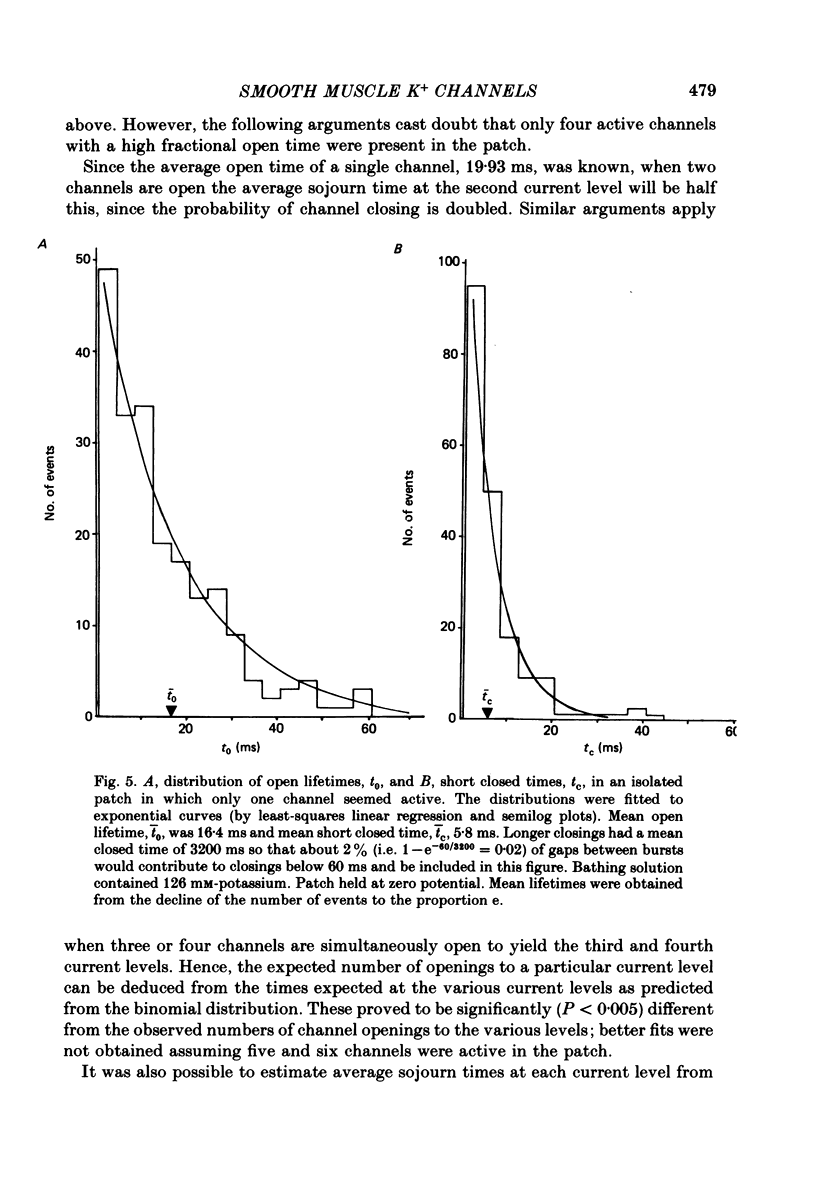
Abstract
1. The patch-clamp technique was used to study single channel currents in membrane patches of longitudinal smooth muscle cells of rabbit jejunum dispersed by collagenase treatment. Recordings were made from both cell-attached and isolated patches.
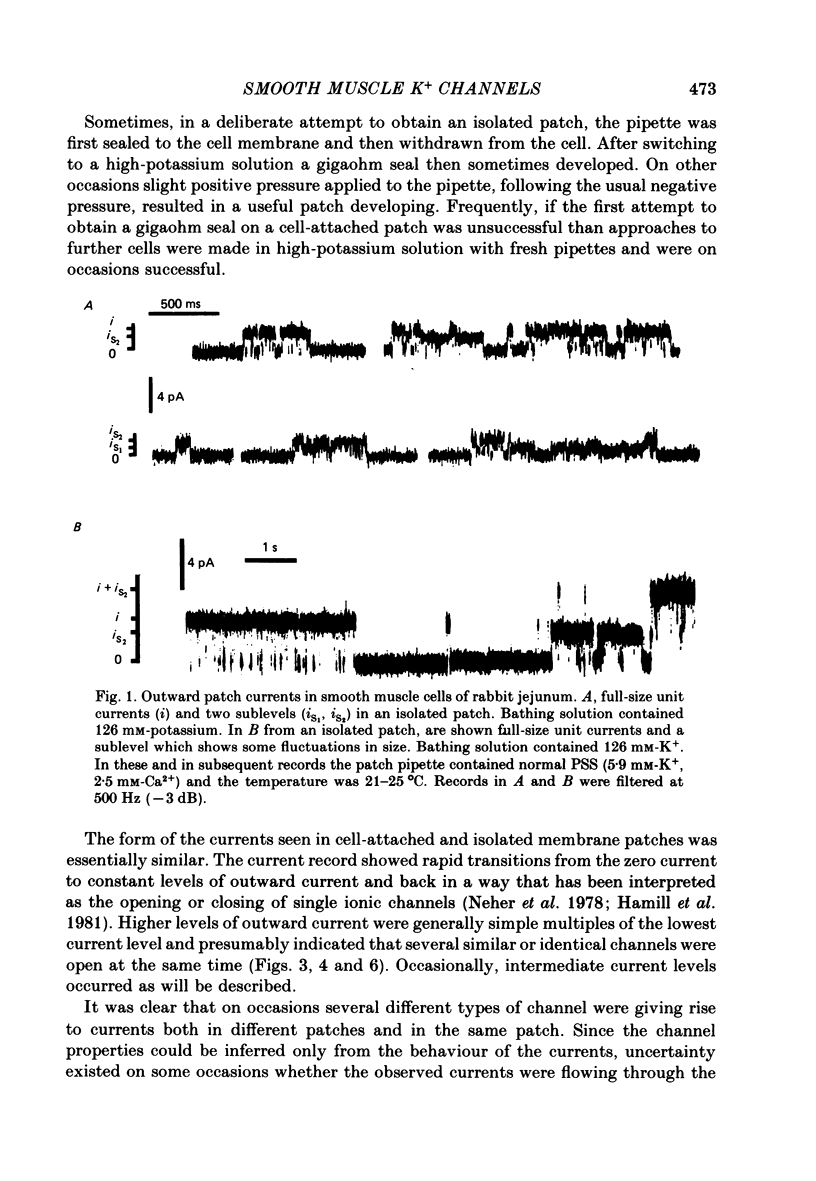
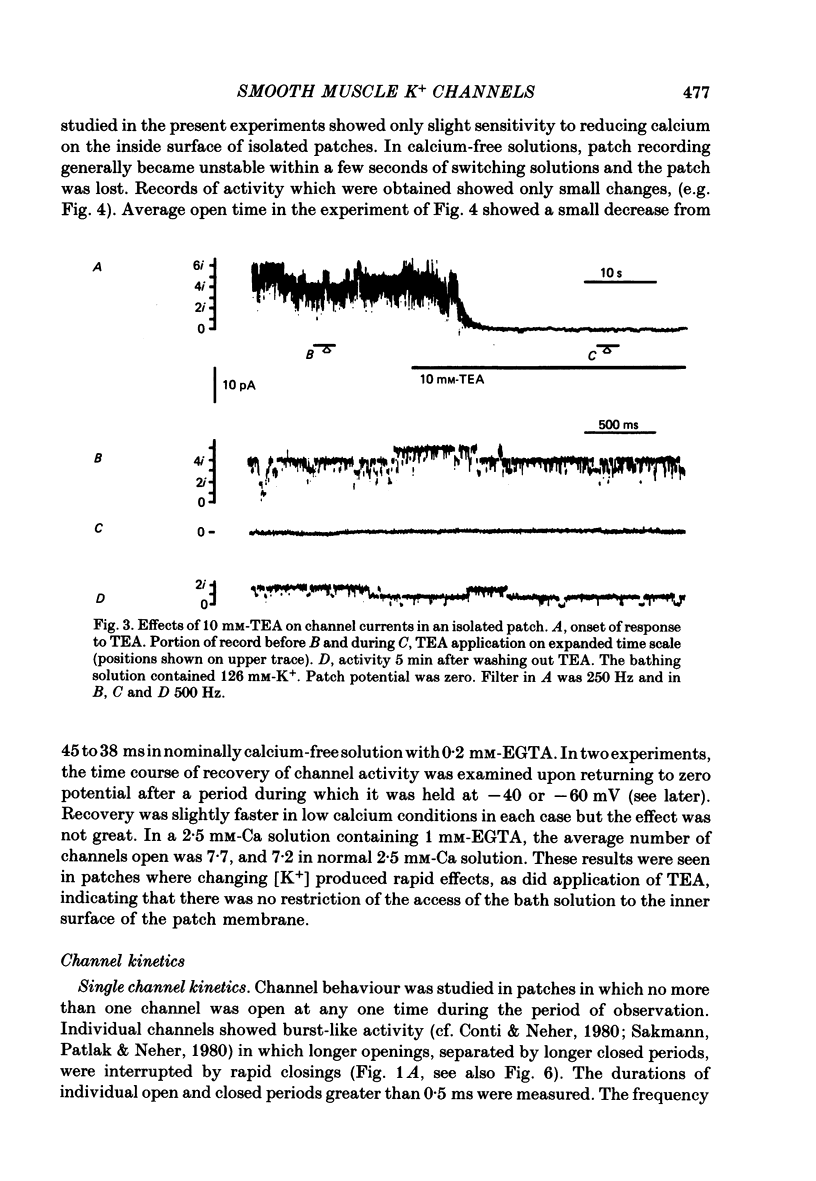
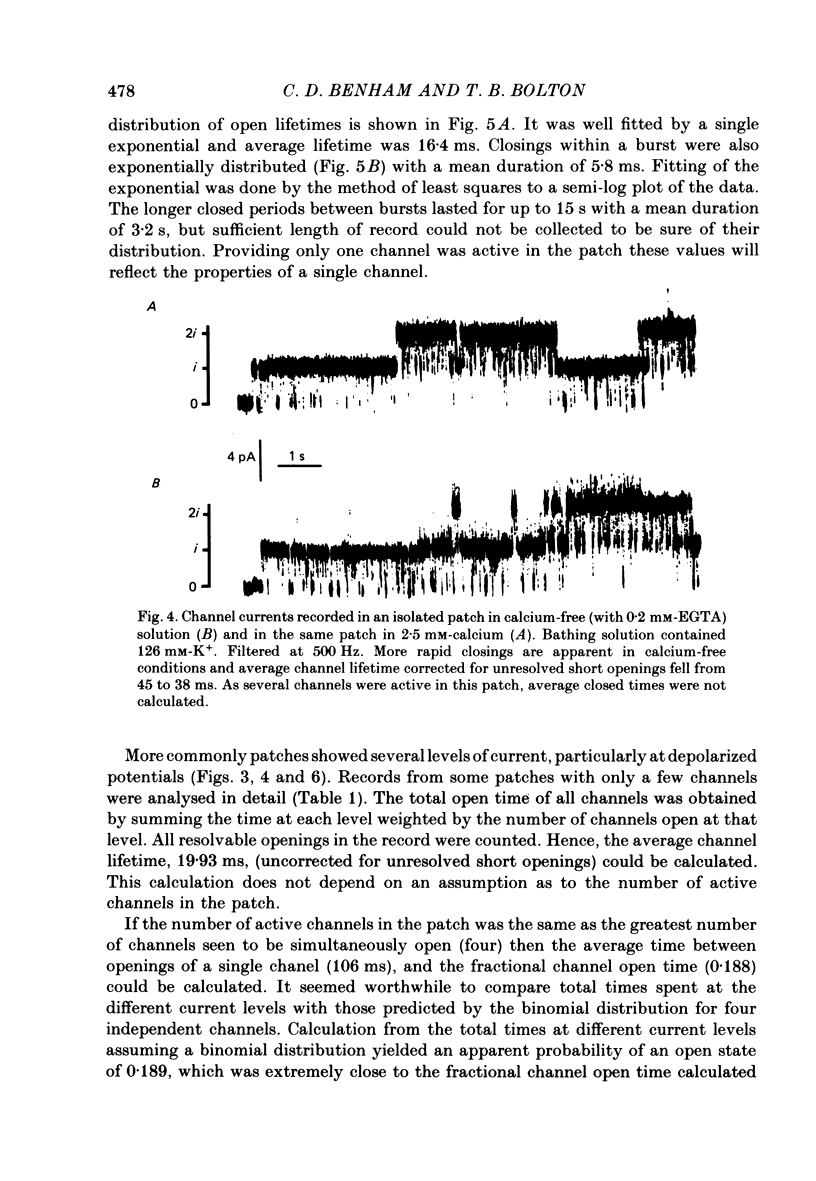
2. The predominant unit currents observed were outward at membrane potentials positive to the potassium equilibrium potential (EK) and they were rapidly and reversibly blocked by tetraethylammonium (TEA). Their size varied as EK was changed but was not noticeably affected by changing ENa, ECl or ECa; it was little altered in calcium-free EGTA solution. Thus, these currents apparently result mainly, if not exclusively, from the movements of potassium ions through channels insensitive to the calcium ion concentration. The present study describes the properties of these potassium channels.
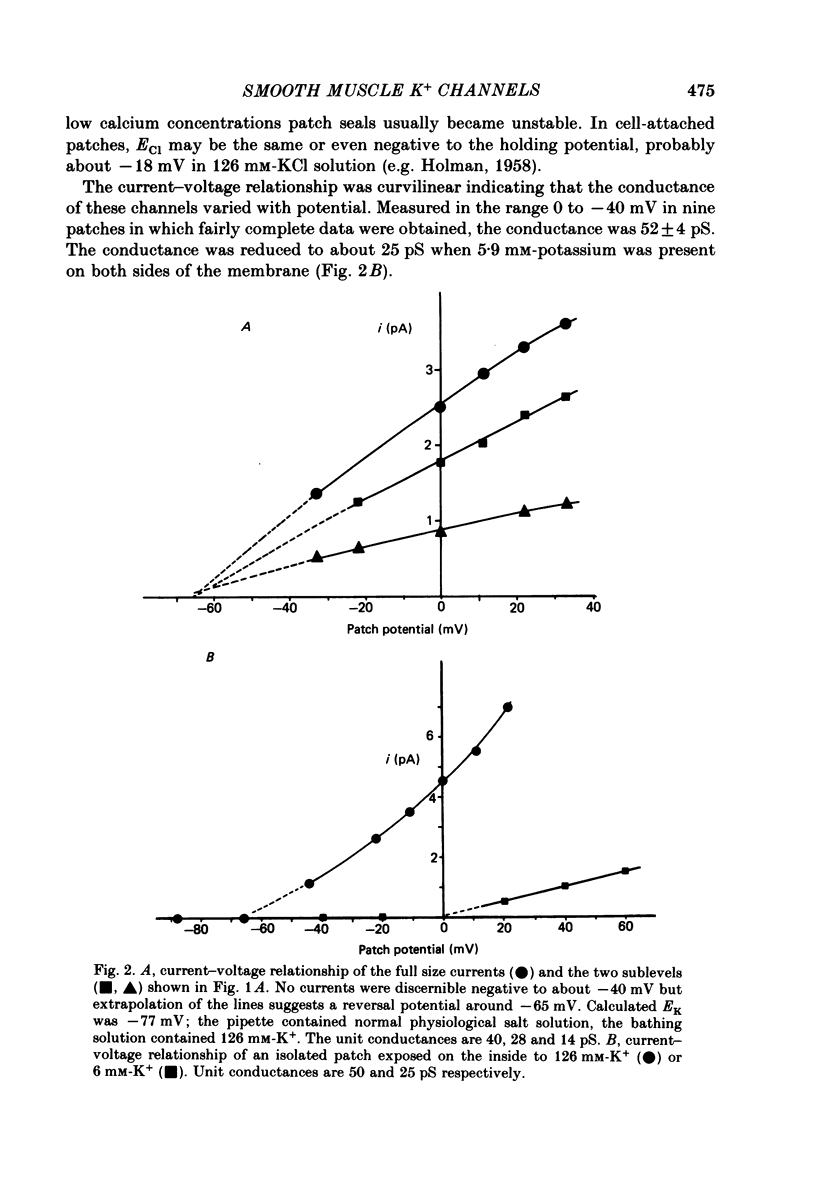
3. The unit conductance varied slightly with potential in most experiments; around zero potential it was about 50 pS. The conductance was dependent upon the potassium, but not the calcium, gradient. Sub levels of conductance of about two-thirds and, less commonly, one-third of the fully conducting channel state were sometimes seen.
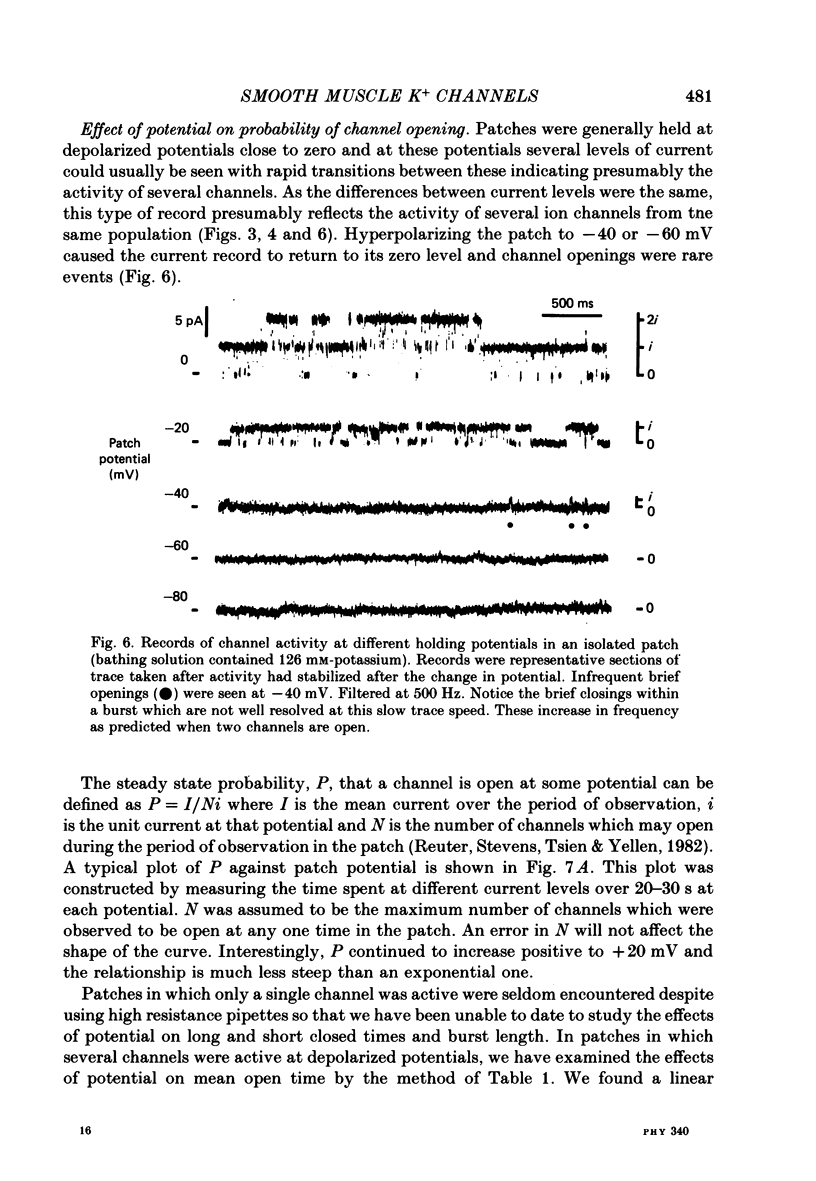
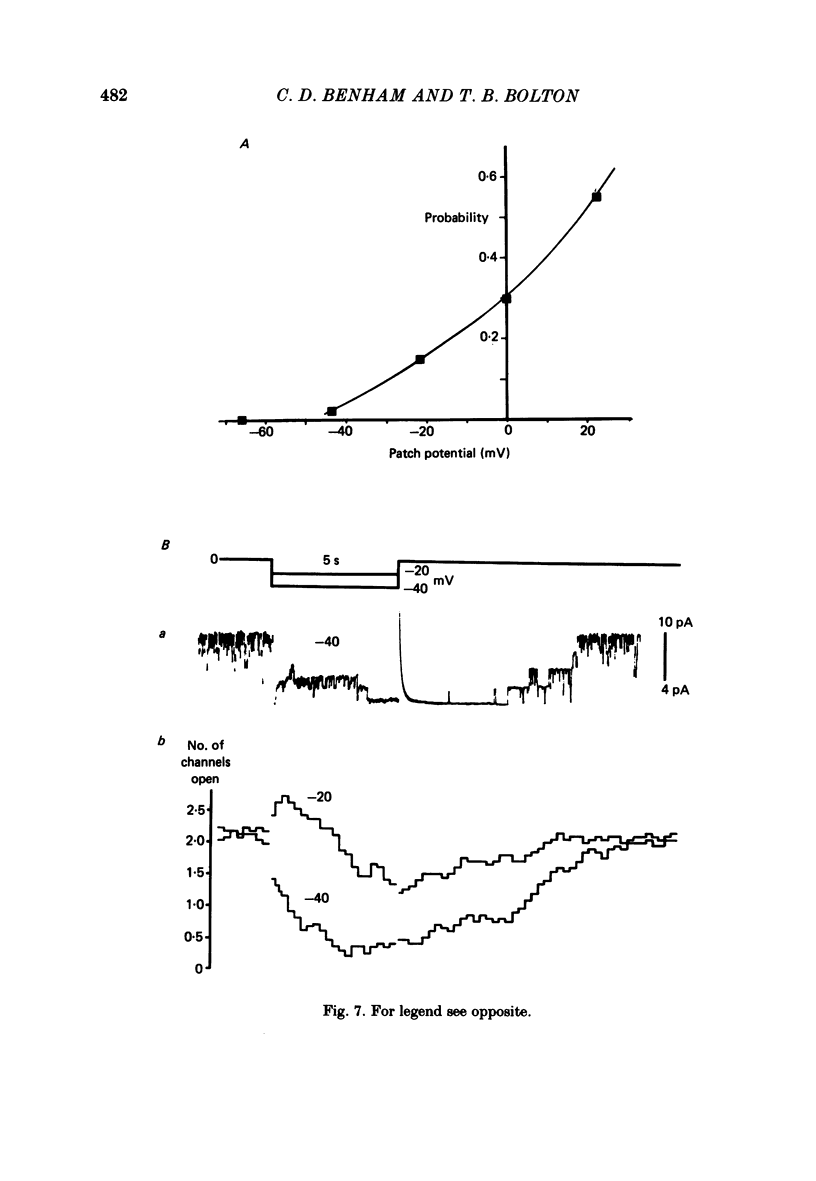
4. Membrane patches were studied which showed one to about twelve levels of outward current which were presumed to result from the opening of up to twelve channels having the same characteristics. The probability of channel open state varied with membrane potential, increasing in the potential range -40 to +40 mV. Channel openings were rare negative to -40 mV. No inward currents through these potassium channels were observed as openings were not seen at membrane potentials negative to EK.
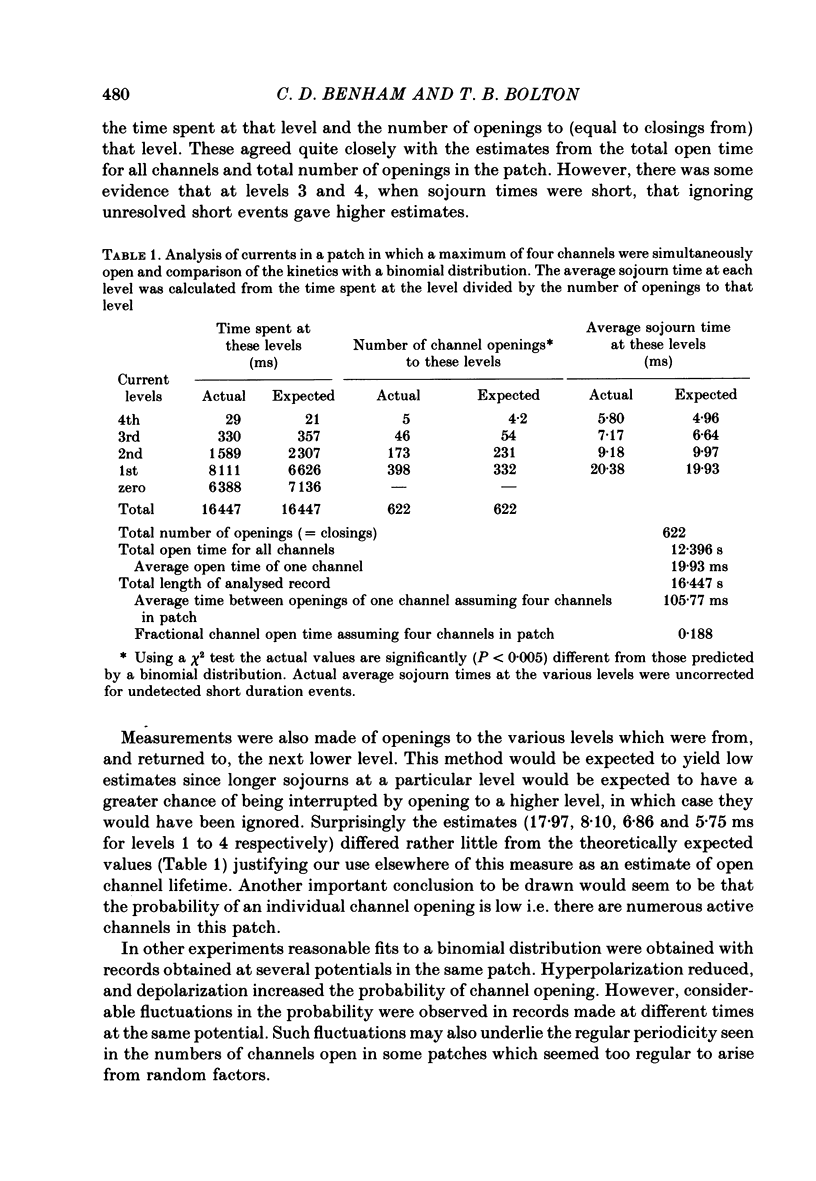
5. When the probability of channel opening was low, channel openings occurred in bursts which could be separated by several seconds. Analysis of the openings of a single channel revealed that open times and short closed times were exponentially distributed with mean durations of 15-45 ms and about 6 ms at zero potential. In some patches regular cyclical openings of several channels occurred. In other patches openings of individual channels appeared to be independent events as they were reasonably fitted by a binomial distribution.
6. Following a step change from negative potentials, where channels were closed, to more positive potentials, channel openings increased during a period of 10 s to reach a steady state. No evidence of inactivation was observed.
7. These results suggest the existence of a population of potential-sensitive potassium-selective ion channels in the smooth muscle cell membrane which are closed at the resting membrane potential and which open upon depolarization with slow (seconds) kinetics; these may be involved in the slow potential (wave) activity of this muscle.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Bagby R. M., Young A. M., Dotson R. S., Fisher B. A., McKinnon K. Contraction of single smooth muscle cells from Bufo marinus stomach. Nature. 1971 Dec 10;234(5328):351–352. doi: 10.1038/234351a0. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Clark J. P. Effects of histamine, high potassium and carbachol on 42K efflux from longitudinal muscle of guinea-pig intestine. J Physiol. 1981 Nov;320:347–361. doi: 10.1113/jphysiol.1981.sp013954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Clark J. P., Kitamura K., Lang R. J. Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea-pig ileum. J Physiol. 1981 Nov;320:363–379. doi: 10.1113/jphysiol.1981.sp013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Parker I. Single glutamate-activated channels recorded from locust muscle fibres with perfused patch-clamp electrodes. J Physiol. 1981 Dec;321:195–210. doi: 10.1113/jphysiol.1981.sp013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Parker I. Rapid kinetics of single glutamate-receptor channels. Nature. 1982 Feb 4;295(5848):410–412. doi: 10.1038/295410a0. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy, Daniel E. E. The electrophysiological basis of the motor inhibitory effect of adrenaline on rabbit small intestinal smooth muscle. Can J Physiol Pharmacol. 1976 Aug;54(4):446–456. doi: 10.1139/y76-064. [DOI] [PubMed] [Google Scholar]
- Fay F. S., Delise C. M. Contraction of isolated smooth-muscle cells--structural changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):641–645. doi: 10.1073/pnas.70.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonella J. Variation de l'activité électrique spontanée du duodénum de Lapin avec le lieu de dérivation. C R Acad Sci Hebd Seances Acad Sci D. 1965 May 17;260(20):5362–5365. [PubMed] [Google Scholar]
- Gration K. A., Lambert J. J., Ramsey R., Usherwood P. N. Non-random openings and concentration-dependent lifetimes of glutamate-gated channels in muscle membrane. Nature. 1981 Jun 4;291(5814):423–425. doi: 10.1038/291423a0. [DOI] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Labarca P. P., Miller C. A K+-selective, three-state channel from fragmented sarcoplasmic reticulum of frog leg muscle. J Membr Biol. 1981;61(1):31–38. doi: 10.1007/BF01870750. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Neher E., Marty A. Single channel activity associated with the calcium dependent outward current in Helix pomatia. Pflugers Arch. 1981 Mar;389(3):293–295. doi: 10.1007/BF00584792. [DOI] [PubMed] [Google Scholar]
- Mills R. G., Taylor G. S. Studies of intestinal slow wave activity with a double sucrose gap apparatus. Life Sci I. 1971 Mar 15;10(6):347–353. doi: 10.1016/0024-3205(71)90134-2. [DOI] [PubMed] [Google Scholar]
- Momose K., Gomi Y. [Studies on isolated smooth muscle cells. VI. Dispersion procedures for acetylcholine-sensitive smooth muscle cells of guinea pig (author's transl)]. Nihon Heikatsukin Gakkai Zasshi. 1980 Mar;16(1):29–36. doi: 10.1540/jsmr1965.16.29. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F., Tsien R. W., Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982 Jun 10;297(5866):501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Schindler H., Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci U S A. 1980 May;77(5):3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Trautmann A. Curare can open and block ionic channels associated with cholinergic receptors. Nature. 1982 Jul 15;298(5871):272–275. doi: 10.1038/298272a0. [DOI] [PubMed] [Google Scholar]