Abstract
We report a magnetic silica bead-based nucleic acid extraction method which is rapid (6 – 7 min) and efficient (extracts nearly all the nucleic acid in the sample). We call this method SHIFT-SP (Silica bead based HIgh yield Fast Tip based Sample Prep). Factors such as pH during binding of nucleic acid to bead, the mode of bead movement during binding, the duration of binding, pH, temperature and duration of elution affect the final yield of the method. We have compared the method developed here to commercially available bead and column-based methods. The commercially available bead-based method took about 40 minutes from lysed sample to extracted nucleic acid and had a similar DNA yield as SHIFT-SP. The commercially available column-based method took 25 minutes with half the DNA yield as SHIFT-SP. In addition to a favorable time and yield, all steps in this developed method are automation compatible. We demonstrated a similar efficiency and speed of extraction for both DNA and RNA and showed that the performance of SHIFT-SP was unaffected by DNA size. SHIFT-SP was also able to extract DNA from low concentrations of microbes from enriched whole blood, for downstream whole genome amplification and sequencing.
Keywords: Nucleic acid extraction, Boom method, RNA extraction, DNA extraction, Rapid nucleic acid extraction, Low volume eluate
Subject terms: Molecular biology, Biotechnology
Introduction
Nucleic acid (NA) based tests are increasingly being used across various disease conditions for diagnosis, prognosis, for treatment decisioning and can be scaled up very quickly in both central lab and point of care testing as evidenced during the COVID-19 pandemic. These molecular diagnostic tests are sensitive enough to detect very low amounts of targets from different types of clinical samples.
NA extraction or sample preparation (SP) is a critical upstream step in various molecular analytical workflows, including PCR, microarray, sequencing, and other hybridization-based workflows. NA extraction can be broadly categorized into chemically driven methods and solid phase extractions1. While the chemically driven extraction methods are based on differences in the biochemical properties of NAs from other components in a cell lysate2, solid phase extraction methods are based on the adsorption of NAs on solid matrices (embedded either on beads or in columns) in the presence of chaotropic salts or other buffers1,3.
Most molecular diagnostic workflows use the solid phase extraction process, which is robust and is automatable. The key steps involved in a solid phase extraction are (1) NA binding to the matrix, usually in the presence of a chaotropic agent or other binding buffer, (2) Washing the matrix to remove non-specifically bound impurities and chaotropic agents, and (3) Eluting the bound NA with an appropriate buffer.
Common examples of solid phase extraction process include the silica based Boom method4, which is the method addressed in this paper or the anion exchange approach which relies on charge-based binding of NA to beads in the absence of chaotropes5–7. The anion exchange method relies on pH change to enable binding, a low pH to enable binding of the negatively charged NA to the positively charged matrix, and a higher pH buffer to enable elution8,9. Boom method on the other hand uses high concentration guanidine salts to facilitate binding between silica beads and DNA. While guanidine is a PCR inhibitor10, and the silica matrix needs thorough washing to eliminate guanidine before eluting NA from the silica surface, it is excellent in denaturing proteins such as DNases11,12 and inactivating viruses13 in samples during NA extraction. Guanidinium thiocyanate based extractions may also result in better inhibitor removal from different sample types than other methods14–16. In a head-to-head comparison study, ion exchange based kits performed worse than silica-based kits when extracting DNA from whole blood/buffy coat both in terms of yield and quality17.
In this paper we have optimized the VERSANT® sample preparation workflow, a magnetic silica bead-based NA extraction method with a goal of minimizing the overall NA extraction time and elution volume. A low NA extraction time reduces molecular diagnostic test turn-around time and can increase throughput and enable timely STAT (from Latin word “statim”; stands for high priority samples) sample processing when implemented in an automated instrument in the central lab. A low elution volume yields a high concentration NA eluate and reduces PCR volumes, thus potentially saving on PCR reagents and enabling rapid amplification on miniaturized PCR chips18.
To optimize the method, we focused on maximizing both NA binding to beads and NA elution from beads in the shortest amount of time. The newly optimized workflow is fast and very efficient, eluting nearly all the NA spiked in the starting sample. This improved efficiency could likely result in better identification of positive samples in molecular testing, even when targets are present in low concentrations thus improving clinical sensitivity of a molecular test, e.g. we hypothesize that an efficient NA extraction would result in better detection of SARS-CoV2 in both symptomatic and asymptomatic infected individuals19,20. Such a high efficiency NA extraction method would also benefit sepsis diagnostic tests where the infection causing pathogen is present at extremely low concentrations21. Furthermore, such a high yield NA extraction method is also well suited for extraction and detection of low amounts of circulating free NAs for oncology and infectious disease applications22.
For workflow optimization, the efficiency of binding and elution were calculated by measuring the amount of input NA in the starting sample, the amount of NA left in solution after binding to silica beads and the amount of NA eluted using a quantitative PCR (qPCR) based approach (Fig. 1).
Fig. 1.
DNA quantification method. (a) Schematic representation of the DNA quantification method for estimation of NA losses at the binding and elution steps in a sample preparation protocol. This quantification method was used to estimate % DNA bound and % DNA eluted for the different binding and elution factors tested during SHIFT-SP protocol development. (b) Standard curves used in the DNA quantification method. Two types of standard curves are used in this study. The curve generated with standards made in diluted LBB is used to estimate % DNA bound whereas the curve generated using standards made in 1X TE is used to estimate % DNA eluted. The standard curve “STD in 1:500 LBB” is similar to the standard curve “STD in 1X TE”, showing that dilution reduces the background effect of guanidine and triton in LBB.
In this development process, we investigated the effect of various factors like binding buffer pH, type of bead movement during nucleic acid binding, duration, and temperature on binding efficiency. Similarly, we evaluated the effect of elution time, temperature, elution buffer (EB) pH, and the number of elution steps on elution efficiency.
We performed the initial method optimizations on DNA samples, which were further adapted to RNA and low quantities of microbes from enriched whole blood samples. This optimized, high yield workflow, SHIFT-SP (Silica bead based HIgh yield Fast Tip based Sample Prep), was completed in a much shorter time than the standard VERSANT® workflow and other commercial workflows tested.
Results
Quantifying DNA samples in guanidine background
We quantified DNA losses during the binding and elution step based on the percent of input DNA that bound the beads and the percent eluted. The quantification approach is described in detail in methods (Fig. 1a, S7). As seen in Fig. 1b, for quantification of DNA bound, when these DNA standards in high chaotrope Lysis binding buffer (LBB) background were diluted 500-fold, the standard curve was found to be very similar to the standard curve of DNA in 1X TE buffer. To test the method, we spiked a known quantity of DNA in LBB and diluted it 500-fold in 1X TE buffer for qPCR quantification. The DNA quantity measured using this method of 500-fold dilution was within 2.4% deviation from the known amount. The known quantity of DNA measured directly by nanodrop was 151 pg vs 154.7 pg (standard deviation or SD = 18.0 pg, n = 3) measured using dilution and qPCR quantification, using the approach outlined in Fig. 1a and S7. Thus, we concluded that a 500-fold dilution in 1X TE buffer was sufficient to eliminate the effect of guanidine and Triton X-100 on PCR and this dilution was used in further experiments.
Effect of pH on binding
To evaluate the effect of pH on DNA binding to silica beads, we used 100 ng of purified ultrasound lysed Mycobacterium smegmatis DNA spiked in LBB from the VERSANT® sample preparation (SP) 1.2 kit, with a pH of 8.2 and VERSANT® SP 1.0 LBB with a pH of 4.1. The LBB from these kits are nearly identical in composition except pH. We evaluated the amount of DNA bound to silica beads at 1, 5, 10 and 15 minutes of binding time. The rest of the extraction steps followed were the same as the “Standard” VERSANT® sample preparation method. As shown in Fig. 2a, the amount of DNA bound to beads increased with time of incubation. In addition, LBB at pH 4.1 showed better DNA binding to silica beads than LBB at pH 8.6. A maximum 84.3% of input DNA is bound at 15 minutes, at pH 8.6. Whereas at pH 4.1, 98.2% input DNA is bound within 10 min (Fig. 2a).
Fig. 2.
Binding condition determination. (a) Effect of binding time and pH on DNA binding efficiency. The figure shows a comparison of percent DNA bound to the beads when incubated with low and high pH LBB with increasing duration of binding, demonstrated for 100 ng. The percent DNA bound to beads is higher at low pH at all the time points tested. Experiments were performed using “orbital shaking” of tubes with DNA/bead/LBB mix. (b) Effect of binding method on DNA binding efficiency. The graph indicates the percent DNA bound by “orbital shaking” method and non-orbital “tip-based” method at 1 min incubation time, for a 100 ng and 1000 ng input DNA. For both quantities of input DNA, the amount of DNA bound by “tip-based” method is higher compared to “orbital shaking” method. The error bar in the graph depicts the SD. n = 6 for each condition in a and b.
A lower pH reduces the negative charge on silica beads thus reducing the electrostatic repulsion between silica and the negatively charged DNA8, thus favoring DNA binding. This data also agrees with Katevatis et al., where pH 8.0 and 5.2 resulted higher DNA losses in the binding step when compared to pH 3.03, i.e. a lower pH favored greater NA binding.
Mode of bead mixing during binding
To evaluate the effect of mode of bead mixing during DNA binding to beads, we compared the currently used “orbital shaking” method with that of a pipette “tip-based” binding as a non-orbital mixing mode. We hypothesize that the tip-based method would allow beads to be rapidly exposed to the LBB and thus increase binding efficiency. Here, the binding mix is aspirated and dispensed repeatedly for 1 or 2 min.
With 100 ng input DNA (M. smegmatis), within 1 min, ~ 85% of DNA was bound to the beads via “tip-based” method, whereas only ~ 61% was bound in the “orbital shaking” method (Fig. 2b). At 1000 ng input DNA, only ~ 47% of the starting DNA quantity was bound by the “orbital shaking”, whereas ~ 62% of the starting DNA was bound with the “tip-based” method, as seen in Fig. 2b. To achieve the same level of binding as 1 min using tip, “orbital shaking” took 5 min (Fig. 2b, S3).
The optimal binding condition for 100 ng was therefore concluded to be 62 ºC for 1 min with 10 µL beads, with LBB at pH 4.1, using “tip-based” binding method. Next, we proceeded to optimize bead amount and the time of binding to further improve the amount of bound DNA, for 1000 ng.
Optimizing “tip-based” binding conditions for a higher input DNA
Next, we extended the binding time of the “tip-based” method to 2 min for 1000 ng input DNA (M. smegmatis), maintaining the bead volume at 10 µL. Approximately 56% of the input DNA is bound to 10 µL beads within 2 min in the “tip-based” binding, as shown in Table S1. To further increase DNA binding, we increased the bead quantity and were able to achieve ~ 92% and ~ 96% of binding with 30 µL and 50 µL of beads respectively (Table S1). The optimal binding conditions for 1000 ng was concluded to be 62 °C for 2 min with 50 µL beads, with LBB at pH 4.1, using the “tip-based” binding method. The increase in bead amount with increase in nucleic acid load in the sample has been introduced in SHIFT-SP to efficiently bind all the nucleic acid in the sample in a short amount of time. This shows that with the right amount of beads, a rapid, near complete binding of nucleic acid is possible even when the starting NA amount is high. If a downstream application needs a smaller quantity of NA, a lower volume of beads can be used, irrespective of the starting NA load in the sample.
Effect of pH and temperature on DNA elution
To understand the effect of pH on DNA elution from silica beads, we tested EB at pH values of 9.3 and 8.0, with 100 ng M. smegmatis DNA in PBS. The standard VERSANT® elution pH is 8.0. To further improve elution going above pH 8 was logical, as a higher pH would increase the net negative charge on the silica bead, thus promoting a more efficient release of the negatively charged DNA from beads8. In early experiments we tested both pH 8.5 and 9.3. pH 9.3 performed significantly better than pH 8.5, p-value < 0.05 (Figure S6). Next, we compared pH 9.3 to pH 8, the standard condition. All conditions except the EB pH remained the same as the “Standard” VERSANT® sample preparation protocol. It was observed that elution at pH 9.3 resulted in almost doubling of DNA yield compared to EB at pH 8.0 (Fig. 3a). The high pH elution buffer has not been tested for long term storage of DNA. The eluate could be neutralized immediately after elution by addition of an appropriate buffer for storage.
Fig. 3.
Elution condition determination. (a) Effect of elution buffer pH: 100 ng input DNA was used in this experiment. The figure indicates the amount of DNA eluted with low and high pH EBs. A higher amount of DNA was eluted at pH 9.3 compared to pH 8.0 at 74 ⁰C. n = 6 samples were tested for each condition. (b) and (c) Effect of elution temperature: The effect of elution temperature on the amount of DNA eluted was tested using 100 ng (b) and 1000 ng (c) of input DNA. As observed in the panels (b) and (c), 90 ⁰C favored better elution. In experiments in (b) and (c) elution was performed at pH 9.3. n = 5 samples were tested for each condition. (d) Effect of binding method on DNA elution: The graph represents the percentage DNA bound, and the corresponding percentage DNA eluted for three different binding conditions. Binding by ‘orbital shaking’ was tested at 15 min and 2 min. The “tip-based” method was tested for 2 min. For 2 min incubation, “tip-based” method, bound the higher amount of DNA. It also eluted the highest amount of DNA among all three conditions. When “orbital shaking” for 15 min is compared to “orbital shaking” for 2 min, the 2-min sample binds less but elutes higher % DNA. The percent DNA eluted from the total input (100 ng) was calculated to be 27.9, 43.82, and 83.78 for sample incubated with beads for 15 min by orbital shaking, 2 min by orbital shaking, and 2 min by “tip-based” method, respectively. The error bar in the graph depicts the SD. n = 6 for each condition.
To investigate the effect of temperature on elution, the elution step was carried out with pH 9.3 EB at 90 °C and 74 °C respectively and DNA yields were compared. A temperature of 74 ⁰C is used in the standard VERSANT® elution, we tested a higher temperature to increase the energy of system and facilitate elution. 90 °C resulted in better yields. Based on these results, pH 9.3 EB and 90 °C elution temperature was identified as favorable for maximum NA elution efficiency (Fig. 3b and 3c).
Effect of binding method on DNA elution
To investigate the effect of binding method and time on DNA elution we considered three test conditions for binding, a) “Orbital shaking” for 15 min, b) “Orbital shaking” for 2 min, and c) “tip-based” binding for 2 min. Binding conditions used were, 10 µL beads, 100 ng input DNA (M. smegmatis), bound at 62 °C with LBB at pH 4.1. Elution was done at pH 9.3, at 74 ⁰C for 1 min in 10 µL EB by “orbital shaking”. We maintained the elution temperature at 74 ºC and elution time at 1 min, as the effect of elution temperature and the adequate elution time was yet to be ascertained. The percent DNA bound was the highest when beads were incubated with sample for 15 min with “orbital shaking” ~ 99.16%, but the corresponding percent DNA eluted was the lowest at ~ 28% (Fig. 3d). Short binding time favored better elution, binding by “orbital shaking” for 2 min yielded more DNA in eluate than binding for 15 min, i.e., 43.82% (SD = 6.8%) vs 27.9% (SD = 6.86%). When binding time is constant, ‘tip-based” binding yielded more DNA than “orbital shaking” based binding, i.e., 83.78% (SD = 17.8%) vs. 43.82% (SD = 6.8%).
Low volume elution and bead separation by air-jump
To address clinical samples with low target abundance, we concentrated the target NAs by a 1st elution in a small volume of 10 µL. The standard bead-eluate separation method in the “Standard” VERSANT® SP protocol, where the eluate is picked up with a pipette tip after bead magnetization, is not suitable for such low volume elutions. Some of the eluate is left behind with the beads in the tube, thus resulting in some eluate volume loss. Hence, we developed the “air-jump” bead-eluate separation method (explained under SHIFT-SP in methods, Fig. 4) where the eluate is left behind in the tip. We observed that when “air-jump” was used to separate beads from eluate, 8.7 µl (SD = 0.18 µl) of eluate is obtained, whereas when the eluate was picked up from the elution tube with a pipette after bead magnetization (as used in VERSANT® SP protocol), only 3.8 µl (SD = 0.24 µl) of eluate was collected (Figure S8). The “air-jump” method of eluate separation minimizes eluate loss and leaves the beads in ‘near dry’ condition.
Fig. 4.
Flow chart illustrating “air-jump” based bead separation during elution in SHIFT-SP. Step 6 shows “air-jump” method implemented to minimize eluate volume loss and the additional Step 8 refers to elution wash, which is done to recover DNA trapped within beads, especially for higher starting DNA amounts. *The bead separation after the Wash 3 is also done using “air-jump”.
Effect of elution wash on the total yield of DNA
Some of the DNA is always trapped between beads after eluate separation, and this is considerable when the input DNA amount is high. We attempted to recover this trapped DNA with a simple bead washing step with EB, i.e., an elution wash. The first eluate was collected by the air-jump method (Fig. 4). For the elution wash, 10 µL of EB was added to the almost dry beads after air-jump, mixed, and the eluate was again separated by air-jump. With the introduction of elution wash, we were able to further improve DNA yield. This additional elution wash significantly improved the yield for 1000 ng input DNA (p-value < 0.05, Table 1, S3), but not for 100 ng input DNA (p-value is ns., Table 1 and S3). With the introduction of elution wash, total DNA yield increased from ~ 80 – 90% to 90 – 100% (for 1000 ng). This wash was not critical for lower input DNA quantities (100 ng), as the first elution alone could recover nearly 100% of the input DNA (Table 1 and S3). We observe that when the input DNA amount is 1000 ng, the elution wash step improves recovery taking it to 90 – 95%. The input DNA amounts in these experiments were ultrasound lysed M. smegmatis DNA.
Table 1.
Performance of “Standard” VERSANT® and SHIFT-SP protocols.
| Starting DNA amount | Method n = 5 for VERSANT; n = 10 for SHIFT-SP |
% DNA bound | % DNA eluted (1st elution) | % DNA eluted (Elution wash) | % Total yield |
|---|---|---|---|---|---|
| 100 ng | VERSANT® | 99.8% (0.3%) | 42.8% (5.2%) | - | 42.8 (5.2) |
| SHIFT-SP | 96.7% (1.5%) | 104.4% (14.1%) | 7.5% (4.2%) | 108.6 (12.8) | |
| 1000 ng | VERSANT® | 96.3% (1.1%) | 51.8% (3.3%) | - | 51.83 (3.3) |
| SHIFT-SP | 98.8% (0.4%) | 87.5% (34.3%) | 14.1% (5.3%) | 112.06 (16.49) |
SHIFT-SP recovers nearly all the starting DNA (100 ng or 1000 ng), while the VERSANT® method recovers only half as much. Elution wash significantly improves DNA yield when input DNA is 1000 ng. p-value = 0.042 (between 1st elution and total yield). p-value is not significant between yield in 1st elution and total yield after elution wash for 100 ng input DNA. The error values in brackets are SD. The % DNA eluted was calculated from a ratio of DNA amount eluted and input DNA, quantified on qPCR. % total yield is calculated as ratio of total DNA eluated (1st elution + Elution wash) to input DNA quantity, quantified on qPCR.
Optimizing SHIFT-SP for RNA extraction
Binding temperature for RNA
Our RNA extraction optimizations were done using non-infectious HIV particles in plasma background. “Standard” VERSANT® uses a lysis/binding temperature of 62 ºC for 15 min with shaking. We maintained 62 ºC as the binding temp for SHIFT-SP for DNA. To check if this condition is sufficient to lyse HIV particles in plasma background, we compared the lysis/binding efficiency at 62 ºC (standard-15 min and 1 min) and 74 ºC (1 min), all with orbital shaking. We observed that 74 ºC with 1 min binding time performed better than 62 ºC (with both 1- and 15-min binding time). We used 74 ºC as the binding temperature for all RNA experiments (Figure S2).
“Standard” VERSANT® vs. SHIFT-SP
We tested RNA elution efficiency on plasma samples spiked with 50 µL of non-infectious HIV-1 virus particles in plasma from 8E5/LAV (Lymphoadenopathy associated virus) which is equivalent to 1 × 105 virus particles23. These samples were lysed and bound to beads by either the tip method (SHIFT-SP, 10 µL beads, 74 ⁰C for 1 min) or the “Standard” VERSANT® SP method (10 µL magnetic silica beads, 62 ⁰C, 15 min at 1100 rpm). Two elution conditions were tested in the SHIFT-SP method (3 min, pH 9.3 at 82 ⁰C (condition 10) and at 74 °C (condition 5), both pH 9.3 conditions were chosen to keep the same EB as DNA (Table S4). Both elution methods showed similar RT-PCR (reverse transcription PCR) Cq values at 26.1 (SD = 0.5) and 26.0 (SD = 0.4) respectively. Elution using the VERSANT® SP method (10 min, pH 8.0, 74 ⁰C, 1100 rpm) yielded a poorer Cq value of 27.4 (SD = 0.5), thus demonstrating the superiority of the SHIFT-SP as shown in the supplementary Table S2.
RNA loss during SHIFT-SP
We tested RNA loss during the extraction process. For this experiment we compared Cq values of purified RNA when directly used in RT-PCR (Cq = 27.1 (SD = 0.1), n = 3), or when spiked in plasma and repurified with SHIFT-SP, and subjected to RT-PCR (Cq = 27.0 (SD = 0.6), n = 3). These methods yielded near identical Cq values, indicating minimum RNA loss during this extraction process.
Comparison of SHIFT-SP with the “Standard” VERSANT® sample preparation method and other commercially available nucleic acid extraction kits
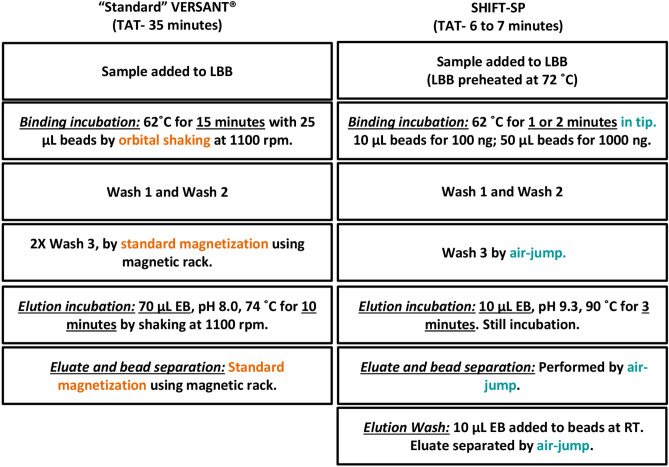
Based on our observations, we consolidated all favorable binding and elution factors and incorporated it in the “Standard” VERSANT® sample preparation protocol. We call this modified method SHIFT-SP (Fig. 5). We compared the DNA yield of SHIFT-SP and “Standard” VERSANT® sample preparation protocols for both 100 ng and 1000 ng using chemically lysed Escherichia coli DNA. The SHIFT-SP protocol resulted in nearly 100% yield compared to the “Standard” VERSANT® sample preparation protocol that resulted in a ~ 40 – 50% yield (Table 1). SHIFT-SP protocol recovers nearly 100% of the 100 ng spike-in or input DNA with the 1st elution itself. Whereas for 1000 ng, SHIFT-SP protocol with its 1st elution followed by an elution wash resulted in nearly 100% yield. Although both methods resulted in almost all the DNA binding to beads, only SHIFT-SP recovered nearly all the DNA bound to beads.
Fig. 5.
Comparison of “Standard” VERSANT® sample preparation method and SHIFT-SP method. The schematic diagram represents the protocols for NA extraction by “Standard” VERSANT® and SHIFT-SP methods. The methods differ in incubation parameters (highlighted above), method of bead mixing and bead separation. In the “Standard” VERSANT® method, NA binding to silica beads is done by orbital shaking at 1100 rpm, whereas in the SHIFT-SP protocol binding is done in a tip by pipetting the mix up and down. Similary the elution or eluate and bead separation step in the “Standard” VERSANT® is done by standard magnetization using a magnetic rack and bead separation in the SHIFT-SP is done through a process called “air-jump”. Additionally, elution buffer pH has been optimized for SHIFT-SP method to be 9.3. Some steps like wash seperations done by magnetic rack is maintained the same across both the methods.
We compared SHIFT-SP to other commercial extraction kits, a bead based one (MagMAX™) and a column based one (QIAamp®). The SHIFT-SP and MagMAX™ kits resulted in recovery of nearly all the input DNA, whereas the QIAamp® kit yielded about 60% of the input DNA (Table 2). The SHIFT-SP was the shortest method while also yielding a roughly tenfold more concentrated nucleic acid eluate which is enabled by a low elution volume. We have compared the “Standard” VERSANT® sample preparation and “SHIFT-SP” methods in Fig. 5.
Table 2.
Comparison of SHIFT-SP protocol with MagMAX™ DNA Multi-Sample Kit and QIAamp® DNA Blood Mini Kit.
| NA extraction method | Average total yield in ng | Method TAT& | Eluate volume | Concentration of NA in the final eluate |
|---|---|---|---|---|
| Experiment 1: SHIFT-SP (n = 4) | 112.8 (24.5) | ~ 6 – 7 min | 20 µL* | 5.64 ng/µL |
| Experiment 2: MagMAX™ DNA Multi-Sample Kit (n = 3) | 105.7 (5.0) | ~ 36 min | 200 µL | 0.53 ng/µL |
| Experiment 3: QIAamp® DNA Blood Mini Kit (n = 3) | 57.8 (6.2) | ~ 14 – 15 min | 200 µL | 0.29 ng/µL |
The yield from SHIFT-SP is comparable with MagMAX™ DNA multi-sample kit protocol, where nearly all the spiked DNA (100 ng) is retrieved after extraction. SHIFT-SP is the shortest of the three and yields a concentrated sample. Error values in brackets are SD. *10 µL eluate is mixed with 10 µL elution wash. &Time from lysed sample to NA. p-value 0.31 is not significant between average total yields of SHIFT-SP and MagMAX™ DNA Multi-Sample kit. p-value 0.01 is significant between average total yields of SHIFT-SP and QIAamp® DNA Blood mini Kit.
Performance of SHIFT-SP with DNA of varying lengths
All the early development work done so far for SHIFT-SP (for DNA) has been done with purified, ultrasound lysed M. smegmatis DNA, where we show that SHIFT-SP results in better yield compared to VERSANT® sample preparation method. Here, we evaluate the performance of SHIFT-SP on mixtures of chemically lysed E. coli (~ 50 Kbp) and M. smegmatis DNA (500 to 15,000 bp with peak at 1544 bp), both purified with VERSANT® SP (Figure S4). These DNA fragments of two varying lengths were mixed in different ratios and used as a starting sample for SHIFT-SP. The DNA amounts correlating to E. coli and M. smegmatis were quantified from the eluate using qPCR. We observe that SHIFT-SP extracts both the long and short DNA fragments efficiently and when there are differently sized DNA fragments in the starting sample, the binding and elution of one fragment length is not affected by the presence of the other. The measured outputs of the long and short DNA fragments in the eluate correlate well with the spiked input amounts of these DNA fragments (Figure S5). Thus, SHIFT-SP is compatible with DNA fragments across a wide size range.
Performance of SHIFT-SP with enriched whole blood
We evaluated if SHIFT-SP can extract DNA from an enriched 8 mL whole blood sample spiked with 5 CFU/mL of E. coli and Candida albicans. We used both whole genome and targeted sequencing to detect the amplified microbe DNA from the SHIFT-SP eluate. This was done to assess if SHIFT-SP protocol can recover extremely low levels of targets in a complex sample type like whole blood. Since whole blood has high human genomic DNA background and other blood-based inhibitors that can hinder the downstream sequencing, we used an enrichment method (MolYsis Basic) to enrich microbes and reduce this human genomic DNA background. SHIFT-SP eluate enables detection of low concentrations of E. coli and C. albicans, when used in a sequencing workflow, using a nanopore sequencer with both whole genome and targeted sequencing techniques (Fig. 6).
Fig. 6.
SHIFT-SP used to extract DNA from enriched whole blood samples. (a) Schematic representation of the workflow details. Enriched whole blood is used as an input to SHIFT-SP protocol. DNA extracted from SHIFT-SP is amplified and sequenced by whole genome sequencing and targeted metagenomic sequencing workflows (b) Whole genome sequencing results correlated to E. coli and C. albicans present at 5 CFU/mL (c) Targeted genomic sequencing results shown for both 16s rDNA and ITS regions correlating with E. coli and C. albicans respectively. SHIFT-SP method enabled the extraction and detection of very low microbial DNA quantities from enriched blood samples.
Discussion
Low starting sample volumes and/or low concentration of targets can reduce assay sensitivity and limit the number of molecular diagnostic tests that can be performed on a given sample. We demonstrate a method that can be used to extract nearly all the nucleic acid from a sample, thus enabling better testing efficiency. We also show that RNA can be extracted at a high efficiency with this method. We have tested various modifications of the “Standard” VERSANT® NA extraction method to achieve a highly efficient extraction method that we call SHIFT-SP.
The differentiating step that makes SHIFT-SP faster and more efficient is the binding of DNA to silica beads by pipette mixing or “tip-based” binding. As seen in Figure S1, “tip-based” method enables maximum NA binding in the time frame tested in the experiment (2 min). This is helpful in two ways; the rapid binding shortens the NA extraction time, and “tip-based” binding also results in better elution (Fig. 3d).
As concluded in Katevatis et al., the silica bead-DNA interaction in a low pH and high chaotrope concentration environment is primarily mediated by hydrophobic and hydrogen bonding interactions3. It is likely that a prolonged interaction between beads and NA under ideal binding conditions further strengthens these interactions by optimal alignment of NA on the silica bead surface, thus making it difficult for NA to separate from the beads during elution.
A contrary hypothesis has been made in Vandeventer et al., where surface adsorption of DNA to a quartz surface (silicon dioxide) was measured in the presence of 6 M sodium perchlorate (a chaotrope)8. Under these strong binding conditions (low pH, high ionic strength), the authors propose a biphasic DNA adsorption process, where the adsorbed DNA layer is initially more rigid but later converts to a more viscoelastic form. They also go on to conclude that the more rigidly adsorbed DNA layer is harder to elute compared to the viscoelastic DNA layer. Since the viscoelastic layer forms only later in the DNA binding process, it would stand to reason that elution would be easier for longer rather than shorter DNA binding durations. The experimental conditions including the binding surface and the chaotrope used in Vandeventer et al., were different from those tested in this work, so the data may not be directly comparable.
The superior DNA binding to silica beads in the “tip-based” method can also be attributed to the fast and comprehensive sampling of the entire DNA/LBB mix by silica beads during the pipetting process, thus providing more “opportunities” for DNA to bind the beads and results in nearly 100% DNA binding in under 2 min. On the other hand, in an orbital mode of mixing, there is limited relative motion between beads and the LBB, likely compromising the binding efficiency.
The second key feature of the SHIFT-SP method is the “air-jump”. This method efficiently removes all buffer remnants from the beads in a separation step. We contend that air-jump improves both the nucleic acid quality by minimizing the wash buffer carry-over to the elution step and the nucleic acid yield by recovering more eluate volume from the beads after the elution step, as discussed in “Low volume elution and bead separation by air-jump” section in Results.
Finally, we compared the performance of SHIFT-SP with other methods such as QIAamp® (silica column) and MagMAX™ (beads with silica-like surface). Both methods were longer and the QIAmp® method had reduced DNA yield. SHIFT-SP also showed superior DNA recovery when compared to what is reported in Katevatis et al., 40.3% and 53.5% recovery from 250 ng/mL and 2.5 µg/mL input DNA respectively compared to near 100% yields with SHIFT-SP at 100 ng/mL and 1 µg/mL of DNA in the LBB3.
Apart from being a high yield method, SHIFT-SP is also extremely rapid, at under 6–7 min. In comparison the “Standard” VERSANT® method takes 25 min (manual operation), and results in 40–50% yield, while the QIAamp® DNA Blood Mini Kit and the MagMAX™ DNA Multi-Sample Kit methods take 25 and 40 min, respectively. In Katevatis et al. the binding incubation alone is lasts one hour3.
This work is a feasibility study to identify conditions for rapid NA extraction and small volume elution. The manual method uses repeated pipette mixing and can only be scaled once automation is established. We have not investigated automation feasibility of SHIFT-SP, although we expect the method to be automation amenable. The ‘air-jump’ method is new and needs to be investigated for automation robustness. Upon testing ‘air-jump’ on an engineering prototype, we observed a smooth movement of beads from the tip to the tube placed below upon magnetization. This gives us confidence about the automatability of the method. The other important step is pipette mixing. Pipette mixing is automated in many electronic pipettes, by aspirating a lower volume (e.g. aspirate only 700 µL when the sample is 1 mL) and an incomplete dispense to prevent bubbles. We think a similar approach can be employed in automating SHIFT-SP as well. The eventual goal of this work, i.e., molecular diagnostics with rapid TAT, will be possible once the method is automated and is able to achieve scale.
SHIFT-SP also uses smaller quantities of lysis buffer (634 µL vs 825 µL in standard VERSANT) and beads (10 µL vs 25 µL in standard VERSANT for 100 ng input DNA). This can have substantial reagent savings over many samples. The NA is eluted in ~ 10 µL, thus enabling low volume PCRs and saving on enzymes, reagents, and probes. Generally, a PCR volume of 20 µL or more are used in different assays. A small PCR volume also engenders the possibility of using PCR chips which offer advantages such as short assay time, low reagent consumption and rapid heating/cooling rates, as well as the potential to integrate multiple processing modules to reduce size and power consumption18.
SHIFT-SP is primarily a nucleic acid extraction process, with optimized binding and elution parameters that result in nearly complete recovery of nucleic acids. We have not tested the lysis capability of this method. The short binding step may not be enough to completely lyse different cells and microorganisms. An earlier lysis step (e.g. using ultrasound for M. smegmatis) or a warmer binding step within SHIFT-SP (e.g. using 74 ⁰C instead of 62 ⁰C for non-infectious HIV in plasma) may be needed for effective lysis of different samples. We have demonstrated our experiments using free NA suspended in buffer or non-infectious HIV in plasma or low concentration bacteria in enriched whole blood. Other difficult sample types such as whole blood and sputum will need further optimization and additional sample pre-processing steps before following up with SHIFT-SP.
Methods
“Standard” VERSANT® sample preparation method
“Standard” VERSANT® sample preparation (SP) 1.0 and 1.2 reagents kit (Siemens Healthineers, SMN: 10286026, 10629800), allow extraction of nucleic acids from routinely used clinical specimens including but not limited to whole blood, plasma, urine, respiratory samples like nasal, nasopharyngeal swabs, bronchoalveolar lavage (BAL) etc. These kits are amenable for use in automated protocols for high throughput requirements and as manual protocols for low throughput needs. SP1.0 kit (commercially sold by Siemens Healthineers) can extract ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) from most sample types, except blood; SP1.2 (currently discontinued) is tailor-made to extract DNA from the above-mentioned sample types and whole blood. Both kits include proprietary silica coated magnetic particles and the associated binding, wash and elution buffers. The buffer compositions of the individual components cannot be shared as it is proprietary. These kits work on the principle of Boom chemistry. In the “Standard” VERSANT® Sample preparation protocol, 634 µL of lysis/binding buffer (LBB – 5 M Guanidine Thiocyanate along with detergents and buffering agents), 25 µL of silica beads and 20 µL of proteinase K is added to 336 µL sample. The mix is incubated at 62 °C, by “orbital shaking”, on Thermomixer® comfort (Thermo Fisher, catalog no: 5382000015, Eppendorf), at 1100 rpm for 15 min. After the lysis/binding step, the mix is magnetized in a magnetic rack- DynaMag™−2 Magnet (Thermo Fisher, catalogue number: 1321D), and the supernatant which has unbound cell lysate is discarded. The beads are then washed with 850 µL of wash buffer1 (3 M Guanidine Thiocyanate, Ethanol, and buffering agents as pH 5.0), 450 µL each of wash buffer 2 (Ethanol, with buffering agents at pH 4.8) and twice with wash buffer 3 (0.1% Triton X-100 buffered to pH 4.0). 70 µL of low salt EB (Tris pH 8.0) is added to the washed beads and incubated at 74 °C in Thermomixer® comfort at 1100 rpm for 10 min. The heated mixture is then magnetized, and the eluate recovered is taken forward for quantitative PCR. Volumes of the “Standard” VERSANT® SP protocol (both sample and reagents) have been scaled down proportionately to a final binding mixture volume of 1 mL.
Quantitative PCR (qPCR)
All the qPCRs were done using Applied Biosystems™ QuantStudio™ 5 Real-time PCR system. The Applied Biosystems Taqman™ gene expression master mix (catalogue number: 4369016) was used in qPCRs for detection and quantification for E. coli, M. smegmatis and HIV targets. qPCR, for E. coli and M. smegmatis was done in a total reaction volume of 10 µL containing 4 µL of template, 5 µL of 2X Taqman master mix, 920 nM of the forward and reverse primer and 250 nM of the Taqman probe. The reaction condition was 95 °C for 10 min of initial denaturation followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s for both targets. The sequence of primers and probes used for E. coli PCRs are 16S rDNA forward primer: AGACTCCTACGGGAGGCAG and 16S rDNA reverse primer: TTACCGCGGCTGCTGGCAC and 16S rDNA TaqMan probe: 5’-VIC-TGACGTTCCCC GCAGAAGAAGCA- BHQ-3’. The sequence of primers and probes used for M. smegmatis are M. smegmatis forward: GGGGTACTCGAGTGGCGAAC and M. smegmatis Reverse: GGCCGGCTACCCGTCGTC and M. smegmatis Taqman probe: 5’-FAM-CACCCTGCTGGTCGCATG- BHQ-1. The E. coli and M. smegmatis amplicons were 204 bp and 205 bp respectively. All the Cq values listed in this manuscript refer to quantification cycle or a threshold cycle threshold and refers to the number of times a machine must copy a piece of genetic material before it can be detected in a PCR test. In general, the lower the Cq value, the higher the amplified NA load that is found in the sample, while the higher the Cq value, the lower the amplified NA load.
DNA quantification
Binding efficiency estimation
We used qPCR for estimating binding and elution efficiencies. This method is explained in Fig. 1a. For example, to quantify losses during the NA binding step, we aliquot 10 µL of mixture from before binding step i.e., the sample mixed with lysis/binding buffer (LBB). We call this the “before binding” (BB) aliquot. After bead incubation, the sample/LBB mixture is magnetized and 10 µl of supernatant is collected as the “after binding” (AB) aliquot. These “BB” and “AB” aliquots are diluted 1:500 in 1X TE (Tris–EDTA, pH 8.0) buffer to decrease the effect of PCR inhibitors such as high concentration guanidine salt and detergent present in LBB. The amount of DNA present in “BB” and “AB” aliquots are measured using qPCR and compared against a standard curve. “BB” and “AB” DNA amounts are used to calculate the binding efficiency (Fig. 1). The DNA standards used to obtain the qPCR are made in the same background as “BB” and “AB” aliquots and diluted 1:500 in 1X TE (Tris–EDTA pH 8.0). A typical quantification of % DNA bound is described in supplementary Figure S7. Every time ‘BB’ and “AB” DNA is quantified, a standard curve in 1:500 diluted LBB is included in the experiment.
Elution quantification
The standard curve generated using standards made in 1X TE is used to estimate eluted DNA amount. % DNA eluted is calculated as amount of DNA in the eluate divided by total input DNA measured in the “BB” aliquot expressed as a percentage. Total yield is calculated as total amount of DNA present in the total eluate volume (1st eluate + EB wash) divided by total input DNA measured in the “BB” aliquot expressed as a percentage.
DNA preparation for method optimization
We used 100 ng and 1000 ng of purified DNA from ultrasound lysed M. smegmatis (ATCC catalogue number: 700084) or chemically lysed E. coli (ATCC catalogue number: 11775) DNA resuspended in PBS, as the starting sample in the sample preparation optimization experiments. For ultrasound lysis of the M. smegmatis, we used UP200St with Vial Tweeter (Hielscher ultrasonics) and a customized sonotrode to fit in standard 2.0 mL Eppendorf tubes. We used this ultrasound setting on the Vial Tweeter—40% amplitude, and an energy target of 500 W-sec with the period clock—1 s ON, 1 s OFF—to lyse 500 µL of overnight grown M. smegmatis culture. The lysed sample was taken through the “Standard” VERSANT® SP 1.2 sample preparation method as described above. The E. coli cells were directly taken through the VERSANT® SP 1.2 sample preparation method. The lysis buffer in the kit was sufficient for lysing E. coli. Subsequently, the purified DNA was treated with RNAse (#EN0531 and 30 min at 37 ºC incubation). This RNAse treated, purified DNA was used in all the optimization experiments.
The choice of 100 ng and 1000 ng as the input DNA quantities for these experiments was chosen by estimating the total amount of NA present in different sample types for a few routinely carried out molecular diagnostic tests. For example, the total NA sample can vary between 8 ng in a 500 µL plasma sample for HIV detection to 8 µg in a 250 µL sputum sample for Mycobacterium tuberculosis detection to 18 µg in a 200 µL blood sample for Candida tropicalis detection. We chose two quantities tenfold apart (100 ng and 1000 ng) within this wide range to carry out the optimization experiments. We also used purified DNA from ultrasound lysed M. smegmatis mixed with purified DNA from chemically lysed E. coli in varying ratios in an experiment to evaluate the performance of the SHIFT-SP protocol with DNA fragments of varying lengths.
Mode of bead mixing during binding experiments
For this experiment, we used 100 ng and 1000 ng ultrasound lysed M. smegmatis DNA spiked in LBB at pH 4.1. For orbital shaking, we add 10 µL of beads to the DNA/LBB mix and shake it at 1100 rpm, at 62 °C, on a Thermomixer comfort®, for binding to occur. In the non-orbital “tip-based” binding the LBB is preheated at 72 °C before adding the DNA. For bead binding we add 10 µL beads and perform pipette mixing at 62 ⁰C. Except changing the mode of bead movement during mixing, all other steps followed are the same as “Standard” VERSANT® sample preparation method outlined above. For both starting amounts of DNA and the binding methods investigated, 10 µL of bead suspension was used.
SHIFT-SP method
Binding and elution parameters are modified based on the various experiments listed in this manuscript. 336 µL of starting sample was added to preheated cocktail of 634 µL of LBB and 20 µL of proteinase K in a 1.5 mL Eppendorf tube. The cocktail was preheated and maintained at 72 °C until sample and bead addition. We added magnetic beads (10 µL for 100 ng and 50 µL for 1000 ng of DNA) to this mixture and binding was performed by aspirating the mixture in a 1000 µL pipette tip (with pipette set at 900 µL) and dispensing it back (incomplete dispense to prevent bubbles), repeatedly for 1 (for 100 ng) or 2 min (for 1000 ng) while the binding mixture tube is placed at 62 °C in a thermomixer comfort. We estimate roughly 60 to 80 cycles of aspiration-dispense in one minute. During this binding step, we ensured that the pipetting did not result in any frothing of the sample. At the end of 2 min of binding, the mixture was collected in a 1.5 mL Eppendorf tube. The tube was magnetized in a magnetic rack (DynaMag™-2 Magnet, cat no: 12321 D, ThermoFisher Scientific) and the supernatant was discarded. The beads were then washed with 450 µL of VERSANT® wash buffers 1, 2, and 3. After addition of each wash buffer, the beads are magnetized in a magnetic rack, supernatant is removed and discarded before washing the beads with the next wash buffer. After addition of wash buffer 3, the entire mix is aspirated in a 1000 µL pipette tip for “air-jump” based bead separation from wash buffer 3. For “air-jump”, the pipette is held on a stand with a clamp for the next steps. A small magnet held firmly on the wall of the tip, is used to gather all the beads towards the end of the pipette tip. A clean 1.5 mL Eppendorf tube is placed immediately underneath the tip; the tip still containing wash buffer 3 with clumped beads at the bottom. A magnet is now placed under the Eppendorf tube. The magnet pulls the beads causing the beads to jump – thus the name “air-jump” – from the 1000 µL tip into the Eppendorf tube, leaving the wash 3 buffer behind in the tip which is then discarded. The “air-jump” method is used to separate beads with minimal liquid carry over. 10 µL of preheated high pH EB (10 mM Tris HCL; 0.1 mM EDTA pH of 9.3, preheated and maintained at 90 °C) is then added to the beads. The tube is incubated at 90 °C for 3 min. The heated mix is then aspirated in a 20 µL pipette tip (when the bead volume was 50 µL, we used a 200 µL tip) and the beads are separated by “air-jump”. The eluate which is retained in the 20 µL tip is now collected separately into a clean tube as 1st eluate. The collected magnetic beads are washed with 10 µL of preheated high pH EB at room temperature. Both eluate and elution wash (total of 20 µL) are pooled to form the final DNA eluate from the SHIFT-SP method.
Consumables used for SHIFT-SP include loRetention Dualfilter 1000 µL PCR clean/Sterile tips (Eppendorf, Cat. No: 022493008) for binding, LoRetention Dualfilter 20 µL PCR clean/ Sterile tips (Eppendorf, Cat. No: 022493002) for “air-jump” elution. We used DNA LoBind tube 1.5 mL PCR clean Safe-lock tubes (Eppendorf, Cat. No: 022431021) for lysis, wash and elution reactions.
DNA extraction kits for comparison
We compared the efficiency of the SHIFT-SP protocol with a commercially available bead based nucleic acid extraction kit, MagMAX™ DNA Multi-Sample Kit (Catalogue number: 4413020, Thermo Fisher Scientific) and a column based nucleic acid extraction kit, QIAamp® DNA Blood Mini Kit (Catalogue number: 51104, QIAGEN). The protocols were followed as per instructions for use manual provided by the manufacturer. The yield, eluate volume and the duration of extraction process were compared.
Low volume elution with air-jump
1000 ng of chemically lysed E. coli DNA was spiked in PBS and then added in LBB and the rest of the SHIFT-SP protocol was followed until wash step. After bead washes, 10 µL of EB at pH 9.3 was added to the beads and incubated at 90 °C for 3 min. Bead-eluate separation was done in five sample replicates using “air-jump” (as described in the SHIFT-SP method) whereas for the rest of the five sample replicates, eluate was separated from beads by magnetization and then the eluate was picked up with a 20 µL pipette tip. All the eluates were collected in empty pre-weighed 1.5 mL Eppendorf tubes (w1). These Eppendorf tubes were weighed again after eluate collection (w2). Each tube was weighed 5 times both empty and after eluate addition. The average of the 5 measures was used to calculate w1 or w2. The difference between the weights (w2-w1) was used to calculate the eluate volume. For example, if w1 = 1008.0 mg and w2 = 1016.6 mg, the difference is 8.6 mg, the eluate volume was 8.6 µL.
Optimizing SHIFT-SP for RNA extraction
Binding temperature for RNA
We tested the effect of bead binding temperature on RNA extraction efficiency in defibrinated human plasma (SeraCon™ II, material number 1800–0013) spiked with non-infectious HIV-1 virus particles from 8E5/LAV cell line23 using VERSANT® SP1.0. The binding step was done at 74 °C or 62 °C for 1 min and the standard 62 ºC for 15 min, all three incubations were done using “orbital shaking”. Elution was done using the standard VERSANT pH 8.0 elution buffer at 72 ⁰C for 3 min. The extracted RNA is amplified using VERSANT® HIV-1 RNA 1.0 Assay. Primer probe sequences are proprietary. The PCR conditions are the following: 7.5 µL of PP (primer – probe) Mix is added to 2.5 µL of enzyme mix to which 27.5 µL template was added. The cDNA synthesis was done at 50 ºC for 40 min followed by a denaturation step at 95 °C for 15 min. This is followed by a PCR of 40 cycles at a denaturation of 95 °C for 15 s followed by annealing at 57 °C for 1 min followed by an extension at 72 °C for 30 s. We used the same qPCR instrument detailed above to carry out the reverse transcription PCRs.
“Standard” VERSANT® sample preparation vs SHIFT-SP method
RNA extraction is performed like DNA extraction as described above under “SHIFT-SP method”, except binding condition was 74 ºC, 1 min, elution condition was 82 ºC, 3 min and eluate is immediately added to a neutralization buffer. As RNA extraction was tested in a plasma background sample, we also added proteinase K (400 µg from the proteinase K provided in the VERSANT® kit) during binding step. We tested RNA extraction efficiency in 316 µL defibrinated human plasma (SeraCon™ II, material number 1800–0013) spiked with 50 µL (93,000 copies) non-infectious HIV-1 virus particles from 8E5/LAV cell line23. SHIFT-SP samples had the following extraction conditions, binding at 74 °C for 1 min, pH 4.1, elution, 3 min, pH 9.3, at 82 °C or 74 °C. The extracted RNA (in 10 µL) is then immediately neutralized by the addition of 23.4 µL of neutralization buffer (30 mM Tris pH 7.5). The extracted RNA is amplified using VERSANT HIV-1 RNA 1.0 Assay.
Some aliquots of the same spiked plasma are extracted with VERSANT® SP1.0 for comparison.
RNA loss estimation with SHIFT-SP extraction
To establish the RNA extraction efficiency with SHIFT-SP we ran the following experiment. HIV RNA was extracted from the calibrator A (1.856 × 106 copies/ml) provided in the VERSANT® HIV-1 RNA 1.0 Assay (SMN number 10375764). An aliquot of 20 µL of this extracted RNA (equivalent to 1.5 × 104 HIV particles) was spiked into 346 µL plasma and extracted using SHIFT-SP in 10 µL EB (binding 1 min, 74 ⁰C on tip, pH 4.1, elution, 3 min, 82 ⁰C, pH 9.3) followed by addition of neutralization buffer to yield a final eluate volume of 33.4 µL. Next, we took another 20 µL aliquot of the initially extracted RNA and added 12.4 µL of EB to make the total volume 32.3 µL to achieve a volume similar as the SHIFT-SP eluate. Next qPCRs were run using 30 µL of the two kinds of the samples as templates to compare any loss in the extraction process using master mixes in the VERSANT® HIV-1 RNA 1.0 Assay by Cq value comparison.
SHIFT-SP method with whole blood samples
We spiked with 5.0 CFU/mL of E. coli (ATCC No: 25922) and C. albicans (ATCC No: SC5314) into 8.0 mL whole blood samples collected from healthy volunteers. The study was approved by the Pranav Diabetes Center, Bangalore, India, ethics committee (ECR ECR/1217 /lnst/KA/2019/RR-22 and in accordance with the provisions of the Declaration of Helsinki. The blood samples were collected from healthy volunteers with informed consent. E. coli and C. albicans were cultured in LB media and YM media respectively as per ATCC guidelines. E. coli was cultured overnight at 37 ºC in LB broth. C. albicans was cultured overnight at 25 ºC from YM agar plate stocks. All overnight cultures were inoculated at 1:5 dilutions in growth media and cultured for another 2–3 h before the start of the experiment. Bacterial cell numbers were estimated using absorption at 600 nm and plating. Fungal cell numbers were estimated using a hemocytometer. Thereafter, ten-fold serial dilutions were made in blood and filter sterilized 0.85% saline to achieve final dilutions of 40 CFU in 8 mL of blood (or 5 CFU/mL final concentration).
These blood samples were enriched using MolYsis Basic™ 10 (D-301–050) as per the manufacturer recommended protocol resulting in a volume of 100 µL. To this, we added 266 µL of pretreatment buffer (a component of VERSANT® SP1.2 Sample preparation kit). Next, DNA was extracted this sample using SHIFT-SP. We changed the bead quantity to 30 µL (we chose 30 µL bead amount specifically here as it is an enriched blood sample, and the DNA quantity would be expected to be higher than 100 ng) and increased the proteinase-K to 600 µg. These are the only changes made to the earlier described SHIFT-SP protocol. The 10 µL eluate was used as template in a 50 µL whole genome amplification following the manufacturers protocol (Truprime WGA kit from 4basebio- SKU: 370,025). From this reaction, we used 25 µL of sheared WGA product (using Covaris g tubes- 520,079; We used 30 s at 11,000 rpm for our desired fragment size and mass of DNA (< 4 µg)) as a template to the library prep kit (Oxford Nanopore technology (ONT): SQK-LSK109) and ran the library on MinION R9.4.1 flow cell FLO-MIN106 (previously sold by ONT. This model is no longer available). We used the rest of the sheared 25 µL as an input for targeted PCR amplification (reaction volume –50 µL using hi-fidelity Phusion Taq Polymerase Kit) in which we used primers to amplify 16S rDNA of bacteria and ITS regions of Fungi (ITSF: TCCGTAGGTGAACCTGCGG bp; ITSR: TCCTCCGCTTATTGATATGC; 16S rDNA F: AGAGTTTGATCMTGGCTCAG; 16S rDNA R: GGTTACCTTGTTACGACTT). The 25 µL targeted PCR product is then used as a template to the library prep kit (Oxford Nanopore technology (ONT): SQK-LSK109) and run on MinION R9.4.1 flow cell. We used MinKNOW Version 18.0 API and software for base calling and analysis.
p-value calculations and errors
All the statistical significance calculations have been done on the Georgiev G.Z., "P-value Calculator", available at: https://www.gigacalculator.com/calculators/p-value-significance-calculator.php. We have used a statistical significance threshold of 0.05 for all our assessments made in the manuscript and supplementary figures. We used a one-tailed T-test for p-value calculation. A p-value > 0.05 was considered non-significant (ns), p-value ≤ 0.05 is significant. p-value ≤ 0.05 and > 0.01 is represented as (*), ≤ to 0.01 and > 0.001 is represented as (**), ≤ 0.001 and > 0.0001 is represented as (***), and ≤ 0.0001 is represented as (****) in all the figures mentioned in main manuscript and supplementary. All errors presented are standard deviations (SD).
Supplementary Information
Supplementary Information 1 (Supplementary tables and figures).
Supplementary Information 2 (RNA stability studies).
Acknowledgements
We would like to thank Paul Patt for constructive discussions during the performance of the experiments. We would like to thank Manimala Sen and Sharath Poojary for running the enrichment, whole genome amplification and sequencing reactions for microbe detection with SHIFT-SP.
Author contributions
NM, MRV, DKS, IC conducted experiments; NM, MRV, DKS analyzed data; NM, MRV, DKS planned the work. NM, MRV, DKS wrote the manuscript. DM was involved in data discussions.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
NM, MRV, DKS, IC are current employees of Siemens Healthcare Pvt. Ltd. DM is a former employee of Siemens Healthineers and has no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in Fig. 2, where the axis line was shifted up, the lighter histogram (“Binding by orbital shaking” was omitted and the p value lines above the histograms were shifted to the right. Additionally, in Fig. 3, the p value line was shifted to the right, the x axis line in Fig. 3b was shifted up and p value line above the histograms was shrunk, the x axis in Fig. 3c was shifted up and p value line above the histograms was shrunk and a data point in in graph in figure 3d was missing “%DNA bound” for “pipette mix 2 minutes”.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/20/2025
A Correction to this paper has been published: 10.1038/s41598-025-04564-6
Contributor Information
Manjula Ramya Vutukuru, Email: ramya.vm@siemens-healthineers.com.
Nivedita Mitra, Email: nivedita.mitra@siemens-healthineers.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-95226-0.
References
- 1.Ali, N., Rampazzo, R. C. P., Costa, A. D. T. & Krieger, M. A. Current Nucleic Acid Extraction Methods and Their Implications to Point-of-Care Diagnostics. Biomed. Res. Int.2017, 9306564. 10.1155/2017/9306564 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janku, F. et al. A novel method for liquid-phase extraction of cell-free DNA for detection of circulating tumor DNA. Sci. Rep.11, 19653. 10.1038/s41598-021-98815-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katevatis, C., Fan, A. & Klapperich, C. M. Low concentration DNA extraction and recovery using a silica solid phase. PLoS One12, e0176848. 10.1371/journal.pone.0176848 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boom, R. et al. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol.28, 495–503. 10.1128/jcm.28.3.495-503.1990 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budelier, K. & Schorr, J. Purification of DNA by anion-exchange chromatography. Curr. Protoc. Mol. Biol.10.1002/0471142727.mb0201bs42 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Jiang, C., Xu, S., Zhang, S. & Jia, L. Chitosan functionalized magnetic particle-assisted detection of genetically modified soybeans based on polymerase chain reaction and capillary electrophoresis. Anal. Biochem.420, 20–25. 10.1016/j.ab.2011.09.004 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Zhang, M. et al. Adsorption of DNA by using polydopamine modified magnetic nanoparticles based on solid-phase extraction. Anal. Biochem.579, 9–17. 10.1016/j.ab.2019.05.004 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Vandeventer, P. E. et al. Multiphasic DNA adsorption to silica surfaces under varying buffer, pH, and ionic strength conditions. J. Phys. Chem. B116, 5661–5670. 10.1021/jp3017776 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker, J. M. Isolation of nucleic acids. United States patent (2005).
- 10.Sur, K. et al. Immiscible phase nucleic acid purification eliminates PCR inhibitors with a single pass of paramagnetic particles through a hydrophobic liquid. J. Mol. Diagn.12, 620–628. 10.2353/jmoldx.2010.090190 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutiu, A. I. & Brandl, C. J. RNA isolation from yeast using silica matrices. J. Biomol. Tech.16, 316–317 (2005). [PMC free article] [PubMed] [Google Scholar]
- 12.Okabe, N., Fujita, E. & Tomita, K. I. The effect of guanidine hydrochloride on the conformation of bovine pancreatic DNAase I as measured with circular dichroism. Biochim. Biophys. Acta700, 165–170. 10.1016/0167-4838(82)90093-0 (1982). [DOI] [PubMed] [Google Scholar]
- 13.Honeywood, M. J. et al. Use of guanidine thiocyanate-based nucleic acid extraction buffers to inactivate poliovirus in potentially infectious materials. J. Virol. Methods297, 114262. 10.1016/j.jviromet.2021.114262 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale, A. D., Green, J. & Brown, D. W. Comparison of four RNA extraction methods for the detection of small round structured viruses in faecal specimens. J. Virol. Methods57, 195–201. 10.1016/0166-0934(95)01966-9 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Shieh, Y. S., Wait, D., Tai, L. & Sobsey, M. D. Methods to remove inhibitors in sewage and other fecal wastes for enterovirus detection by the polymerase chain reaction. J. Virol. Methods54, 51–66. 10.1016/0166-0934(95)00025-p (1995). [DOI] [PubMed] [Google Scholar]
- 16.Schrader, C., Schielke, A., Ellerbroek, L. & Johne, R. PCR inhibitors - occurrence, properties and removal. J. Appl. Microbiol.113, 1014–1026. 10.1111/j.1365-2672.2012.05384.x (2012). [DOI] [PubMed] [Google Scholar]
- 17.Psifidi, A. et al. Comparison of eleven methods for genomic DNA extraction suitable for large-scale whole-genome genotyping and long-term DNA banking using blood samples. PLoS One10, e0115960. 10.1371/journal.pone.0115960 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, C. & Xing, D. Miniaturized PCR chips for nucleic acid amplification and analysis: latest advances and future trends. Nucleic Acids Res.35, 4223–4237. 10.1093/nar/gkm389 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun, Y. et al. Digital PCR assay for the effective detection of COVID-19 patients with SARS-CoV-2 low viral load. J. Virol. Methods295, 114185. 10.1016/j.jviromet.2021.114185 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puhach, O., Meyer, B. & Eckerle, I. SARS-CoV-2 viral load and shedding kinetics. Nat. Rev. Microbiol.21, 147–161. 10.1038/s41579-022-00822-w (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha, M. et al. Emerging Technologies for Molecular Diagnosis of Sepsis. Clin. Microbiol. Rev.10.1128/CMR.00089-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polatoglou, E., Mayer, Z., Ungerer, V., Bronkhorst, A. J. & Holdenrieder, S. Isolation and Quantification of Plasma Cell-Free DNA Using Different Manual and Automated Methods. Diagnostics (Basel)10.3390/diagnostics12102550 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folks, T. M. et al. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J. Exp. Med.164, 280–290. 10.1084/jem.164.1.280 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information 1 (Supplementary tables and figures).
Supplementary Information 2 (RNA stability studies).
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.