Abstract
A group of 252 cattle without clinical signs of paratuberculosis (paraTB) in 10 herds infected with paraTB and a group of 117 cattle in 5 herds without paraTB were selected. Whole-blood samples were stimulated with bovine, avian, and johnin purified protein derivative (PPD) and examined for gamma interferon (IFN-γ) release. For diagnosis of paraTB, satisfactory estimated specificities (95 to 99%) could be obtained by johnin PPD stimulation irrespective of interpretation relative to bovine PPD or no-antigen stimulation alone, but numbers of test positives in the infected herds varied from 64 to 112 with different interpretation criteria. For a limited number of test-positive animals, no change in the test results could be observed with increasing antigen concentrations but IFN-γ responses were significantly reduced (P < 0.0001) and four out of seven reactors tested negative when stimulation was performed on day-old samples. Denmark is free of bovine tuberculosis, but cross-reactivity with paraTB could be documented for cattle more than 14 months old in paraTB-infected herds compared with those in non-paraTB-infected herds. In both paraTB-free and paraTB-infected herds, false positives were observed when the test was applied to calves less than 15 months of age. Until novel antigen formulations more specific for these diseases are available, interpretation of the IFN-γ test must be individually adjusted to fit specific needs and the context within which the test is applied and, for paraTB, the test seems most appropriate for use as a supportive tool for evaluation of disease-preventive measures in young stock.
Paratuberculosis (paraTB) or Johne's disease is a slowly progressive chronic granulomatous enteritis of ruminants caused by Mycobacterium avium subsp. paratuberculosis (39). In Denmark, paraTB is a serious economic and animal welfare problem with an estimated prevalence of up to 47% in the Danish milk-producing cattle population (26, 27). In contrast, the zoonotic bovine tuberculosis (TB), caused by M. bovis, was eradicated in the Danish cattle population half a century ago and control is maintained by meat inspection at slaughterhouses and skin testing of export animals as required.
M. avium subsp. paratuberculosis is closely related to the opportunistic pathogen M. avium subsp. avium and also has many antigens in common with M. bovis (16). Although M. avium subsp. paratuberculosis can be cultured from fecal samples, shedding is intermittent until the late stages of infection and growth is exceedingly slow, making diagnostic tests for early diagnosis highly warranted (10). Of the serological assays available, the M. phlei absorbed enzyme-linked immunosorbent assays (ELISAs) are the most sensitive and specific. However, as with other mycobacterial infections, the immune response in paraTB seems to be regulated by a Th1-type cellular immune response well into the progression of the disease (37), which makes serological detection of subclinical cases difficult (38). Assessments of delayed-type hypersensitivity cell-mediated immune (CMI) responses by skin tests with purified protein derivative (PPD) preparations similar to human and bovine TB have been used (3), often with PPD prepared from the more easily culturable organism M. avium subsp. avium (PPDa) replacing johnin M. avium subsp. paratuberculosis PPD (PPDj) (16).
During the last decade, in vitro tests using released gamma interferon (IFN-γ) as a specific way to measure CMI against bovine TB and paraTB have been introduced (5, 11, 32, 35, 50). As PPDj is not commercially available, the IFN-γ response to PPDa relative to the response to M. bovis PPD (PPDb) stimulation has been used to examine M. avium subsp. paratuberculosis-specific CMI responses for early diagnosis of Johne's disease (1) or the relationship between M. avium subsp. paratuberculosis and Crohn's disease (12). The commercial tuberculin IFN-γ test is also a candidate for replacement of the skin test in human TB testing (19, 30). However, it is evident from the literature that wide differences exist in terms of antigen concentration during stimulation and not least in interpretation of the IFN-γ results. Furthermore, the influence of M. avium subsp. paratuberculosis infection on the IFN-γ test for bovine TB has not been investigated.
In this study, the IFN-γ test of whole-blood stimulation with mycobacterial PPD preparations was preliminarily evaluated as a possible test for the diagnosis of subclinical paraTB with single samples from different age groups of a dairy cow population. Different interpretation criteria are discussed, and by virtue of the TB-free status of Denmark, the IFN-γ test for bovine TB in a non-TB-infected population is evaluated with respect to possible cross-reactions with M. avium subsp. paratuberculosis or other environmental mycobacteria in herds infected or not infected with paraTB. The influences of antigen concentration and stimulation of day-old samples from a limited number of paraTB-reactive animals were also examined.
MATERIALS AND METHODS
Animals.
In a well-defined area of Denmark, a large (approximately 125 cows) cattle herd research project (the Kongeaa project [http://www.kongeaaprojektet.dk]) has been initiated with a focus on milk quality, cattle health, and cattle diseases relevant to the modern dairy industry, including paraTB. Among the herds in the Kongeaa project, herds included in the present study were selected as follows. First, a serological screening of individual milk samples from all lactating cows was performed with an in-house indirect M. phlei absorbed ELISA with a commercial M. avium subsp. avium antigen (M. paratuberculosis strain 18; Allied Monitor Laboratories) with performance comparable to that of the Paracheck ELISA (CSL), which employs an M. avium subsp. paratuberculosis antigen (25). From these results, 10 dairy herds with a high prevalence of test-positive reactors (5 to 21%) were selected as the infected herds. From these herds, several cows with clinical paraTB were culled each year but none of the farmers had exact knowledge of the prevalence of infection in their herds. To identify noninfected herds, fecal samples were collected from the older half of the cows in 18 herds with no or only a few serologic reactors and grown for 12 weeks on modified Löwenstein-Jensen medium (18) following decontamination as described by Beerwerth (2). Three serology- and culture-negative herds were selected, and an additional two presumably paraTB-free herds were selected based on the initial serologic screening without reactors and on historical information from the farmers, local veterinarians, and milk quality advisors. The status of these five herds as not infected with M. avium subsp. paratuberculosis has since been supported by two yearly fecal cultures of all of the cows without any culture-positive samples and by serological examination of individual milk or serum samples. At the second serologic examination, 11 cows (out of 230 sampled) showed a positive reaction in the M. avium subsp. avium ELISA described but all were negative by the Paracheck ELISA. All five noninfected herds had been closed without introduction of new animals for a minimum of 5 years.
Within the infected and noninfected herds, 252 and 119 animals, respectively, aged 1 to 60 months were selected to evaluate the interpretation criteria and specificity of the IFN-γ test in an area where paraTB is endemic. None of the selected animals had a history, or showed clinical symptoms, of paraTB at the time of sampling, but (based on historical information) in the infected herds, offspring of cows known to be paraTB infected were preferentially selected to give a high number of subclinical infections.
Antigens.
PPDb and PPDa, both at 0.3 mg/ml, were purchased from CSL. Lyophilized PPDj prepared in 1975 from M. avium subsp. paratuberculosis strain Promise (21, 22) was reconstituted at 4°C overnight in 6.4 parts of sterile 1/15 M secondary phosphate and adjusted with 1.6 parts of 1/15 M primary phosphate and 2 parts of 24-g/liter saline to give a PPD concentration of 1 mg/ml (wt/vol) in phosphate-buffered saline (PBS). Working solutions of 0.3 mg/ml were prepared by dilution in similarly prepared PBS, which was also used for no-antigen stimulations.
Whole-blood culture.
Heparinized whole-blood samples were collected by the Vacutainer system, packed, and shipped to the laboratory in a thermos box (without cooling). At arrival, 6 to 8 h after sampling, 1.5-ml cultures were stimulated in 24-well culture plates (Greiner) with previously added PPDb, PPDa, PPDj, and PBS (50 μl) and two positive control stimulations with the mitogen concanavalin A (ConA; Sigma-Aldrich) and the superantigen Staphylococcus enterotoxin B (SEB; Alexis), respectively. All PPD preparations and ConA were added to a final concentration of 10 μg/ml, and SEB was added to a final concentration of 1 μg/ml unless otherwise specified. Cultures were incubated for 18 h at 37°C and 5% CO2 in air. After incubation, the plates were centrifuged and approximately 0.8 ml of supernatant was collected into 96-well 1-ml polypropylene storage plates (Greiner) and frozen at −20°C until analysis. Two samples of one of the negative herds were lost following stimulation, bringing the total number of samples used in this study to 369.
Effects of antigen concentration and delayed stimulation.
To test if the chosen concentration of PPD in cell cultures was adequate, a series of six twofold dilutions of PPDb (0.125 to 40 μg/ml) and PPDj (0.25 to 80 μg/ml) was incubated with the whole blood of 11 animals from one of the infected herds. Similarly, three fivefold SEB dilutions (0.2, 1.0, and 5.0 μg/ml) were tested. The general recommendation of incubation within 8 h of sampling is a major problem for implementation of the IFN-γ test under field conditions. Therefore, a test of the effect of stimulation on the following day was performed on 10 of these animals: Additional heparinized samples were left at room temperature overnight, and a second antigen titration was performed 26 h after sampling. These cultures were then incubated for 24 h before supernatant collection.
Bovine IFN-γ ELISA.
IFN-γ contents of supernatants were measured in duplicate by the Bovigam ELISA kit (CSL) in accordance with the manufacturer's instructions. The tetramethylbenzidine reaction was stopped with H2SO4, and optical density (OD) at 450 nm was measured with 650-nm-background correction. The negative and positive controls included in the Bovigam kit produced OD readings ranging from 0.026 to 0.097 and 1.390 to 3.295 on the 53 plates, respectively. With this broad range of the positive control (accepted kit limits of 0.7 and greater), the OD readings were calibrated to reduce the effects of plate-to-plate variation. For this calibration, the standard formula used was ODC = [(sample OD − NegC) × (mean Pos − mean Neg)/(PosC − NegC)] + mean Neg, where ODC is the calibrated OD, PosC and NegC are the positive and negative kit controls on the plate and mean Pos and mean Neg are the mean values of the positive and negative kit controls in the 53 plates (2.406 and 0.046, respectively). Samples with calibrated no-antigen minus negative kit control values of greater than 0.1 were deemed invalid and excluded from the analyses.
The analysis of PPD titration and delayed stimulation of samples was performed with an in-house anti-bovine IFN-γ ELISA. Plates (MaxiSorp; Nunc) were coated with an anti-bovine IFN-γ monoclonal antibody (clone cc302 at 1 μg/ml; Serotec) and blocked with 1% casein in PBS-0.05% Tween 20. Samples (50 μl) were added to an equal amount of blocking buffer, incubated for 2 h at room temperature, and incubated with a purified polyclonal rabbit anti-recombinant bovine IFN-γ antibody and subsequently with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin (ZyMAX; Zymed). This ELISA correlates with the Bovigam kit for dilutions of both recombinant (r = 0.9932, P < 0.0001) and natural (r = 0.9989, P < 0.0001) bovine IFN-γ and has the same detection limits (data not shown).
Statistical analyses.
All statistical analyses were performed with GraphPad Prism version 3.02 for Windows (GraphPad Software). To obtain Gaussian distributions of IFN-γ responses, ODC values were log transformed before analysis when appropriate.
RESULTS
SEB is superior to ConA as a positive control.
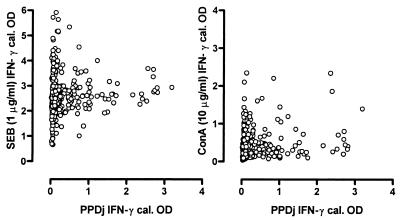
In Table 1, the overall IFN-γ response to positive control stimulation with either SEB or ConA is presented. The variation in response to SEB or ConA stimulation could not be attributed to age (both P = 0.21). While ConA responses were somewhat correlated with herd/sampling date (r2 = 0.45, P = 0.001; data not shown), this was not the case for SEB (P = 0.28). In Fig. 1, the SEB and ConA results are presented relative to the PPDj-specific response, showing how, even when PPD stimulation resulted in substantial IFN-γ production, some samples did not respond to ConA stimulation.
TABLE 1.
IFN-γ production in 369 positive control stimulated whole-blood cultures
| Stimulus (concn [μg/ml]) | Raw OD
|
ODC
|
||||
|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | |
| SEB (1) | 2.508 | 0.485 | 4.000 | 2.588 | 0.656 | 5.906 |
| ConA (10) | 0.414 | 0.035 | 2.486 | 0.417 | −0.036 | 2.345 |
FIG. 1.
Relationship between SEB or ConA as positive control stimulators and M. avium subsp. paratuberculosis PPDj as antigen-specific stimulation. OD readings were calibrated (cal.) with the positive and negative kit controls.
Higher responses to PPDa compared to PPDj.
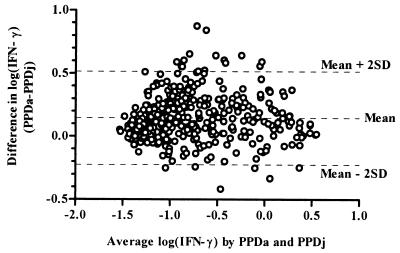
IFN-γ production following PPDj stimulation of samples and that after PPDa stimulation of samples were highly correlated (r2 = 0.84, P < 0.0001). However, the difference between the log-transformed OD readings obtained by stimulation with PPDj and PPDa was plotted against their mean value, as recommended by Bland and Altman (6), a lack of agreement between the two stimulations was displayed in the OD range of 0.1 to 1.0 (log −1 to 0), i.e., not at the maximal CMI response (Fig. 2). In general, the response to PPDa was greater than that to PPDj (a mean of 56% greater, as measured by the antilog of the mean difference in log OD values of infected herds). The mean ODC values of the PPDj stimulation minus no antigen (PBS) were 0.390 ± 0.649 and 0.046 ± 0.106 in the paraTB-infected and noninfected herds, respectively. Similarly, 0.529 ± 0.750 and 0.104 ± 0.178 were obtained following stimulation with PPDa. Responses to PPDb were also correlated with PPDj responses, although at a lower level (r2 = 0.65, P < 0.0001).
FIG. 2.
Lack of agreement between IFN-γ induction by M. avium subsp. avium PPDa or PPDj as shown by the difference in IFN-γ levels plotted against the average value following logarithmic transformation of OD values. SD, standard deviation.
Interpretation of the IFN-γ test.
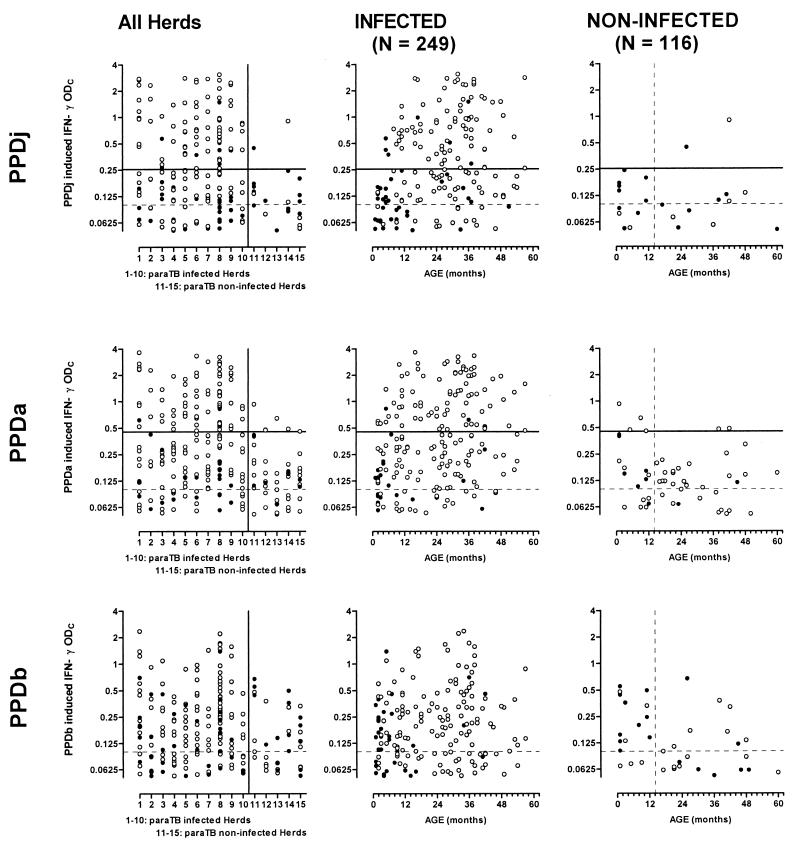
In Fig. 3, calibrated IFN-γ test results following stimulation with PPDj, PPDa, or PPDb are shown in relation to herd and age in paraTB-infected and noninfected populations. IFN-γ test-positive reactors for paraTB were present in all of the infected herds, but as animals were deliberately sampled to include animals with subclinical infections, any inferred herd prevalence estimates would be heavily biased. In the noninfected herds, IFN-γ responses to antigenic stimulation were significantly reduced (t test with Welch's correction on either PPDj, PPDa, or PPDb response: P < 0.0001). Estimates of numbers of paraTB test-positive animals with different interpretation criteria are presented in Table 2. Interpretation of the IFN-γ test has most often been accomplished similar to that of proliferation assays by comparing the specific response to the no-antigen response, in terms of the absolute difference between either OD values (5, 14, 31, 44) or IFN-γ indices (13, 34). Interpretation by absolute difference is often fixed at, e.g., a 0.05- or 0.1-U difference from the no-antigen level. However, given the observed stronger response to the PPDa preparation compared to PPDj, the 95% cutoff points were estimated to be 0.254 and 0.453 for PPDj-PBS and PPDa-PBS, respectively, calculated as the mean response plus 1.96 times the standard deviation in the noninfected herds (data given above).
FIG. 3.
Individual IFN-γ responses in herds infected or not infected with paraTB following whole-blood stimulation with PPDj, PPDa, or PPDb. Responses are presented as ODC responses following specific stimulation minus no-antigen stimulation. Open circles indicate PPDj > PPDb in the top panel or PPDa > PPDb in the lower two panels. Closed circles indicate PPDb responses greater than or equal to the PPDj or PPDa response, respectively. The broken horizontal line at ODC = 0.1 indicates the standard cutoff level at test interpretation for bovine paraTB or TB (bottom panel). The solid horizontal lines at 0.254 and 0.453 indicate the estimated 95% paraTB cutoff points for interpretation of PPDj or PPDa responses, respectively. Only IFN-γ ODC levels of greater than 0.05 are shown; thus, e.g., results for 80 and 93 PPDj-stimulated animals in the infected and noninfected herds, respectively, are not shown (Table 2).
TABLE 2.
Number of animals positive for subclinical paraTB by IFN-γ test and estimated specificity with different antigen stimulations and different interpretation criteria
| Interpretation criterion for paraTB | No. of cows positive (% specificity)
|
|||||
|---|---|---|---|---|---|---|
| 5 noninfected herds (n = 116)
|
10 infected herds (n = 249)
|
|||||
| PPDj | PPDa | PPDj + PPDaa | PPDj | PPDa | PPDj + PPDa | |
| Fixed difference from no-antigen stimulation | ||||||
| 1. PPDxb-PBS > 0.05 | 23 (80)c | 53 (54) | 20 | 169 | 193 | 164 |
| 2. PPDx-PBS > 0.1 | 12 (90)c | 36 (69) | 12 | 137 | 163 | 135 |
| 3. Estimated difference from no-antigen stimulation | ||||||
| PPDj-PBS > 0.254 | 2 (98) | 83 | ||||
| PPDa-PBS > 0.453 | 6 (95) | 1 | 84 | 74 | ||
| 4. Relative to no-antigen stimulation, 2 and PPDx/PBS > 4 | 7 (95) | 24 (79) | 5 | 114 | 143 | 109 |
| Relative to control antigen | ||||||
| 5. 1 and PPDx > PPDb | 6 (95) | 42 (64) | 4 | 127 | 171 | 124 |
| 6. 2 and PPDx > PPDb | 2 (98) | 27 (77) | 2 | 112 | 149 | 109 |
| 7. 3 and PPDx > PPDb | 1 (99) | 6 (95) | 1 | 75 | 82 | 67 |
| IDEXX | ||||||
| 8. 1 and PPDb/PPDx < 0.71 | 3 (97) | 29 (75) | 2 | 93 | 141 | 90 |
| 9. 2 and PPDb/PPDx < 0.71 | 2 (98) | 22 (81) | 2 | 89 | 127 | 85 |
| 10. 3 and PPDb/PPDx < 0.71 | 1 (99) | 4 (97) | 1 | 64 | 75 | 57 |
PPDj + PPDa indicates the number of animals with paraTB-positive results in both PPDj and PPDa tests.
PPDx relates to either PPDj or PPDa as a stimulant in whole-blood cultures.
An estimate of test specificity for bovine paraTB was calculated as [N −n(pos)]/N × 100, where N is the number of tested animals (N = 116) and n(pos) is the number of (false) test-positive animals.
Rather than a comparison with no-antigen stimulation alone, it is often recommended that the response to a closely related antigenic stimulus also be taken into account, thus calculating an IFN-γ index for specific interpretation of responsive samples. The strong response to PPDa resulted in only 13 animals producing a PPDj-induced IFN-γ response that was greater than 0.1 and higher than that induced by PPDa. Of these 13 animals, 8 were from the noninfected herds, thus invalidating the use of PPDa as a reference antigen for PPDj stimulation. PPDj and PPDa were then evaluated as paraTB-specific antigens against PPDb as a control antigen. In other studies, specific indices have been interpreted with a PPDb/PPDa ratio of less than 1, i.e., PPDa > PPDb (13), or by the “IDEXX criterion,” according to which a PPDb/PPDa ratio of less than 0.71 is indicative of paraTB (11, 23). Irrespective of which of these criteria was applied, a number of animals in the noninfected herds tested positive following stimulation with PPDa, while responses to PPDj revealed that only a few animals were test positive (Fig. 3; Table 2). Not surprisingly, the highest estimated specificities were obtained when the specific indices were interpreted in combination with the estimated cutoff points (Table 2, criteria 7 and 10), although interpretation by the estimated PPDj-PBS cutoff point alone (criterion 3) showed that only two animals from the noninfected herds were test positive. Likewise, only 2 animals were test positive by more commonly used criterion 6 following PPDj stimulation, but this criterion revealed as many as 112 test-positive animals in the infected herds, compared with 83 by criterion 3 (of these, 75 were test positive by both criteria). One 3.5-year-old cow from a noninfected herd was positive by both criteria. This cow was later culled for other reasons, and a number of cultures were set up from its intestinal lining and lymph nodes without any mycobacteria being identified, strongly indicating that this cow was indeed a false-positive IFN-γ reactor.
Although both populations are, without a doubt, free of bovine TB, a number of animals reacted more strongly to PPDb than to PPDj or PPDa (Fig. 3, closed circles). This was most prominent in the young age groups of both paraTB-infected and noninfected herds. In the infected population, a gradual increase in CMI against paraTB with age was reflected in a significant (P < 0.001) positive correlation with age for all PPD stimulations (including PPDb, due to the observed cross-reactivity, but not PBS). However, this unspecific (innate) IFN-γ production in response to PPDb in young animals resulted in a significant negative correlation (r = −0.22, P = 0.02) between age and PPDb, but not PPDj or PPDa, in the noninfected population.
Other studies have reported a high number of animals exhibiting IFN-γ test OD values of greater than 0.1 following no-antigen (PBS) stimulation, making the interpretation of antigen-specific IFN-γ induction invalid (23, 28). In the present study, all samples from animals showing a PBS value of >0.1 were retested with the ELISA, whereafter only four animals (aged 3 months to 3 years) were excluded on the basis of “circulatory IFN-γ.”
Effect of antigen concentration.
The PPD concentration used varies between laboratories, although 10 μg/ml seems to be the most widely used concentration. To test the effect of the PPD concentration in cell cultures on interpretation of results, a series of six twofold dilutions of PPDb (0.125 to 40 μg/ml) and PPDj (0.25 to 80 μg/ml) was incubated with the whole blood of 11 animals from herd 7 (Table 3). There was no significant difference in IFN-γ induction following stimulation with PPDb at 10 or 20 μg/ml (P = 0.17 in a paired t test), while responses at the chosen PPDj concentration of 10 μg/ml, as used in the large study, were significantly less than that observed with PPDj at 20 or 40 μg/ml (P = 0.036 or 0.0011, respectively) but not at 80 μg/ml (P = 0.17, data not shown). However, optimal PPDj concentrations of 40 to 80 μg/ml only increased IFN-γ responses by 16% compared to that achieved with 10 μg/ml. Thus, a suboptimal concentration of PPDj cannot account for the observed 56% higher response to PPDa compared to PPDj. Three fivefold dilutions of SEB (0.2, 1.0, and 5.0 μg/ml) all induced comparable levels of IFN-γ (P > 0.05; data not shown). No titrations of PPDa or ConA were performed.
TABLE 3.
IFN-γ responses to SEB, PPDb, and PPDj in whole-blood cultures of fresh (8-h-old) or day-old samplesa
| Stimulus concn (μg/ml) | IFN-γ response of cow:
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1031
|
1053
|
1075
|
1089
|
1101
|
1104
|
1112
|
1122
|
|||||||||||||||||
| SEB | PPDb | PPDj | SEB | PPDb | PPDj | SEB | PPDb | PPDj | SEB | PPDb | PPDj | SEB | PPDb | PPDj | SEB | PPDb | PPDj | SEB | PPDb | PPDj | SEB | PPDb | PPDj | |
| Day 1 | ||||||||||||||||||||||||
| 1 | 3.16 | 3.11 | 2.25 | 1.18 | 2.87 | 1.89 | 3.17 | 1.75 | ||||||||||||||||
| 2.5 | 0.13 | 0.64b | 0.16 | 0.81b | 0.01 | 0.02 | 0.08 | 0.05 | 0.78 | 1.58b | 0.02 | 0.44b | 1.07 | 1.65b | 0.02 | 0.25b | ||||||||
| 5 | 0.21 | 0.46b | 0.17 | 0.64b | 0.02 | 0.05 | 0.02 | 0.05 | 0.38 | 1.77b | 0.04 | 0.27b | 1.22 | 2.10b | 0.04 | 0.31b | ||||||||
| 10 | 0.30 | 1.58b | 0.24 | 0.74b | 0.01 | 0.16b | 0.02 | 0.11b | 0.48 | 2.05b | 0.05 | 0.38b | 1.43 | 2.81b | 0.06 | 0.38b | ||||||||
| 20 | 0.34 | 1.85b | 0.22 | 0.87b | 0.04 | 0.13b | −0.02 | 0.10b | 0.59 | 2.22b | 0.07 | 0.44b | 1.53 | 2.96b | 0.08 | 0.40b | ||||||||
| 40 | 0.20 | 2.07b | 0.16 | 0.96b | 0.05 | 0.16b | −0.03 | 0.14b | 0.54 | 2.25b | 0.08 | 0.53b | 1.51 | 3.07b | 0.09 | 0.47b | ||||||||
| Day 2 | ||||||||||||||||||||||||
| 1 | 3.02 | 0.92 | 1.69 | 0.77 | 1.23 | 1.07 | 0.88 | |||||||||||||||||
| 2.5 | 0.02 | 0.62b | 0.00 | 0.28b | 0.00 | −0.01 | −0.04 | 0.04 | 0.01 | 0.09 | 0.13 | 0.24b | 0.01 | 0.03 | ||||||||||
| 5 | 0.02 | 0.52b | 0.02 | 0.09 | −0.02 | 0.00 | 0.01 | −0.07 | 0.01 | 0.06 | 0.20 | 0.46b | 0.01 | 0.06 | ||||||||||
| 10 | 0.06 | 0.59b | 0.06 | 0.14b | −0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.08 | 0.26 | 0.69b | 0.01 | 0.08 | ||||||||||
| 20 | 0.07 | 0.63b | 0.05 | 0.19b | −0.01 | 0.03 | −0.06 | 0.06 | 0.02 | 0.09 | 0.28 | 0.82b | 0.02 | 0.09 | ||||||||||
| 40 | 0.06 | 0.70b | 0.06 | 0.15b | −0.01 | 0.03 | −0.18 | −0.18 | 0.04 | 0.10b | 0.30 | 0.90b | 0.04 | 0.11b | ||||||||||
All results are expressed as the OD of stimulation − no-antigen (PBS) stimulation. Three additional animals produced IFN-γ levels with PPD-PBS of <0.1 in all stimulations (positive control [SEB] >1.5) and were omitted from the table.
Positive test result for paraTB by the IDEXX criterion: PPDj-PBS >0.100 and PPDb-PPDj < 0.71.
Effect of delayed stimulation.
The general recommendation of incubation within 8 h of sampling is a major problem for implementation of the IFN-γ test under field conditions. Therefore, a test of the effect of stimulation on the following day was performed on 10 of these animals: Additional heparinized samples were left at room temperature overnight, and a second antigen titration was performed 26 h after sampling. These cultures were then incubated for 24 h before collection of supernatants. No-antigen-stimulated day-old samples contained slightly higher levels of IFN-γ compared with fresh samples (mean 119% of fresh levels), while the IFN-γ contents of the SEB positive control were 30 to 95% (mean 57%) of the levels obtained with fresh samples. As the IFN-γ contents of many fresh samples were around the saturation point of the IFN-γ ELISA, this reduction may be underestimated. IFN-γ production by PPD-responsive samples was reduced to 25 to 60% of the contents of fresh stimulated samples (e.g., P < 0.0001in a paired t test for PPDj at 10 μg/ml). The response to PPDj was reduced slightly more than the response to PPDb, resulting in a higher number of day-old samples that tested negative for paraTB by the IDEXX criterion (Table 3).
DISCUSSION
The results of the present study showed that the IFN-γ test can identify animals with possible subclinical paraTB infection before the animals become positive by fecal culture. However, the results also clearly demonstrate how the IFN-γ test for M. avium subsp. paratuberculosis and M. bovis is highly susceptible to cross-reactivity and that interchanging PPDj and PPDa changes the test's sensitivity and specificity. Furthermore, application of different criteria for interpretation of the IFN-γ test in relation to no-antigen stimulation or stimulation with a control antigen demonstrates how the test can be adjusted to achieve a high level of specificity. As with other diagnostic tests, it must be anticipated that an increase in specificity is achieved at the expense of test sensitivity.
The IFN-γ test is the only diagnostic test with a potential for diagnosing subclinical paraTB in live animals, while animals with clinical paraTB may exhibit low cellular immune responses and thus present negative IFN-γ test results at stages of the disease at which they are positive by other tests. It is generally accepted that (i) susceptibility to paraTB is highly increased when calves are exposed to M. avium subsp. paratuberculosis early in life and (ii) a very large number of calves in infected herds are exposed to the infection while only a small number of infected calves eventually develop disease (9). Hence, it must be anticipated that some animals showing a true paraTB-specific positive CMI response by the IFN-γ test early in life may contain, or even eliminate, the infection (47) and thus will remain undiagnosed by other available tests. Other subclinically paraTB-infected animals may, at some point, enter the clinical stage of disease with bacterial shedding and seroconversion, giving retrospective confirmation of a previous IFN-γ test result. Thus, no “gold standard” is available for evaluation of the sensitivity of the IFN-γ test for subclinical paraTB. Thorough pre- and posttesting documents the status of the noninfected herds in the present study. Therefore, it was found to be appropriate to choose an interpretation criterion giving acceptable estimates of IFN-γ test specificity and compare the number of test-positive animals in the infected herds in the context of different antigenic stimulations and interpretation criteria.
The results of the IFN-γ test with PPDb clearly demonstrate how cross-reactivity with paraTB negatively influences the specificity of the IFN-γ test for bovine TB. When a test interpretation criterion of PPDb-PBS > 0.1 as positive for bovine TB was used for animals greater than 14 months old, test specificities of 44 and 89% were calculated for paraTB-infected and noninfected herds, respectively (data not shown). Only when PPDb responses were additionally interpreted relative to PPDa responses was the IFN-γ test specificity for bovine TB greater than 97%, irrespective of paraTB infection status. To our knowledge, this cross-reactivity with paraTB has not been documented before. Other studies have obtained IFN-γ test specificities for bovine TB comparable to what was seen in the non-paraTB-infected herds of the present study (24, 48, 49), with estimates of sensitivity increasing as the stringency of interpretation is reduced (45). In addition to the observed cross-reactivity, a high degree of unspecific response to PPDb was observed in young animals in both paraTB-infected and non-paraTB-infected herds. Even with the most stringent interpretation criterion for bovine TB, 12 out of 153 calves less than 15 months of age were IFN-γ test positive. This is in agreement with previous observations of some false-positive TB (and paraTB) reactors identified when the IFN-γ test was applied to young animals (11, 23). These age-related false-positive responses were related to low specific responses to PPDa and PPDj and high unspecific PPDb responses. On this basis, it must be concluded that the IFN-γ test for mycobacterial infections is unsuitable for young cattle, at least with existing antigen preparations.
A positive control stimulation is recommended for documentation of the correct handling of samples or to aid in the interpretation of a negative IFN-γ result when an animal in a late stage of paraTB infection may have entered a state of complete anergy or immune deactivation (41). An antigen-specific IFN-γ result may also be presented as relative to the positive control, as suggested for the testing of human TB (19), if both values are within the measurement range of the ELISA. In the present study, many SEB-induced IFN-γ levels were above the saturation point of the Bovigam kit and would thus have to be diluted prior to such an analysis. Furthermore, in some situations, control stimulations are a means by which to detect fraudulent immunosuppression of animals prior to sampling. In the present material, the superantigen SEB proved to be a far more reliable indicator of IFN-γ secretion potential than the more commonly used mitogen ConA (Table 1; Fig. 1). Many animals failed to respond to stimulation with ConA by producing IFN-γ, including animals in which PPD stimulation resulted in substantial IFN-γ production. SEB is a superantigen that directly activates large fractions of bovine T cells by coupling to the Vβ (or Vγ) T-cell receptor elements outside the antigen binding groove without a need for prior internalization and processing by antigen-presenting cells (15). By bromodeoxyuridine flow cytometry proliferation analysis (BD Biosciences), the major proliferating cell subpopulation following SEB stimulation of paraTB-infected or noninfected control animals are CD4+ T cells (data not shown) while B cells and monocytes are not activated by SEB. ConA is a lectin and a universal stimulator of T cells (and monocytes), but not B cells, acting by induction of multiple cross-linkings between T cells and macrophages that lead to activation similar to that seen with antigen induction of the CMI (17). In M. bovis-infected cattle, it has recently been shown that CD4+ T cells are the predominate subsets of lymphocytes responding to PPDb by proliferation (43), while other studies have shown that in paraTB, CD4+ and, to a lesser extent, CD8+, but not γδ, T cells are the major producers of IFN-γ following in vitro stimulation (1). In humans, γδ T cells are, however, capable of producing IFN-γ in response to mycobacterial antigens (40) and they may be responsible for some of the innate IFN-γ responses observed in young calves, where numbers of γδ T cells are relatively large (28). Thus, both SEB and ConA activate relevant lymphocyte subsets by mechanisms that are similar to the antigen-specific induction measured by the IFN-γ test. The surprisingly low responses to ConA mitogenicity in the present study warrant that caution be exerted when nonresponsive whole-blood stimulations are interpreted as anergic on the basis of a ConA positive control stimulation. This may relate to the presence of red blood cells in the whole-blood stimulation assay, as this has previously been reported to inhibit ConA responses (51).
Previous studies on the antigen concentration have estimated the optimal concentration to be 20 μg/ml (32), 4 μg/ml (1, 29), or 10 μg/ml with or without information on previous titration experiments (20, 36, 46). We performed titrations of PPD on both fresh and day-old samples, and although a significant effect was observed when the PPDj concentration in stimulated samples was increased, this increase was not great. Thus, increasing the PPDb and PPDj concentration to greater than 10 μg/ml did not change the test result of any of the eight test-positive animals tested by antigen titration of fresh samples. While 10 μg/ml thus appeared to be sufficient for fresh samples, an increase in the PPD concentration returned more positive test results with day-old samples (Table 3). However, even with an antigen concentration of 20 μg/ml, four out of seven reactors were test negative when day-old samples were used. These results are supportive of the original observations on the optimal conditions for the IFN-γ test (32), while more recent reports have revealed no differences in sensitivity or specificity when day-old samples were compared with fresh samples (33).
Interpretation of the IFN-γ test by a specific stimulation index is preferable, as false positive-reactions due to, e.g., other mycobacterial infections are reduced. However, the results of the present study demonstrate large individual differences between responses to antigen cocktails such as PPD preparations and the possibility that some animals in the paraTB-infected herds found to give higher responses to PPDb than to PPDj are indeed infected with paraTB is very likely. When interpretation is done on the basis of the ratio of PPDb to PPDa or PPDj, a positive test result for paraTB excludes the possibility that the animal can simultaneously test positive for bovine TB. Thus, how the test can be safely interpreted for animals possibly infected with both Johne's disease and bovine TB is obviously controversial and highlights the need for more specific antigen candidates like heat shock proteins (20), ESAT-6 (7, 29), or synthetic peptide cocktails (42) that may be directly evaluated against no-antigen stimulation.
These observations of how the IFN-γ test is subject to cross-reactivity with other mycobacteria and how the interpretation criteria can be adjusted with respect to specificity and sensitivity is important when this new test is applied. However promising the IFN-γ test may be for the diagnosis of infected animals at an earlier stage than with other available tests, the results also emphasize that new and more specific antigens are needed in order to fully exploit the potential of this in vitro test for specific cellular immune responses to mycobacterial infections. The greatest potential for the IFN-γ test (with the currently available antigens) seems to be as a decision-supportive tool that, at a much earlier time than any other available test, can provide an indication of the efficacy of current on-farm preventive measures. In running eradication programs, such a guideline is offered by ELISA and fecal culture of animals greater than 2 years old (4, 8), with expected sensitivities of ELISA and fecal culture of 25 and 40%, respectively. Thus, farmers may wait several years before they have uncertain indications of whether new stock is raised in a disease-free environment. With the very distinct difference between IFN-γ responses in paraTB-infected and paraTB-free herds presented here, we believe that an IFN-γ test of a subsample of young stock, e.g., 16 to 20 months of age, may provide very good indications of how well preventive measures perform on a given farm.
Acknowledgments
We thank the milk quality advisers at the Danish Dairy Board and the veterinary practitioners involved for careful collection and handling of blood samples. Recombinant bovine IFN-γ was obtained from L. A. Babiuk of VIDO, Saskatoon, Saskatchewan, Canada. PPDj was prepared by M. Magnusson at Statens Serum Institut, Copenhagen, Denmark.
This study was supported by CEPROS and The Novo Nordisk Foundation.
REFERENCES
- 1.Bassey, E. O., and M. T. Collins. 1997. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect. Immun. 65:4869-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beerwerth, W. 1967. The culture of mycobacteria from feces of domestic animals and their significance for epidemiology and control of tuberculosis. Prax. Pneumol. 21:189-202. [PubMed] [Google Scholar]
- 3.Bendixen, P. H. 1978. Immunological reactions caused by infection with Mycobacterium paratuberculosis. A review. Nord. Veterinaermed. 30:163-168. [PubMed]
- 4.Benedictus, G., J. Verhoeff, Y. H. Schukken, and J. W. Hesselink. 1999. Dutch paratuberculosis programme: history, principles and development, p. 9-21. In E. J. B. Manning and M. T. Collins (ed.), Proceedings of the Sixth International Colloquium on Paratuberculosis. The International Association for Paratuberculosis, Madison, Wis.
- 5.Billman-Jacobe, H., M. Carrigan, F. Cockram, L. A. Corner, I. J. Gill, J. F. Hill, T. Jessep, A. R. Milner, and P. R. Wood. 1992. A comparison of the interferon gamma assay with the absorbed ELISA for the diagnosis of Johne's disease in cattle. Aust. Vet. J. 69:25-28. [DOI] [PubMed] [Google Scholar]
- 6.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. [PubMed] [Google Scholar]
- 7.Buddle, B. M., T. J. Ryan, J. M. Pollock, P. Andersen, and G. W. de Lisle. 2001. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37-46. [DOI] [PubMed] [Google Scholar]
- 8.Bulaga, L. L., and M. T. Collins. 1999. U.S. voluntary Johne's disease herd status program for cattle, p. 39-47. In E. J. B. Manning and M. T. Collins (ed.), Proceedings of the Sixth International Colloquium on Paratuberculosis. The International Association for Paratuberculosis, Madison, Wis.
- 9.Cocito, C., P. Gilot, M. Coene, M. de Kesel, P. Poupart, and P. Vannuffel. 1994. Paratuberculosis. Clin. Microbiol. Rev. 7:328-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, M. T. 1996. Diagnosis of paratuberculosis. Vet. Clin. N. Am. Food. Anim. Pract. 12:357-371. [DOI] [PubMed] [Google Scholar]
- 11.Collins, M. T., and B. Y. Zhao. 1994. Comparison of the commercial serum antibody ELISA, δ-interferon test kit, and radiometric fecal culture for early diagnosis of paratuberculosis in experimentally infected Holstein calves, p. 67-76. In R. J. Chiodini, M. T. Collins, and E. O. E. Bassey (ed.), Proceedings of the Fourth International Colloquium on Paratuberculosis. The International Association for Paratuberculosis, Rehoboth, Mass.
- 12.Collins, M. T., G. Lisby, C. Moser, D. Chicks, S. Christensen, M. Reichelderfer, N. Hoiby, B. A. Harms, O. O. Thomsen, U. Skibsted, and V. Binder. 2000. Results of multiple diagnostic tests for Mycobacterium avium subsp. paratuberculosis in patients with inflammatory bowel disease and in controls. J. Clin. Microbiol. 38:4373-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz, F., F. Masso, A. Paez, E. Varela, F. Suarez-Guemes, and L. F. Montano. 1999. Secretion of IFN-gamma by bovine peripheral blood mononuclear cells stimulated with Mycobacterium bovis protein fractions obtained by isoelectric-focusing. Vet. Immunol. Immunopathol. 67:203-212. [DOI] [PubMed] [Google Scholar]
- 14.Eamens, G. J., T. Jessep, M. J. Carrigan, and R. Webb. 1996. Long term evaluation of Johne's absorbed ELISA and gamma interferon (γ-IFN) assays in an endemically infected beef herd in New South Wales, Australia, p. 280-285. In R. J. Chiodini, M. E. Hines, and M. T. Collins (ed.), Proceedings of the Fifth International Colloquium on Paratuberculosis. The International Association for Paratuberculosis, Rehoboth, Mass.
- 15.Fikri, Y., O. Denis, P. P. Pastoret, and J. Nyabenda. 2001. Purified bovine WC1+ gamma delta T lymphocytes are activated by staphylococcal enterotoxins and toxic shock syndrome toxin-1 superantigens: proliferation response, TCR V gamma profile and cytokine expression. Immunol. Lett. 77:87-95. [DOI] [PubMed] [Google Scholar]
- 16.Gilot, P., and C. Cocito. 1993. Comparative analysis of three sensitins used in cutaneous testing for tuberculosis and paratuberculosis in cattle. FEMS Microbiol. Lett. 110:307-311. [DOI] [PubMed] [Google Scholar]
- 17.Heegaard, P. M. H., and K. Müller. 1988. Lectins and the immune system. J. Immunol. Immunopharmacol. 8:239-247.
- 18.Jorgensen, J. B. 1982. An improved medium for culture of Mycobacterium paratuberculosis from bovine faeces. Acta Vet. Scand. 23:325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katial, R. K., J. Hershey, T. Purohit-Seth, J. T. Belisle, P. J. Brennan, J. S. Spencer, and R. J. M. Engler. 2001. Cell-mediated immune response to tuberculosis antigens: comparison of skin testing and measurement of in vitro gamma interferon production in whole-blood culture. Clin. Diagn. Lab. Immunol. 8:339-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koets, A. P., V. P. Rutten, A. Hoek, D. Bakker, F. van Zijderveld, K. E. Muller, and W. van Eden. 1999. Heat-shock protein-specific T-cell responses in various stages of bovine paratuberculosis. Vet. Immunol. Immunopathol. 70:105-115. [DOI] [PubMed] [Google Scholar]
- 21.Magnusson, M. 1961. Specificity of mycobacterial sensitins. I. Studies on guinea pigs with purified “tuberculin” prepared from mammalian and avian tubercle bacilli, Mycobacterium balnei, and other acid-fast bacilli. Am. Rev. Respir. Dis. 83:57-68. [DOI] [PubMed]
- 22.Magnusson, M., and M. W. Bentzon. 1958. Preparation of purified tuberculin RT-23. Bull. W. H. O. 19:829-843. [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald, W. L., S. E. Ridge, A. F. Hope, and R. J. Condron. 1999. Evaluation of diagnostic tests for Johne's disease in young cattle. Aust. Vet. J. 77:113-119. [DOI] [PubMed] [Google Scholar]
- 24.Monaghan, M., P. J. Quinn, A. P. Kelly, K. McGill, C. McMurray, K. O'Crowley, H. F. Bassett, E. Costello, F. Quigley, J. S. Rothel, P. R. Wood, and J. D. Collins. 1997. A pilot trial to evaluate the g-interferon assay for the detection of Mycobacterium bovis-infected cattle under Irish conditions. Irish Vet. J. 50:229-232. [Google Scholar]
- 25.Nielsen, S. S., H. Houe, S. M. Thamsborg, and V. Bitsch. 2001. Comparison of two enzyme-linked immunosorbent assays for serologic diagnosis of paratuberculosis (Johne's disease) in cattle using different subspecies strains of Mycobacterium avium. J. Vet. Diagn. Investig. 13:164-166. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen, S. S., S. M. Thamsborg, H. Houe, and V. Bitsch. 2000. Bulk-tank milk ELISA antibodies for estimating the prevalence of paratuberculosis in Danish dairy herds. Prev. Vet. Med. 44:1-7. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen, S. S., S. M. Thamsborg, H. Houe, and V. Bitsch. 2000. Corrigendum to “Bulk-tank milk ELISA antibodies for estimating the prevalence of paratuberculosis in Danish dairy herds” [Prev. Vet. Med. 44:1-7, 2000]. Prev. Vet. Med. 46:297.. [DOI] [PubMed] [Google Scholar]
- 28.Olsen, I., and A. K. Storset. 2001. Innate IFN-gamma production in cattle in response to MPP14, a secreted protein from Mycobacterium avium subsp. paratuberculosis. Scand. J. Immunol. 54:306-313. [DOI] [PubMed] [Google Scholar]
- 29.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 30.Pottumarthy, S., A. J. Morris, A. C. Harrison, and V. C. Wells. 1999. Evaluation of the tuberculin gamma interferon assay: potential to replace the Mantoux skin test. J. Clin. Microbiol. 37:3229-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes, S. G., N. Palmer, S. P. Graham, A. E. Bianco, R. G. Hewinson, and H. M. Vordermeier. 2000. Distinct response kinetics of gamma interferon and interleukin-4 in bovine tuberculosis. Infect. Immun. 68:5393-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1992. The gamma-interferon assay for diagnosis of bovine tuberculosis in cattle: conditions affecting the production of gamma-interferon in whole blood culture. Aust. Vet. J. 69:1-4. [DOI] [PubMed] [Google Scholar]
- 33.Ryan, T. J., B. M. Buddle, and G. W. de Lisle. 2000. An evaluation of the gamma interferon test for detecting bovine tuberculosis in cattle 8 to 28 days after tuberculin skin testing. Res. Vet. Sci. 69:57-61. [DOI] [PubMed] [Google Scholar]
- 34.Smyth, A. J., M. D. Welsh, R. M. Girvin, and J. M. Pollock. 2001. In vitro responsiveness of γδ T cells from Mycobacterium bovis-infected cattle to mycobacterial antigens: predominant involvement of WC1+ cells. Infect. Immun. 69:89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stabel, J. R. 1996. Production of gamma-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J. Vet. Diagn. Investig. 8:345-350. [DOI] [PubMed] [Google Scholar]
- 36.Stabel, J. R. 2000. Cytokine secretion by peripheral blood mononuclear cells from cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 61:754-760. [DOI] [PubMed] [Google Scholar]
- 37.Stabel, J. R. 2000. Transitions in immune responses to Mycobacterium paratuberculosis. Vet. Microbiol. 77:465-473. [DOI] [PubMed] [Google Scholar]
- 38.Sweeney, R. W., R. H. Whitlock, C. L. Buckley, and P. A. Spencer. 1995. Evaluation of a commercial enzyme-linked immunosorbent assay for the diagnosis of paratuberculosis in dairy cattle. J. Vet. Diagn. Investig. 7:488-493. [DOI] [PubMed] [Google Scholar]
- 39.Thorel, M. F., M. Krichevsky, and V. V. Levy-Frebault. 1990. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int. J. Syst. Bacteriol. 40:254-260. [DOI] [PubMed] [Google Scholar]
- 40.Tsukaguchi, K., K. N. Balaji, and W. H. Boom. 1995. CD4+ alpha beta T cell and gamma delta T cell responses to Mycobacterium tuberculosis. Similarities and differences in Ag recognition, cytotoxic effector function, and cytokine production. J. Immunol. 154:1786-1796. [PubMed] [Google Scholar]
- 41.Valentin-Weigand, P., and R. Goethe. 1999. Pathogenesis of Mycobacterium avium subspecies paratuberculosis infections in ruminants: still more questions than answers. Microbes. Infect. 1:1121-1127. [DOI] [PubMed] [Google Scholar]
- 42.Vordermeier, H. M., P. C. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waters, W. R., M. V. Palmer, B. A. Pesch, S. C. Olsen, M. J. Wannemuehler, and D. L. Whipple. 2000. Lymphocyte subset proliferative responses of Mycobacterium bovis-infected cattle to purified protein derivative. Vet. Immunol. Immunopathol. 77:257-273. [DOI] [PubMed] [Google Scholar]
- 44.Waters, W. R., J. R. Stabel, R. E. Sacco, J. A. Harp, B. A. Pesch, and M. J. Wannemuehler. 1999. Antigen-specific B-cell unresponsiveness induced by chronic Mycobacterium avium subsp. paratuberculosis infection of cattle. Infect. Immun. 67:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whipple, D. L., C. A. Bolin, A. J. Davis, J. L. Jarnagin, D. C. Johnson, R. S. Nabors, J. B. Payeur, D. A. Saari, A. J. Wilson, and M. M. Wolf. 1995. Comparison of the sensitivity of the caudal fold skin test and a commercial gamma-interferon assay for diagnosis of bovine tuberculosis. Am. J. Vet. Res. 56:415-419. [PubMed] [Google Scholar]
- 46.Whist, S. K., A. K. Storset, and H. J. Larsen. 2000. The use of interleukin-2 receptor expression as a marker of cell-mediated immunity in goats experimentally infected with Mycobacterium avium ssp. paratuberculosis. Vet. Immunol. Immunopathol. 73:207-218. [DOI] [PubMed] [Google Scholar]
- 47.Whitlock, R. H., and C. Buergelt. 1996. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet. Clin. N. Am. Food. Anim. Pract. 12:345-356. [DOI] [PubMed] [Google Scholar]
- 48.Wood, P. R., L. A. Corner, J. S. Rothel, C. Baldock, S. L. Jones, D. B. Cousins, B. S. McCormick, B. R. Francis, J. Creeper, and N. E. Tweddle. 1991. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust. Vet. J. 68:286-290. [DOI] [PubMed] [Google Scholar]
- 49.Wood, P. R., L. A. Corner, J. S. Rothel, J. L. Ripper, T. Fifis, B. S. McCormick, B. Francis, L. Melville, K. Small, and K. de Witte. 1992. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet. Microbiol. 31:71-79. [DOI] [PubMed] [Google Scholar]
- 50.Wood, P. R., K. Kopsidas, A. R. Milner, J. Hill, I. Gill, R. Webb, W. N. Mack, and K. Coates. 1989. The development of an in vitro cellular assay for Johne's disease in cattle, p. 164-167. In A. R. Milner and P. R. Wood (ed.), Johne's disease. Current trends in research, diagnosis and management. CSIRO Publications, Melbourne, Australia.
- 51.Yachnin, S., L. W. Allen, J. M. Baron, and R. H. Svenson. 1972. The potentiation of phytohemagglutinin-induced lymphocyte transformation by cell-cell interaction: a matrix hypothesis. Cell. Immunol. 3:569-589. [DOI] [PubMed] [Google Scholar]