin | In-vivo assay using guinea pigs to estimate the plant extract hydrogel (200 mg with concentration 50%) for 30 days | The hydrogel enhanced the organization and regeneration of the new tissue but did not shorten the time of the closure | (Leite et al. 2002) |
| Wedelia trilobata (L.) | Leaves | Flavonoids, terpenoids, saponin and polyphenolics | In-vitro study to evaluate the effect of studied plants on L929 Fibroblasts cells to Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Staphylococcus epidermidis | The plant provides promising wound-healing properties that associated with antibacterial effect and promoted L929 Fibroblasts cells | (Balekar, et al. 2012) |
| Yucca aloifolia | Seeds |
Saturated Fatty acids; azelaic, stearic and palmetic Unsaturated fatty acids; linoleic and palmitoleic γ-sitosterol and phenolics |
In-vivo study was designed to assess the effect of Yucca aloifolia seed gel and its nanoformulated gel | Significant suppress in biomarkers; TNF-α, TGF-β and VEGFA & with significar elevation in TNF-α and COX-2 | (Bassam et al. 2024) |
| Zataria multifora Boiss | Aerial part | Essential oil; Thymol and Carvacrol | Nanogel of the plant oil was tried on wounded skin of rats for 21 days | Significant reduction of vascularity, inflammation, and edema with parallel increasing of fibrosis, re-epithelialization, and collagen deposition | (Osanloo et al. 2024) |
| Zingiber officinale | Rhizome | Oleoresin; gingerol, volatile oil and starch | Ginger assessed to treat incisional wound of rats | Ginger can improve the epithelialization of incisional wounds, decrease neutrophil counts, and raise fibroblast counts | (Rahayu et al. 2020) |
In addition, the data from clinical trials demonstrating the efficacy of natural products for venous diseases were displayed in Table S1.
Isolated natural compounds in venous disorders: anti-inflammatory effects, toxicity, and therapeutic potential
Diosmin and hesperidin are two citrus-derived flavonoids that have shown potent anti-inflammatory activities through the modulation of their inflammatory mediators (Harasstani et al. 2010). Diosmin has been reported to suppress NO, PGE2, and TNF-α production in lipopolysaccharide (LPS)-activated macrophages, indicating its anti-inflammatory activity (Harasstani et al. 2010; Abohashem et al. 2024). Comparisons with conventional anti-inflammatory drugs suggest that diosmin, particularly in association with other flavonoids, can possess comparable activity in inhibition of inflammatory processes. In addition, diosmin exhibits a good toxicity profile with low toxicity levels and protective effects against drug-induced organ injury, which is an attractive therapeutic implication (Harasstani et al. 2010; Wu et al. 2022).
Also, hesperidin has been shown to decrease NO, PGE2, TNF-α, and IL-6 production in inflammatory models (Choi et al. 2022). Although direct statistical comparison with traditional anti-inflammatory drugs is restricted, the anti-inflammatory potentiality of hesperetin, the aglycone form of hesperidin, is reported to be greater at low doses (Li et al. 2019b). In addition, hesperidin derivatives, such as hesperidin glucoside, maintain similar efficacy while offering improved solubility. The compound is typically safe with low level of acuteantub-chronic toxicity, but at high doses, slight physiological effects may occur. Given its anti-inflammatory effects and low cytotoxicity, hesperidin is a promising agent for therapeutic use (Li et al. 2019b).
Quercetin, a dietary flavonoid, is increasingly being studied for its possible therapeutic applications in the treatment of venous diseases (i.e., chronic venous insufficiency [CVI]. Its anti-inflammatory and antioxidant activities are at the core of its suggested role (Zhang et al. 2024).
In terms of inflammatory mediator production, quercetin has been shown to inhibit key pro-inflammatory cytokines such as TNF-α and IL-6. This inhibition is realized by blocking the JAK1/STAT3/HIF-1α signaling pathway, resulting in decreased inflammation and oxidative stress. These mechanisms are of relevance for venous pathologies in which chronic inflammation is a key factor in disease evolution (Miguel et al. 2025).
When compared to standard pharmacological treatments for CVI, such as diosmin and hesperidin, quercetin's efficacy in modulating inflammatory responses appears promising. However, direct statistical comparisons are limited. Pharmacological strategies for CVI under development have been tested with a view to clinical outcomes such as pain, oedema, and quality of life. Although quercetin's anti-inflammatory properties are well established, further systematic assessment is needed to confirm the comparative efficiency of quercetin compared to marketed venotonic agents (Huang et al. 2024).
Regarding toxicity, quercetin has been safely assumed with an advantageous profile. High doses in animal studies (up to 6 g/kg) did not result in organ damage or mortality. The subacute tests did not show any significant difference in histomorphometry, blood biochemistry, and blood counts. In human clinical studies, daily oral intake of 5000 mg of quercetin for 28 consecutive days in individuals with chronic hepatitis C did not lead to adverse effects.
Resveratrol, a polyphenolic compound present in grapes and red wine, has been studied for possible advantages in venous diseases on the basis of anti-inflammatory and antioxidant activities (Cottart et al. 2014). Evidence supports that resveratrol can also regulate inflammatory media such as the blocking of TNF-α and IL-6, that are central to the inflammatory process. Toxicity wise, resveratrol is [generally] well tolerated at low doses; however, at doses higher than 1 g/d, resveratrol can cause gastrointestinal problems, and paradoxically can cause hematological abnormalities on very rare occasions (Cottart et al. 2010). These results suggest that resveratrol could provide therapeutic potential for the treatment of venous conditions, but it is necessary to determine whether it is superior to conventional treatments (Meng et al. 2021).
Tranoiden saponins of Centella asiatica, Asiaticoside and Madecassoside, oleanane triterpenoid saponins, have been investigated on therapeutic effects of venous insufficiency (Tan et al. 2021). These compounds shows anti-inflammatory activity through inhibition of inflammatory mediators such as NO, TNF-α, and IL-6. Comparative analyses show that asiaticoside and madecassoide are able to reduce oxidative stress and inflammatory processes in different cell lines including macrophages and endothelial cells (Tan et al. 2021). In terms of toxicity, both compounds are regarded as safe but, some may have allergic reactions or intestinal upset. The effectiveness of their use in ameliorating signs and symptoms of venous insufficiency, including varicose veins has been validated by clinical practice, but direct statistical comparisons with conventional pharmacotherapy are sparse (Tan et al. 2021).
Ruscogenins, steroidal saponins isolated from Ruscus aculeatus (butcher's broom), have been used for the treatment of chronic venous insufficiency (Abascal and Yarnell 2002). It is thought that they have an anti-inflammatory effect by antagonizing elastase activity and by limiting vascular permeability resulting in reduction of edema and enhancement of venous tonicity (Bone 2003). While ruscogenins have been reported to be effective in alleviating symptoms such as leg swelling and discomfort, comprehensive data on their impact on specific inflammatory mediators and direct statistical comparisons with standard venotonic drugs are scarce (Lichota et al. 2019). Toxicity profiles indicate that ruscogenins are safe with rare reports of adverse events of clinical use (Ercan et al. 2020).
Ginkgolides and bilobalide, terpene lactones in Ginkgo biloba leaf extract, have been studied for their vascular effects (potentially with therapeutic use in venous system disorders) (Tian et al. 2017). These compounds show anti-inflammatory activity by blocking platelet-activating factor (PAF) and regulating cytokine production (Dziwenka and Coppock 2021). The clinical studies have found that ginkgolides can down regulate the expression of pro-inflammatory cytokines, which is related to enhancement of vasculoprotection (Silva and Martins 2022). Bilobalide also demonstrates neuroprotective and anti-inflammatory effects. Regarding toxicity Ginkgo biloba extracts are in general nontoxic if used properly but may interact with anticoagulant drugs and cause bleeding disorders (Liu et al. 2022).
Commercially available preparations for venous diseases
Numerous products of biological origin have been developed in regions around the globe to help treat conditions relating to veins, like chronic venous insufficiency and varicose leg veins. Their healing properties and safety make these natural treatments stand out.
Miconized purified flavonoid fraction (MPFF)
MPFF is known under the trade mark Daflon. It is blended diosmin and hesperidin and derived from citrus fruits. It is meant for use in improvement of venous tone, inflammation and alleviation of symptoms of chronic venous disease (Awad et al. 2024; Lurie and Branisteanu 2023).
Horse chestnut seed extract
It is marketed as Venastaat or Aesculaforce. Horse chestnut seed extract contains aescin as its active ingredient. This extract is said to modify the tone of veins, decrease swelling of legs, and improve the venous inflow (Max and Pittler 2012).
Red vine leaf extract
Antistax is the commercial name under which it is sold. Red vine leaf extract is enriched with polyphenols such as quercetin and resveratrol. It is used to protects blood vessels, reduces edema, and enhances circulation (Stücker et al. 2019).
Gotu kola (Centella asiatica) extract
Sellers use the trademark Madecassol or Centellase. Gotu Kola extract is meant for healing wounds because it contains asiaticoside and madecassoside. It is meant also as an aid to microcirculation and to strengthen walls of veins (Park 2021).
Grape seed extract
Products containing Endotelon and Masquelier’s Original OPCs contain grape seed extract which carries oligomeric proanthocyanidin complexes. It is believed to have antioxidant capabilities and is also useful in improving venous tone along with alleviating the symptoms of venous insufficiency (Weseler and Bast 2017).
Ruscus aculeatus (Butcher’s broom) extract
Butcher’s broom extract is sold with the brand names Cyclo 3 Fort and Venoruton and contains ruscogenins. This herbal medicine is used for treating symptoms of chronic venous insufficiency like swelling and pain in the legs by improving venous tone as well as inflammation (Boyle et al. 2003).
Ginkgo biloba extract
Tanakan and Tebonin are the marketed names for products containing Ginkgo Biloba extract, which consists of ginkgolides and bilobalide. Its vasodilator and antioxidant properties make it useful for blood flow and symptoms of various venous disorders (Kleijnen and Knipschild 1992).
Pycnogenol (Maritime pine bark extract)
The extract contains procyanidins and is sold under the name Pycnogenol. It strengthens the walls of capillaries, and helps to reduce swelling in the legs while improving circulation in people suffering from chronic venous insufficiency (Schoonees et al. 2012).
Conclusions
CVDs are prevalent and progressive health issues, particularly affecting the aging population. Its incidence escalates with age, leading to a significant decline in quality of life due to symptoms associated with varicose veins and severe complications such as venous ulcers. Recent advances in the treatment of CVDs highlight the potential of natural products. Clinical studies have demonstrated that these products exert their effects through multiple mechanisms: they inhibit enzymes responsible for degrading the structural integrity of the venous wall, exhibit anti-inflammatory and antioxidant activities, possess vasoactive properties that enhance vascular tone, and have good ulcer healing potentials. Despite the therapeutic potential of these natural products, caution is warranted. The interaction between herbal extracts and conventional medications can lead to adverse effects, necessitating thorough investigation into their safety profiles. Furthermore, there is a pressing need for well-designed clinical trials that focus on specific formulations, dosages, and detailed compositions of these herbal remedies.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AI
Artificial intelligence
- BMI
Body mass index
- BRBNS
Bleb Nevus Syndrome
- CEAP
Clinical, etiological, anatomical, and pathophysiological
- CVD
Cardiovascular disease
- CVI
Chronic venous insufficiency
- CVMs
Congenital venous malformations
- DVT
Deep venous thrombosis
- EDS
Ehlers-Danlos syndrome
- EVLA
Endovenous laser ablation
- KTS
Klippel-Trenaunay syndrome
- LDS
Lymphedema distichiasis syndrome
- MPFF
Micronized purified flavonoid fraction
- OPCs
Oligomeric proanthocyanidins
- PE
Pulmonary thromboembolism
- PTA
Percutaneous transluminal angioplasty
- PTS
Post-thrombotic syndrome
- PWS
Parkes Weber syndrome
- RFA
Radiofrequency ablation
- SVT
Superficial venous thrombosis
- VV
Varicose veins
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data are available within the manuscript.
Declarations
Conflict of interest
The authors declare no competing interests.
Animal and human rights
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abascal K, Yarnell E (2002) Butcher’s broom: herb’s potentials too-often swept under the rug. Alternative Complement Therapies 8:177–185 [Google Scholar]
- Abohashem RS, Ahmed HH, Sayed AH, Effat H (2024) Primary protection of diosmin against doxorubicin cardiotoxicity via inhibiting oxido-inflammatory stress and apoptosis in rats. Cell Biochem Biophys. 10.1007/s12013-024-01289-7 [DOI] [PubMed] [Google Scholar]
- Agnese M, Cipolletta L, Bianco M, Quitadamo P, Miele E, Staiano A (2010) Blue rubber bleb nevus syndrome. Acta Paediatr 99:632–635 [DOI] [PubMed] [Google Scholar]
- Ain QU (2022) Anticoagulant and thrombolytic activities of leaf extract of mangifera indica in smokers. Tob Regul Sci (TRS) 8:1189–1201 [Google Scholar]
- Akkol EK, Koca U, Pesin I, Yilmazer D (2011) Evaluation of the wound healing potential of Achillea biebersteinii Afan. (Asteraceae) by In vivo excision and incision models. Evidence-Based Complement Alter Med 2011:474026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam P, Ansari MJ, Anwer MK, Raish M, Kamal YK, Shakeel F (2017) Wound healing effects of nanoemulsion containing clove essential oil. Artificial Cells Nanomed Biotechnol 45:591–597 [DOI] [PubMed] [Google Scholar]
- Alaraky, N.E.E., 2018. Effect of allium sativum (garlic) intake on prothrombin time and international normalize ratio, Omkalthoum Osman Hamad.
- Aleksandrowicz H, Owczarczyk-Saczonek A, Placek W (2021) Venous leg ulcers: advanced therapies and new technologies. Biomedicines 9:1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaigh T, Fukaya E (2021) Varicose veins and chronic venous disease. Cardiol Clin 39:567–581 [DOI] [PubMed] [Google Scholar]
- Altiok D, Altiok E, Tihminlioglu F (2010) Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J Mater Sci - Mater Med 21:2227–2236 [DOI] [PubMed] [Google Scholar]
- Ameri A, Rajive B, Vaidya J, Apte K, Deokule S (2013) Anti-staphylococcal and wound healing activities of Ganoderma praelongum and Glycyrrhiza glabra formulation in mice. CABI Databases 6:27–31 [Google Scholar]
- Awad N, Hetzel JD, Bhupalam V, Nestor MS (2024) Stasis dermatitis: pathophysiology, current treatment paradigms, and the use of the flavonoid diosmin. J Clin Aesthetic Dermatol 17:15 [PMC free article] [PubMed] [Google Scholar]
- Bairy K, Rao C (2001) Wound healing profiles of Ginkgo biloba. J Nat Remedies 1:25–27 [Google Scholar]
- Balekar N, Katkam NG, Nakpheng T, Jehtae K, Srichana T (2012) Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. J Ethnopharmacol 141:817–824 [DOI] [PubMed] [Google Scholar]
- Banzic I, Brankovic M, Maksimović Ž, Davidović L, Marković M, Rančić Z (2017) Parkes Weber syndrome—Diagnostic and management paradigms: A systematic review. Phlebology 32:371–383 [DOI] [PubMed] [Google Scholar]
- Barstow C, Kassop D (2019) Cardiovascular Disease: Chronic Venous Insufficiency and Varicose Veins. FP Essentials 479:16–20 [PubMed] [Google Scholar]
- Bashir A, Saeed B, Mujahid TY, Jehan N (2011) Comparative study of antimicrobial activities of Aloe vera extracts and antibiotics against isolates from skin infections. Afr J Biotech 10:3835–3840 [Google Scholar]
- Bashor CJ, Hilton IB, Bandukwala H, Smith DM, Veiseh O (2022) Engineering the next generation of cell-based therapeutics. Nat Rev Drug Discovery 21:655–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass A (2007) The effect of standing in the workplace and the development of chronic venous insufficiency. Harefuah 146(675–676):734 [PubMed] [Google Scholar]
- Bassam SM, Ali DE, Awwad ZM, Mahmoud SA, Abou-Taleb BA (2024) Development and characterization of plant derived wastes nano-formulation loaded in thermo-reversible gel for burn healing: an effort towards sustainable development. J Drug Delivery Sci Technol 95:105543 [Google Scholar]
- Basudkar V, Gujrati G, Ajgaonkar S, Gandhi M, Mehta D, Nair S (2024) Emerging Vistas for the Nutraceutical Withania somnifera in Inflammaging. Pharmaceuticals 17:597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis RA, Smith NL, Klarin D, Fukaya E (2021) Epidemiology and genetics of venous thromboembolism and chronic venous disease. Circ Res 128:1988–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcaro G, Cesarone MR, Errichi BM, Ledda A, Di Renzo A, Stuard S, Dugall M, Pellegrini L, Rohdewald P, Ippolito E, Ricci A, Cacchio M, Ruffini I, Fano F, Hosoi M (2005) Venous ulcers: microcirculatory improvement and faster healing with local use of Pycnogenol. Angiology 56:699–705 [DOI] [PubMed] [Google Scholar]
- Bencsik T, Balázs VL, Farkas Á, Csikós E, Horváth A, Ács K, Kocsis M, Doseděl M, Fialová SB, Czigle S (2024) Herbal drugs in chronic venous disease treatment: an update. Fitoterapia 179:106256. [DOI] [PubMed]
- Bharath V, Kahn SR, Lazo-Langner A (2014) Genetic polymorphisms of vein wall remodeling in chronic venous disease: a narrative and systematic review. Blood J Am Soc Hematol 124:1242–1250 [DOI] [PubMed] [Google Scholar]
- Bhaskar A, Nithya V (2012) Evaluation of the wound-healing activity of Hibiscus rosa sinensis L (Malvaceae) in Wistar albino rats. Indian J Pharmacol 44:694–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar M, Parwani L, Sharma V, Ganguli J, Bhatnagar A (2013) Hemostatic, antibacterial biopolymers from Acacia arabica (Lam.) Willd. and Moringa oleifera (Lam.) as potential wound dressing materials. NISCAIR-CSIR 51(10):804–810 [PubMed] [Google Scholar]
- Bihari I, Guex J-J, Jawien A, Szolnoky G (2022) Clinical Perspectives and Management of Edema in Chronic Venous Disease—What about Ruscus? Medicines 9:41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijak M, Saluk J, Szelenberger R, Nowak P (2016) Popular naturally occurring antioxidants as potential anticoagulant drugs. Chem Biol Interact 257:35–45 [DOI] [PubMed] [Google Scholar]
- Bone K (2003) Butcher's broom: evidence-based phytotherapy for venous conditions. Townsend Letter Doctors Patients 1 (241–242):66–71
- Boyle P, Diehm C, Robertson C (2003) Meta-analysis of clinical trials of Cyclo 3 Fort in the treatment of chronic venous insufficiency. Int Angiol 22:250 [PubMed] [Google Scholar]
- Cai PL, Hitchman LH, Mohamed AH, Smith GE, Chetter I, Carradice D (2023) Endovenous ablation for venous leg ulcers. Cochrane Database Syst Rev. 10.1002/14651858.CD009494.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Zhang L, Sun ZB, Cheng XH, Zhang Y, Zou HB (2015) Salvia miltiorrhiza prevents deep vein thrombosis via antioxidative effects in endothelial cells. Mol Med Rep 11:3593–3600 [DOI] [PubMed] [Google Scholar]
- Carroll B, Piazza G, Goldhaber S (2019) Sulodexide in venous disease. J Thromb Haemost 17:31–38 [DOI] [PubMed] [Google Scholar]
- Castro-Ferreira R, Cardoso R, Leite-Moreira A, Mansilha A (2018) The role of endothelial dysfunction and inflammation in chronic venous disease. Ann Vasc Surg 46:380–393 [DOI] [PubMed] [Google Scholar]
- Chandika P, Tennakoon P, Kim T-H, Kim S-C, Je J-Y, Kim J-I, Lee B, Ryu B, Kang HW, Kim H-W (2022) Marine biological macromolecules and chemically modified macromolecules; potential anticoagulants. Mar Drugs 20:654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P, Yadav E, Mani M, Ghosh AK, Sachan N (2015) Protective effect of Lygodium flexuosum (family: Lygodiaceae) against excision, incision and dead space wounds models in experimental rats. Toxicol Ind Health 31:274–280 [DOI] [PubMed] [Google Scholar]
- Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Sukma M, Opanasopit P (2013) Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int J Pharm 452:333–343 [DOI] [PubMed] [Google Scholar]
- Chase C, Doyle A, St John S, Laurent T, Griffith S (2022) Post-operative haemorrhage secondary to cinnamon use. A case report. Int J Surg Case Rep 95:107179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-W, Tseng Y-H, Lin C-C, Kao C-C, Wong MY, Lin B-S, Huang Y-K (2020) Novel diagnostic options without contrast media or radiation: Triggered angiography non-contrast-enhanced sequence magnetic resonance imaging in treating different leg venous diseases. Diagnostics 10:355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yang Y, Wang S, Zhang K (2023) Crocin improves lower extremity deep venous thrombosis by regulating the PIM1/FOXO3a axis. Cell Mol Biol (Noisy-Le-Grand) 69:183–188 [DOI] [PubMed] [Google Scholar]
- Choi S-S, Lee S-H, Lee K-A (2022) A comparative study of hesperetin, hesperidin and hesperidin glucoside: antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants 11:1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JH, Heo S (2023) Varicose veins and the diagnosis of chronic venous disease in the lower extremities. J Chest Surg 57:109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke-Barber J, Kreimer S, Patel M, Dasgupta R, Jeng M (2020) Venous malformations, Seminars in Pediatric Surgery, Elsevier, p. 150976. [DOI] [PubMed]
- Costa D, Andreucci M, Ielapi N, Serraino GF, Mastroroberto P, Bracale UM, Serra R (2023) Molecular determinants of chronic venous disease: a comprehensive review. Int J Mole Sci 24:1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL (2010) Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res 54:7–16 [DOI] [PubMed] [Google Scholar]
- Cottart CH, Nivet-Antoine V, Beaudeux JL (2014) Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol Nutr Food Res 58:7–21 [DOI] [PubMed] [Google Scholar]
- Dai M, Zheng X, Xu X, Kong X, Li X, Guo G, Luo F, Zhao X, Wei Y, Qian Z (2009) Chitosan-alginate sponge: preparation and application in curcumin delivery for dermal wound healing in rat. Biomed Res Int 2009:595126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AH (2019) The seriousness of chronic venous disease: a review of real-world evidence. Adv Therapy 36:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HO, Popplewell M, Singhal R, Smith N, Bradbury AW (2017) Obesity and lower limb venous disease–the epidemic of phlebesity. Phlebology 32:227–233 [DOI] [PubMed] [Google Scholar]
- De Maeseneer MG, Kakkos SK, Aherne T, Baekgaard N, Black S, Blomgren L, Giannoukas A, Gohel M, de Graaf R, Hamel-Desnos C (2022) Editor’s choice–European Society for Vascular Surgery (ESVS) 2022 clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg 63:184–267 [DOI] [PubMed] [Google Scholar]
- de-Abreu GCG, Camargo O, de-Abreu MFM, de-Aquino JLB (2017) Ultrasound-guided foam sclerotherapy for severe chronic venous insufficiency. Rev Col Bras Cir 44:511–520 [DOI] [PubMed] [Google Scholar]
- DiGiorgio AM, Tsolinas R, Alazzeh M, Haefeli J, Talbott JF, Ferguson AR, Bresnahan JC, Beattie MS, Manley GT, Whetstone WD (2017) Safety and effectiveness of early chemical deep venous thrombosis prophylaxis after spinal cord injury: pilot prospective data. Neurosurg Focus 43:E21 [DOI] [PubMed] [Google Scholar]
- Ding T-B, Jian L-G, Zhao J-T, Liu S-C, Zhang J-Y, Wu SH (2015) Ethanol extract of Carthamus tinctoriusL. shows anti-thrombosis activity in rats. Afr J Tradit Complement Altern Med 12:120–124 [Google Scholar]
- Dissemond J, Storck M, Kröger K, Stücker M (2018) Indications and contraindications for modern compression therapy. Wien Med Wochenschr 168:228–235 [DOI] [PubMed] [Google Scholar]
- Dziwenka M, Coppock RW (2021) Ginkgo biloba, Nutraceuticals. Elsevier, pp 835–852 [Google Scholar]
- Eberhardt RT, Raffetto JD (2005) Chronic venous insufficiency. Circulation 111:2398–2409 [DOI] [PubMed] [Google Scholar]
- Elamrawy S, Darwish I, Moustafa S, Elshaer N, Ahmed N (2021) Epidemiological, life style, and occupational factors associated with lower limb varicose veins: a case control study. J Egypt Public Health Assoc 96:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbialy ZI, Atiba A, Abdelnaby A, Al-Hawary II, Elsheshtawy A, El-Serehy HA, Abdel-Daim MM, Fadl SE, Assar DH (2020) Collagen extract obtained from Nile tilapia (Oreochromis niloticus L.) skin accelerates wound healing in rat model via up regulating VEGF, bFGF, and α-SMA genes expression. BMC Vet Res 16:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbialy ZI, Assar DH, Abdelnaby A, Asa SA, Abdelhiee EY, Ibrahim SS, Abdel-Daim MM, Almeer R, Atiba A (2021) Healing potential of Spirulina platensis for skin wounds by modulating bFGF, VEGF, TGF-ß1 and α-SMA genes expression targeting angiogenesis and scar tissue formation in the rat model. Biomed Pharmacother 137(10):1016 [DOI] [PubMed] [Google Scholar]
- Ercan G, Tartar RI, Solmaz A, Gulcicek OB, Karagulle OO, Meric S, Cayoren H, Kusaslan R, Kemik A, Kayali DG (2020) Potent therapeutic effects of ruscogenin on gastric ulcer established by acetic acid. Asian J Surg 43:405–416 [DOI] [PubMed] [Google Scholar]
- Fernández-Colino A, Jockenhoevel S (2021) Advances in engineering venous valves: the pursuit of a definite solution for chronic venous disease. Tissue Eng Part B Rev 27:253–265 [DOI] [PubMed] [Google Scholar]
- Fernández-Rojas M, Rodríguez L, Trostchansky A, Fuentes E (2022) Regulation of platelet function by natural bioactive compounds. Food Biosci 48:101742 [Google Scholar]
- Fikru A, Makonnen E, Eguale T, Debella A, Abie Mekonnen G (2012) Evaluation of in vivo wound healing activity of methanol extract of Achyranthes aspera L. J Ethnopharmacol 143:469–474 [DOI] [PubMed] [Google Scholar]
- Frade MA, Assis RV, Coutinho Netto J, Andrade TA, Foss NT (2012) The vegetal biomembrane in the healing of chronic venous ulcers. An Bras Dermatol 87:45–51 [DOI] [PubMed] [Google Scholar]
- Francis P, Masimba PJ, Mwakigonja AR (2018) Evaluation of the wound healing activity of formulated ointments and water preparation from Sida rhombifolia leaf extract. Tanzania J Health Res. 10.4314/thrb.v20i4.4 [Google Scholar]
- Franks PJ, Barker J, Collier M, Gethin G, Haesler E, Jawien A, Laeuchli S, Mosti G, Probst S, Weller C (2016) Management of patients with venous leg ulcers: challenges and current best practice. J Wound Care 25:S1–S67 [DOI] [PubMed] [Google Scholar]
- Fukaya E, Flores AM, Lindholm D, Gustafsson S, Zanetti D, Ingelsson E, Leeper NJ (2018) Clinical and genetic determinants of varicose veins: prospective, community-based study of≈ 500 000 individuals. Circulation 138:2869–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshkumar M, Ponrasu T, Krithika R, Iyappan K, Gayathri VS, Suguna L (2012) Topical application of acalypha indica accelerates rat cutaneous wound healing by up-regulating the expression of type I and III collagen. J Ethnopharmacol 142:14–22 [DOI] [PubMed] [Google Scholar]
- Gao R-D, Qian S-Y, Wang H-H, Liu Y-S, Ren S-Y (2022) Strategies and challenges in treatment of varicose veins and venous insufficiency. World J Clin Cases 10:5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawande VR, Mukhmale VS, Bhatkar VD, Tayade SP, Katekar V, Deshmukh SP (2024) Varicose veins: a systematic review of the diagnosis and treatment of varicose veins. GSCBPS 27:114–124
- Ghosh SK, Al Mamun A, Majumder A (2023) Clinical presentation of varicose veins. Indian J Surg 85:7–14 [Google Scholar]
- Guguloth SK, Malothu N, Ganta NM, Ramakrishna K, Guntupalli C (2023) Antiplatelet and antithrombotic properties of methanolic leaf extract of plumbago zeylanica L.: GC-MS and HR-LCMS metabolite profiling. S Afr J Bot 159:627–634 [Google Scholar]
- Gulati OP (2014) Pycnogenol® in chronic venous insufficiency and related venous disorders. Phytother Res 28:348–362 [DOI] [PubMed] [Google Scholar]
- Hadisi Z, Nourmohammadi J, Nassiri SM (2018) The antibacterial and anti-inflammatory investigation of Lawsonia Inermis-gelatin-starch nano-fibrous dressing in burn wound. Int J Biol Macromol 107:2008–2019 [DOI] [PubMed] [Google Scholar]
- Hajiali H, Summa M, Russo D, Armirotti A, Brunetti V, Bertorelli R, Athanassiou A, Mele E (2016) Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J Mater Chem B 4:1686–1695 [DOI] [PubMed] [Google Scholar]
- Han S-Y, Kim J-H, Jo E-H, Kim Y-K (2021) Eleutherococcus sessiliflorus inhibits receptor activator of nuclear factor kappa-B ligand (RANKL)-induced osteoclast differentiation and prevents ovariectomy (OVX)-induced bone loss. Molecules 26:1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasstani OA, Moin S, Tham CL, Liew CY, Ismail N, Rajajendram R, Harith HH, Zakaria ZA, Mohamad AS, Sulaiman MR (2010) Flavonoid combinations cause synergistic inhibition of proinflammatory mediator secretion from lipopolysaccharide-induced RAW 264.7 cells. Inflamm Res 59:711–721 [DOI] [PubMed] [Google Scholar]
- Harenberg J (2008) Drugs affecting blood coagulation, fibrinolysis, and hemostasis, side effects of drugs annual. Elsevier, pp 399–422 [Google Scholar]
- Harnarayan P, Harnanan D (2022) The Klippel-Trenaunay syndrome in 2022: unravelling its genetic and molecular profile and its link to the limb overgrowth syndromes. Vasc Health Risk Manag 18:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W, Toonder I, Wittens C (2004) Value of the Trendelenburg tourniquet test in the assessment of primary varicose veins. Phlebology 19:77–80 [Google Scholar]
- Huan Y, Peng K-J, Wang Q-L, Gu Z-Y, Lu Y-Q, Jun Z, Fang X, Liu Y-L, Ying T, Deng F-M (2013) Effect of pomegranate peel polyphenol gel on cutaneous wound healing in alloxan-induced diabetic rats. Chin Med J 126:1700–1706 [PubMed] [Google Scholar]
- Huang M, Liu X, Ren Y, Huang Q, Shi Y, Yuan P, Chen M, (2024) Quercetin: a flavonoid with potential for treating acute lung injury, drug design. Develop Therapy 18:5709–5728 [DOI] [PMC free article] [PubMed]
- Ilie-Ene A, Eşanu V, Toşa V, Dindelegan G (2023) Cyanoacrylate adhesives used for topical hemostasis–a systematic review. Chirurgia 118:596–608 [DOI] [PubMed] [Google Scholar]
- Islam MA, Alam F, Khalil I, Haryo Sasongko T, Hua Gan S (2016) Natural products towards the discovery of potential future antithrombotic drugs. Curr Pharm des 22:2926–2946 [DOI] [PubMed] [Google Scholar]
- Jacobs BN, Andraska EA, Obi AT, Wakefield TW (2017) Pathophysiology of varicose veins. J Vasc Surg Venous Lymphat Disord 5:460–467 [DOI] [PubMed] [Google Scholar]
- Jain V, Kunwar B, Verma S (2023) A review on thrombolysis enhancing indian edible plants. Biomed Pharmacol J 16:1283–1302 [Google Scholar]
- Jarald E, Narendra N, Manish M, Anurekha J, Sheeja E (2009) Determination of rutin content and antioxidant activity of extracts of Butea monosperma flowers extracted using various extraction methods. Phcog J 1:126–129 [Google Scholar]
- Jarošíková R, Roztočil K, Husáková J, Dubský M, Robert B, Wosková V, Fejfarová V (2023) Chronic venous disease and its intersections with diabetes mellitus. Physiol Res 72:280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawien A (2003) The influence of environmental factors in chronic venous insufficiency. Angiology 54:S19–S31 [DOI] [PubMed] [Google Scholar]
- Jayaraj A, Noel C, Kuykendall R, Raju S (2021) Long-term outcomes following use of a composite Wallstent-Z stent approach to iliofemoral venous stenting. J Vasc Surg Venous Lymphat Disord 9(393–400):e392 [DOI] [PubMed] [Google Scholar]
- Karakaya PS, Oktay A, Seventekin N, Yesil-Celiktas O (2021) Design of a new generation wound dressing with pine bark extract. J Ind Text 50:1193–1204 [Google Scholar]
- Kelechi TJ, Johnson JJ, Yates S (2015) Chronic venous disease and venous leg ulcers: an evidence-based update. J Vasc Nurs 33:36–46 [DOI] [PubMed] [Google Scholar]
- Khan MM, Begum M, Anees A, Mishra A (2024) Vein unveiled: an overview of varicose vein. World J Curr Med Pharmaceutical Res 6(1):47–52
- Khidyrova N, Shakhidoyatov KM (2002) Plant polyprenols and their biological activity. Chem Nat Compd 38:107–121 [Google Scholar]
- Khosropanah A, Mehri Ardestani M, Rostami N, Hashemi F, Pasalar M, Hunter J, Heydarirad G (2023) Effects of chicory and fumitory on hot flashes among breast cancer survivors: a randomized, double-blind placebo-controlled trial. J Integrative Complement Med 29:31–41 [DOI] [PubMed] [Google Scholar]
- Kienzl P, Deinsberger J, Weber B (2024) Chronic venous disease: pathophysiological aspects, risk factors, and diagnosis. Hämostaseologie 44 (4):277–286 [DOI] [PubMed]
- Kimura Y, Sumiyoshi M, Samukawa K-I, Satake N, Sakanaka M (2008) Facilitating action of asiaticoside at low doses on burn wound repair and its mechanism. Eur J Pharmacol 584:415–423 [DOI] [PubMed] [Google Scholar]
- Kleijnen J, Knipschild P (1992) Ginkgo biloba for cerebral insufficiency. Br J Clin Pharmacol 34:352–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koca U, Süntar I, Akkol EK, Yılmazer D, Alper M (2011) Wound repair potential of Olea europaea L. leaf extracts revealed by in vivo experimental models and comparative evaluation of the extracts’ antioxidant activity. J Med Food 14:140–146 [DOI] [PubMed] [Google Scholar]
- Koehler A, Rao R, Rothman Y, Gozal YM, Struve T, Alschuler L, Sengupta S (2022) A case study using papaya leaf extract to reverse chemotherapy-induced thrombocytopenia in a GBM patient. Integr Cancer Ther 21:15347354211068416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konschake W, Riebe H, Pediaditi P, Haase H, Jünger M, Lutze S (2017) Compression in the treatment of chronic venous insufficiency: Efficacy depending on the length of the stocking. Clin Hemorheol Microcirc 64:425–434 [DOI] [PubMed] [Google Scholar]
- Krizanova O, Penesova A, Hokynkova A, Pokorna A, Samadian A, Babula P (2024) Chronic venous insufficiency and venous leg ulcers: aetiology, on the pathophysiology-based treatment. Int Wound J 21:e14405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger-Genge A, Blocki A, Franke R-P, Jung F (2019) Vascular endothelial cell biology: an update. Int J Mol Sci 20:4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Khan IA, Das A, Shah H (2022) Chronic venous disease. Part 1: pathophysiology and clinical features. Clin Exp Dermatol 47:1228–1239 [DOI] [PubMed] [Google Scholar]
- Kundaković T, Milenković M, Zlatković S, Nikolić V, Nikolić G, Binić I (2012) Treatment of venous ulcers with the herbal-based ointment Herbadermal®: a prospective non-randomized pilot study. Forschende Komplementärmedizin/res Complement Med 19:26–30 [DOI] [PubMed] [Google Scholar]
- Labropoulos N, Gasparis AP, Pefanis D, Leon LR Jr, Tassiopoulos AK (2009) Secondary chronic venous disease progresses faster than primary. J Vasc Surg 49:704–710 [DOI] [PubMed] [Google Scholar]
- Lammoglia-Ordiales L, Vega-Memije ME, Herrera-Arellano A, Rivera-Arce E, Agüero J, Vargas-Martinez F, Contreras-Ruiz J (2012) A randomised comparative trial on the use of a hydrogel with tepescohuite extract (Mimosa tenuiflora cortex extract-2G) in the treatment of venous leg ulcers. Int Wound J 9:412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamponi S (2021) Potential use of plants and their extracts in the treatment of coagulation disorders in COVID-19 disease: a narrative review. Longhua Chin Med 4:26 [Google Scholar]
- Leenstra B, Wijnand J, Verhoeven B, Koning O, Teraa M, Verhaar MC, de Borst GJ (2020) Applicability of transcutaneous oxygen tension measurement in the assessment of chronic limb-threatening ischemia. Angiology 71:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite SN, Palhano G, Almeida S, Biavatti MW (2002) Wound healing activity and systemic effects of Vernonia scorpioides extract in guinea pig. Fitoterapia 73:496–500 [DOI] [PubMed] [Google Scholar]
- Leite PM, Freitas A, Amorim J, Figueiredo RCD, Bertolucci S, Faraco A, Martins M, Carvalho MG, Castilho R (2022) In vitro anticoagulant activity of selected medicinal plants: Potential interactions with warfarin and development of new anticoagulants. J Basic Clin Physiol Pharmacol 33:499–510 [DOI] [PubMed] [Google Scholar]
- Li J, Liang Q, Sun G (2019a) Interaction between traditional Chinese medicine and anticoagulant/antiplatelet drugs. Curr Drug Metab 20:701–713 [DOI] [PubMed] [Google Scholar]
- Li Y, Kandhare AD, Mukherjee AA, Bodhankar SL (2019b) Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats. Regul Toxicol Pharmacol 105:77–85 [DOI] [PubMed] [Google Scholar]
- Li D, Sheppard SE, March ME, Battig MR, Surrey LF, Srinivasan AS, Matsuoka LS, Tian L, Wang F, Seiler C (2023) Genomic profiling informs diagnoses and treatment in vascular anomalies. Nat Med 29:1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichota A, Gwozdzinski L, Gwozdzinski K (2019) Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur J Med Chem 176:68–91 [DOI] [PubMed] [Google Scholar]
- Lichota A, Szewczyk EM, Gwozdzinski K (2020) Factors affecting the formation and treatment of thrombosis by natural and synthetic compounds. Int J Mol Sci 21:7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xin H, Zhang Y, Che F, Shen N, Cui Y (2022) Leaves, seeds and exocarp of Ginkgo biloba L.(Ginkgoaceae): a comprehensive review of traditional uses, phytochemistry, pharmacology, resource utilization and toxicity. J Ethnopharmacol 298:115645 [DOI] [PubMed] [Google Scholar]
- López-Pérez A, Hernández-Juárez J, Solano R, Majluf-Cruz A, Hernández-Cruz PA, Lagunez-Rivera L (2022) Laelia furfuracea Lindl.: an Endemic Mexican orchid with anticoagulant activity. J Mex Chem Soc 66:1–16 [Google Scholar]
- Lurie F, Branisteanu DE (2023) Improving chronic venous disease management with micronised purified flavonoid fraction: new evidence from clinical trials to real life. Clin Drug Investig 43:9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie F, Passman M, Meisner M, Dalsing M, Masuda E, Welch H, Bush RL, Blebea J, Carpentier PH, De Maeseneer M (2020) The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord 8:342–352 [DOI] [PubMed] [Google Scholar]
- Lutsey PL, Zakai NA (2023) Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol 20:248–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli O, Alunno A, Drudi FM, Malaj A, Irace L (2021) Duplex ultrasound versus CT angiography for the treatment planning of lower-limb arterial disease. J Ultrasound 24:471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder T, Salam M, Mitra S, Hossain S, Hussain M (2022) Current antithrombotic therapies and prospects of natural compounds in the management of the thrombotic disorder. Nat Res Human Health 3:134–175 [Google Scholar]
- Mazzolai L, Ageno W, Alatri A, Bauersachs R, Becattini C, Brodmann M, Emmerich J, Konstantinides S, Meyer G, Middeldorp S (2022) Second consensus document on diagnosis and management of acute deep vein thrombosis: updated document elaborated by the ESC Working Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur J Prev Cardiol 29:1248–1263 [DOI] [PubMed] [Google Scholar]
- Max H, Pittler Edzard, Ernst (2012) Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev 2012(12). 10.1002/14651858.CD003230.pub4 [DOI] [PMC free article] [PubMed]
- McEwen BJ (2015) The influence of herbal medicine on platelet function and coagulation: a narrative review Seminars in Thrombosis and Hemostasis. Thieme Medical Publishers, pp 300–314 [DOI] [PubMed] [Google Scholar]
- Meissner MH, Gloviczki P, Bergan J, Kistner RL, Morrison N, Pannier F, Pappas PJ, Rabe E, Raju S, Villavicencio JL (2007a) Primary chronic venous disorders. J Vasc Surg 46:S54–S67 [DOI] [PubMed] [Google Scholar]
- Meissner MH, Eklof B, Smith PC, Dalsing MC, DePalma RG, Gloviczki P, Moneta G, Neglén P, O’Donnell T, Partsch H (2007b) Secondary chronic venous disorders. J Vasc Surg 46:S68–S83 [DOI] [PubMed] [Google Scholar]
- Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J (2021) Anti-inflammatory action and mechanisms of resveratrol. Molecules 26:229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel CB, Andrade RS, Mazurek L, Martins-de-Abreu MC, Miguel-Neto J, Barbosa AdM, Silva GP, Ges-Neto A, Soares SdC, Lazo-Chica JE (2025) Emerging pharmacological interventions for chronic venous insufficiency: a comprehensive systematic review and meta-analysis of efficacy, safety, and therapeutic advances. Pharmaceutics 17:59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S-K, Kim S-Y, Park YJ, Lee W, Jung IM, Lee T, Ha J, Kim SJ (2010) Role of three-dimensional computed tomography venography as a powerful navigator for varicose vein surgery. J Vasc Surg 51:893–899 [DOI] [PubMed] [Google Scholar]
- Mokhtari M, Yousefi M, Bazaz MM, Rakhshandeh H, Vahid H, Ariamanesh AS (2020) The efficacy of topical red clover oil on knee osteoarthritis: A pilot prospective randomized triple-blind placebo-controlled clinical trial. Phytother Res 34:1687–1695 [DOI] [PubMed] [Google Scholar]
- Murti K, Kumar U (2012) Enhancement of wound healing with roots of Ficus racemosa L. in albino rats. Asian Pac J Trop Biomed 2:276–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi SF, Habtemariam S, Ahmed T, Sureda A, Daglia M, Sobarzo-Sánchez E, Nabavi SM (2015) Polyphenolic composition of Crataegus monogyna Jacq.: from chemistry to medical applications. Nutrients 7:7708–7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete S, Solar C, Tapia R, Pereira J, Fuentes E, Palomo I (2023) Pathophysiology of deep vein thrombosis. Clin Exp Med 23:645–654 [DOI] [PubMed] [Google Scholar]
- Nayak SB, Isik K, Marshall JR (2017) Wound-healing potential of oil of hypercium perforatum in excision wounds of male sprague dawley rats. Adv Wound Care 6:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides AN (2020) The most severe stage of chronic venous disease: an update on the management of patients with venous leg ulcers. Adv Ther 37:19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides A, Kakkos S, Baekgaard N, Comerota A, de Maeseneer M, Eklof B, Giannoukas AD, Lugli M, Maleti O, Myers K (2018) Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence part I. Int Angiol: a J Int Union Angiol 37:181–254 [DOI] [PubMed] [Google Scholar]
- Olsen GM, Nackers A, Drolet BA (2020) Infantile and congenital hemangiomas, Seminars in Pediatric Surgery, Elsevier, p. 150969. [DOI] [PubMed]
- Orhurhu V, Chu R, Xie K, Kamanyi GN, Salisu B, Salisu-Orhurhu M, Urits I, Kaye RJ, Hasoon J, Viswanath O (2021) Management of lower extremity pain from chronic venous insufficiency: a comprehensive review. Cardiol Ther 10:111–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega MA, Fraile-Martínez O, García-Montero C, Álvarez-Mon MA, Chaowen C, Ruiz-Grande F, Pekarek L, Monserrat J, Asúnsolo A, García-Honduvilla N (2021) Understanding chronic venous disease: a critical overview of its pathophysiology and medical management. J Clin Med 10:3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega MA, Gómez-Lahoz AM, Sánchez-Trujillo L, Fraile-Martinez O, García-Montero C, Guijarro LG, Bravo C, De Leon-Luis JA, Saz JV, Bujan J (2022) Chronic venous disease during pregnancy causes a systematic increase in maternal and fetal proinflammatory markers. Int J Mol Sci 23:8976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanloo M, Noori F, Varaa N, Tavassoli A, Goodarzi A, Moghaddam MT, Ebrahimi L, Abpeikar Z, Farmani AR, Safaei M (2024) The wound healing effect of polycaprolactone-chitosan scaffold coated with a gel containing Zataria multiflora Boiss volatile oil nanoemulsions. BMC Complement Med Therapies 24:56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas PJ, Lakhanpal S, Nguyen KQ, Vanjara R (2018) The Center for Vein Restoration Study on presenting symptoms, treatment modalities, and outcomes in Medicare-eligible patients with chronic venous disorders. J Vasc Surg Venous Lymphat Disord 6:13–24 [DOI] [PubMed] [Google Scholar]
- Park KS (2021) Pharmacological effects of Centella asiatica on skin diseases: Evidence and possible mechanisms. Evidence-Based Complementary and Alternative Medicine 2021:5462633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin M, Ramelet A-A (2011) Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg 41:117–125 [DOI] [PubMed] [Google Scholar]
- Qin Y, Guo XW, Li L, Wang HW, Kim W (2013) The antioxidant property of chitosan green tea polyphenols complex induces transglutaminase activation in wound healing. J Med Food 16:487–498 [DOI] [PubMed] [Google Scholar]
- Rabe E, Partsch H, Morrison N, Meissner MH, Mosti G, Lattimer CR, Carpentier PH, Gaillard S, Jünger M, Urbanek T (2020) Risks and contraindications of medical compression treatment–A critical reappraisal. IntConsensus Statement Phlebol 35:447–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radović J, Suručić R, Niketić M, Kundaković-Vasović T (2022) Alchemilla viridiflora Rothm.: the potent natural inhibitor of angiotensin I-converting enzyme. Mol Cell Biochem 477:1893–1903 [DOI] [PubMed] [Google Scholar]
- Raffetto JD (2018) Pathophysiology of chronic venous disease and venous ulcers. Surgical Clinics 98:337–347 [DOI] [PubMed] [Google Scholar]
- Rahayu, K., Suharto, I., Etika, A., Nurseskasatmata, S., 2020. The effect of ginger extract (zingiber officinale roscoe) on the number of neutrophil cells, fibroblast and epithelialization on incision wound, Journal of Physics: Conference Series, IOP Publishing, p. 032063.
- Raina AK, Jackson MD, Severson RF (1997) Increased pheromone production in wild tobacco budworm (Lepidoptera: Noctuidae) exposed to host plants and host chemicals. Environ Entomol 26:101–105 [Google Scholar]
- Raja A, Varalakshmi B, Santhi K (2020) Antibacterial, antioxidant and anticoagulant efficacy of C. verum Mediated Silver Nanoparticles. Int J Life Sci 9:214 [Google Scholar]
- Raju S, Hollis K, Neglen P (2006) Obstructive lesions of the inferior vena cava: clinical features and endovenous treatment. J Vasc Surg 44:820–827 [DOI] [PubMed] [Google Scholar]
- Rastogi S, Pandey MM, Rawat A (2016) Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine 23:1082–1089 [DOI] [PubMed] [Google Scholar]
- Rhone P, Ruszkowska-Ciastek B, Bielawski K, Brkic A, Zarychta E, Góralczyk B, Roszkowski K, Rość D (2018) Comprehensive analysis of haemostatic profile depending on clinicopathological determinants in breast cancer patients. Biosci Rep 38:20171657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risto J, Hamiti A, Rrapaj E (2024) Alternative therapies for viral infections caused by SARS-Cov-2. Fisioterapia Em Movimento 37:e37201 [Google Scholar]
- Rivera-Arce E, Chavez-Soto MA, Herrera-Arellano A, Arzate S, Agüero J, Feria-Romero IA, Cruz-Guzmán A, Lozoya X (2007) Therapeutic effectiveness of a Mimosa tenuiflora cortex extract in venous leg ulceration treatment. J Ethnopharmacol 109:523–528 [DOI] [PubMed] [Google Scholar]
- Robertson L, Evans Ca, Fowkes F (2008) Epidemiology of chronic venous disease. Phlebology 23:103–111 [DOI] [PubMed] [Google Scholar]
- Romero-Cerecero O, Zamilpa-Álvarez A, Jiménez-Ferrer E, Tortoriello J (2012) Exploratory study on the effectiveness of a standardized extract from Ageratina pichinchensis in patients with chronic venous leg ulcers. Planta Med 78:304–310 [DOI] [PubMed] [Google Scholar]
- Sadaf F, Saleem R, Ahmed M, Ahmad SI, Navaid ul Z (2006) Healing potential of cream containing extract of Sphaeranthus indicus on dermal wounds in Guinea pigs. J Ethnopharmacol 107:161–163 [DOI] [PubMed] [Google Scholar]
- Saha P, Black S, Breen K, Patel A, Modarai B, Smith A (2016) Contemporary management of acute and chronic deep venous thrombosis. British Med Bull 117:107 [DOI] [PubMed] [Google Scholar]
- Salim S, Machin M, Patterson BO, Onida S, Davies AH (2021) Global epidemiology of chronic venous disease: a systematic review with pooled prevalence analysis. Ann Surg 274:971–976 [DOI] [PubMed] [Google Scholar]
- Sánchez-Ferrer A, Engelhardt M, Richter K (2023) Anisotropic wood–water interactions determined by gravimetric vapor sorption experiments. Cellulose 30:3869–3885 [Google Scholar]
- Sannikov A, Emelyanenko V, Drozdova I (2020) Review of the plethysmographic methods for studying hemodynamic disorders in patients with chronic lower extremites venous diseases. Ambulatornaya Khirurgiya Ambulatory Surg (Russia). 10.21518/1995-1477-2020-1-2-58-70 [Google Scholar]
- Scholz I, Liakoni E, Hammann F, Grafinger KE, Duthaler U, Nagler M, Krähenbühl S, Haschke M (2021) Effects of Hypericum perforatum (St John’s wort) on the pharmacokinetics and pharmacodynamics of rivaroxaban in humans. Br J Clin Pharmacol 87:1466–1474 [DOI] [PubMed] [Google Scholar]
- Schoonees A, Visser J, Musekiwa A, Volmink J (2012) Cochrane database of systematic reviews. John Wiley & Sons Ltd, Chichester, UK [Google Scholar]
- Shahriar M, Bhuiyan M, Rana M (2018) Screening of antibacterial, thrombolytic, membrane stabilizing, anti-inflammatory and antitumor activity of citrus assamensis leaf extracts. J Sci Res 10:195 [Google Scholar]
- Shahzad MN, Ahmed N (2013) Effectiveness of Aloe Vera gel compared with 1% silver sulphadiazine cream as burn wound dressing in second degree burns, JPMA. J Pak Med Assoc 63:225–230 [PubMed] [Google Scholar]
- Sharma UK, Singh A, Sharma U, Kumar M, Rai D, Agrahari P (2010) Wound healing activity of Kigelia pinnata bark extract. Asian J Pharm Clin Res 3:73–75 [Google Scholar]
- Shenoy RR, Sudheendra AT, Nayak PG, Paul P, Kutty NG, Rao CM (2011) Normal and delayed wound healing is improved by sesamol, an active constituent of Sesamum indicum (L.) in albino rats. J Ethnopharmacol 133:608–612 [DOI] [PubMed] [Google Scholar]
- Shi C-C, Chen T-R, Zhang Q-H, Wei L-H, Huang C, Zhu Y-D, Liu H-B, Bai Y-K, Wang F-J, Guo W-Z (2020) Inhibition of human thrombin by the constituents of licorice: Inhibition kinetics and mechanistic insights through in vitro and in silico studies. RSC Adv 10:3626–3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukhman D, Bernstein P (2024) Tamarind-Vitex agnus-castus, a Clinician’s evidence-based guide to supplements. Springer, pp 171–197 [Google Scholar]
- Silva H, Martins FG (2022) Cardiovascular activity of Ginkgo biloba—An insight from healthy subjects. Biology 12:15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparreboom A, Cox MC, Acharya MR, Figg WD (2004) Herbal remedies in the United States: potential adverse interactions with anticancer agents. J Clin Oncol 22:2489–2503 [DOI] [PubMed] [Google Scholar]
- Speroni E, Govoni P, Guizzardi S, Renzulli C, Guerra MC (2002) Anti-inflammatory and cicatrizing activity of Echinacea pallida Nutt. root extract. J Ethnopharmacol 79:265–272 [DOI] [PubMed] [Google Scholar]
- Stafforini NA, Singh N (2023) Management of Vascular Injuries in Penetrating Trauma. Surgical Clinics 103:801–825 [DOI] [PubMed] [Google Scholar]
- Stücker M, Rabe E, Meyer K, Ottillinger B, Schütt T (2019) Therapeutic approach to chronic venous insufficiency–clinical benefits of red-vine-leaf-extract AS 195 (Antistax®). Die Pharmazie-an Int J Pharmaceutical Sci 74:193–200 [DOI] [PubMed] [Google Scholar]
- Süntar IP, Akkol EK, Yalçın FN, Koca U, Keleş H, Yesilada E (2010) Wound healing potential of Sambucus ebulus L. leaves and isolation of an active component, quercetin 3-O-glucoside. J Ethnopharmacol 129:106–114 [DOI] [PubMed] [Google Scholar]
- Suryamathi M, Ruba C, Viswanathamurthi P, Balasubramanian V, Perumal P (2019) Tridax procumbens extract loaded electrospun PCL nanofibers: a novel wound dressing material. Macromol Res 27:55–60 [Google Scholar]
- Tan SC, Bhattamisra SK, Chellappan DK, Candasamy M (2021) Actions and therapeutic potential of madecassoside and other major constituents of Centella asiatica: a review. Appl Sci 11:8475 [Google Scholar]
- Tang T, Yin L, Yang J, Shan G (2007) Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur J Pharmacol 567:177–185 [DOI] [PubMed] [Google Scholar]
- Tansey EA, Montgomery LE, Quinn JG, Roe SM, Johnson CD (2019) Understanding basic vein physiology and venous blood pressure through simple physical assessments. Adv Physiol Educ 43:423 [DOI] [PubMed] [Google Scholar]
- Tantiwatcharothai S, Prachayawarakorn J (2020) Property improvement of antibacterial wound dressing from basil seed (O. basilicum L.) mucilage- ZnO nanocomposite by borax crosslinking. Carbohyd Polym 227:115360 [DOI] [PubMed] [Google Scholar]
- Taylor AM, Bordoni B (2020) Histology, blood vascular system. StatPearls Publishing, Treasure Island (FL), PMID:31985998
- Thomas AB, Dapkekar M, Nagore D, Doke R, Bankar N, Surve N (2024) Herbal Emulgel Containing Azadirachta indica (Neem) and Nigella Sativa L. (Black Cumin) Oils in Wound Management: Preclinical Investigations. Anc Sci Life 38:141–149 [Google Scholar]
- Tian J, Liu Y, Chen K (2017) Ginkgo biloba extract in vascular protection: molecular mechanisms and clinical applications. Curr Vasc Pharmacol 15:532–548 [DOI] [PubMed] [Google Scholar]
- Todd M (2012) Strategies to prevent the progression of venous and lymphovenous disease. Br J Community Nurs 17:3–14 [Google Scholar]
- Udombhornprabha A (2018) The efficacy and safety of herbal medicine combination for management of leg symptoms due to chronic venous diseasethai patients: a double blind ed. randomized controlled trial. College of Public Health Sciences (thesis). https://digiverse.chula.ac.th/Info/item/dc:83409
- Valente IVB, Garcia D, Abbott A, Spruill L, Siegel J, Forcucci J, Hanna G, Mukherjee R, Hamann M, Hilliard E (2024) The anti-proliferative effects of a frankincense extract in a window of opportunity phase ia clinical trial for patients with breast cancer. Breast Cancer Res Treat 204:521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlajinac H, Radak ÐJ, Marinković J, Maksimović MŽ (2012) Risk factors for chronic venous disease. Phlebology 27:416–422 [DOI] [PubMed] [Google Scholar]
- Wang Y, Li G, Yang L, Luo R, Guo G (2022) Development of innovative biomaterials and devices for the treatment of cardiovascular diseases. Adv Mater 34:2201971 [DOI] [PubMed] [Google Scholar]
- Weseler AR, Bast A (2017) Masquelier’s grape seed extract: From basic flavonoid research to a well-characterized food supplement with health benefits. Nutr J 16:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Auyeung G, Lamprou S, Vuong T, Baker I (2020) What is the risk of DVT/PE in patients diagnosed with superficial vein thrombosis? Evidence-Based Practice 23:33–34 [Google Scholar]
- Wittens C, Davies A, Bækgaard N, Broholm R, Cavezzi A, Chastanet S, de Wolf M, Eggen C, Giannoukas A, Gohel M (2015) Editor’s choice–management of chronic venous disease: clinical practice guidelines of the European society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg 49:678–737 [DOI] [PubMed] [Google Scholar]
- Wolberg AS, Rosendaal FR, Weitz JI, Jaffer IH, Agnelli G, Baglin T, Mackman N (2015) Venous thrombosis. Nat Rev Dis Primers 1:1–17 [DOI] [PubMed] [Google Scholar]
- Wong M, Parsi K, Myers K, De Maeseneer M, Caprini J, Cavezzi A, Connor DE, Davies AH, Gianesini S, Gillet J-L (2023) Sclerotherapy of lower limb veins: Indications, contraindications and treatment strategies to prevent complications–a consensus document of the international union of phlebology-2023. Phlebology 38:205–258 [DOI] [PubMed] [Google Scholar]
- Wu XB, Luo XQ, Gu SY, Xu JH (2012) The effects of Polygonum cuspidatum extract on wound healing in rats. J Ethnopharmacol 141:934–937 [DOI] [PubMed] [Google Scholar]
- Wu C, Liang C, Sun J, Zhang Z, Pan X (2022) Effects of Diosmin on IL-1 beta-Induced Inflammatory Response in Primary Rat Chondrocytes. Int J Pharmacol 18:667–672 [Google Scholar]
- Yang T-Z, Liu Y, Liu Y-Y, Ding X-F, Chen J-X, Kou M-J, Zou X-J (2017) The use of Rheum Palmatum L. in the treatment of acute respiratory distress syndrome: a meta-analysis of randomized, controlled trials. Afr J Tradit Complement Altern Med 14:334–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang X, Xue B (2024) New insights into the role of cellular senescence and chronic wounds. Front Endocrinol 15:1400462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn YJ, Lee J (2018) Chronic venous insufficiency and varicose veins of the lower extremities. Korean J Intern Med 34:269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn YJ, Lee J (2019) Chronic venous insufficiency and varicose veins of the lower extremities. Korean J Intern Med 34:269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepon KM, Martins MM, Marques MS, Heckler JM, Dal Pont Morisso F, Moreira MG, Ziulkoski AL, Kanis LA (2019) Smart wound dressing based on κ–carrageenan/locust bean gum/cranberry extract for monitoring bacterial infections. Carbohyd Polym 206:362–370 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang J, Cheng X, Yi B, Zhang X, Li Q (2015) Apigenin induces dermal collagen synthesis via smad2/3 signaling pathway. European Journal of Histochemistry : EJH 59:2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhang Y, Zhou J, Cai Y, Li Z, Sun J, Xie Z, Hao G (2024) Metabolic effects of quercetin on inflammatory and autoimmune responses in rheumatoid arthritis are mediated through the inhibition of JAK1/STAT3/HIF-1α signaling. Mol Med 30:170 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available within the manuscript.