Abstract
Sepsis, a life-threatening condition characterized by a dysregulated immune response to infection, remains a significant cause of mortality in both humans and veterinary patients. This study explores oxylipins as potential indicators of sepsis in dogs through in vivo plasma analysis and an ex vivo lipopolysaccharide (LPS)-treated skin organ culture model. By employing a robust analytical platform, 52 oxylipins and 4 polyunsaturated fatty acids were profiled in plasma and skin cultures. Results revealed distinct biochemical and morphological changes, with LPS triggering capillary vasodilation and time-dependent increases in pro-inflammatory mediators such as PGE2 and isoprostanes. Importantly, PGE2 exhibited consistent trends across both models, highlighting its potential as a diagnostic biomarker. This study underscores the utility of the skin organ culture model in mimicking early inflammatory events, offering novel insights into oxylipin dynamics during sepsis and their implications for disease resolution.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-97460-y.
Subject terms: Analytical chemistry, Biomarkers, Experimental models of disease, Biomarkers, Molecular medicine
Introduction
Sepsis, defined in 2016 by the Sepsis-3 consensus as life-threatening organ dysfunction caused by a dysregulated host response to infection1, affects millions of people annually, accounting for approximately 20% of global deaths2. Its incidence continues to rise, prompting the World Health Organization to recognize it as a global health emergency3,4. Despite resolutions aimed at enhancing efforts to prevent, diagnose, and treat this condition5, sepsis remains for humans a leading cause of death in intensive care units6. Data on the incidence and impact of sepsis in veterinary medicine remain limited. Mortality rates for dogs with septic peritonitis range from 21–68%7,8 while cats experience sepsis-related mortality rates around 40%9. These findings suggest that sepsis is a major contributor to mortality also among hospitalized dogs and cats, paralleling its impact on humans.
Sepsis is a complex systemic inflammatory condition involving immune dysfunction, mitochondrial damage, coagulation disorders, and neuroendocrine-immune dysregulation and endoplasmic reticulum stress10. Given the severity of the condition and its increasing incidence, identifying reliable early biomarkers remains an urgent challenge in veterinary and human healthcare.
Canine serum-free skin organ culture (SOC) has recently emerged as a promising model to test therapeutics that modulate mast cell degranulation and counteract allergic inflammation11. As the largest organ in mammals, the skin plays a critical role in maintaining homeostasis and protecting the body from environmental damage. Beyond its physical barrier function, the skin possesses significant immunomodulatory properties, contributing to defence mechanisms against pathogens12.
Due to its structural and cellular complexity, skin organ culture often represents a viable approach to explore clinically relevant processes. The skin comprises a diverse array of cell types, including keratinocytes, Langerhans cells, Merkel cells, melanocytes, endothelial cells, nerve endings, Schwann cells, fibroblasts, smooth muscle cells, lymphocytes, mast cells, plasma cells, macrophages, dendritic cells, sebocytes, sweat gland cells, adipocytes, and at least 15 distinct interacting cell populations within hair follicles13.
Lipopolysaccharide (LPS) is widely used to simulate septic conditions in both in vitro and ex vivo models14. In human skin organ culture models, LPS has been shown to upregulate pro-inflammatory cytokines, including IL-6, IL-8, TNFα, and IL-1β15. The development of ex vivo skin cultures dates back to the 1960s in humans16 and the late 1980s in veterinary medicine17. Early efforts in this field focused on cultivating skin in chemically defined media18, eliminating serum to create a more suitable model for testing pharmacologically inducible changes and studying skin physiology19–21.
Mutations in Toll-like receptor-4 (TLR-4) have been associated with variations in LPS responsiveness in humans, highlighting significant differences in host immune responses to sepsis that can lead to unpredictable outcomes22. This variability arises from the intricate balance between pro-inflammatory and anti-inflammatory mediators, which collectively determine the severity of infection, the extent of tissue injury, and the likelihood of recovery23. The intracellular signalling processes involved in sepsis are highly complex. For example, the cGAS-STING pathway triggers the expression of NF-κB, TNF-α, IL-6, and other pro-inflammatory cytokines. These cytokines activate phospholipase A2 (PLA2), leading to the release of polyunsaturated fatty acids (PUFAs). These PUFAs act as precursors to both pro-inflammatory and anti-inflammatory oxylipins, as well as specialized pro-resolving lipid mediators24. Consequently, PUFAs and oxylipins play a critical role in modulating the inflammatory response and promoting its resolution during sepsis.
In this work, we measured plasma and culture media levels of 52 oxylipins (including isoprostanoids, prostanoids, hydroxy- and epoxy-fatty acids, lipoxins, resolvins, protectins, and maresins) and 4 PUFAs (AA, EPA, DHA, and DPAn−3 to identify compounds related to canine sepsis both ex vivo and in vivo.
Materials and methods
This study is performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Skin organ culture
Animals and culture procedure Skin stripes were collected from 4 dogs during mastectomy from the lower abdominal area at the periphery of surgical site. The dogs presented no history of dermatological diseases or drug treatments, and a written informed consent was obtained from owners before surgery. Under general anaesthesia the surgical site was cleaned with consecutive passages of chlorhexidine solution and povidone iodine, the shin was collected and it was placed in the isolation medium (99% Williams’ E medium-L-glutamine-free and 1% antibiotic antimycotic solution) at 4 °C before being transported to the laboratory. Upon arrival, the excess subcutaneous fat was carefully removed, and skin stripes were sectioned in 4 mm-biopsies using a biopsy punch. A total of 21 biopsies were obtained from each dog. Of these, three were immediately fixed in neutral buffered formalin for histological analysis, serving as the control at time 0. The remaining 18 biopsies were cultured in triplicate, submerged in culture medium (consisting of Williams’ E medium supplemented with 1% 10,000 IU/mL penicillin/10 mg/mL streptomycin, 1% ITS + 3 Liquid Media Supplement, 1% MEM Non-essential Amino Acid Solution, 0.02% 10 ng/mL hydrocortisone and 1% 200 mM L-glutamine). The cultures were maintained in a humidifier incubator at 37 °C with 5% CO2.
Treatments After 24 h, half of the biopsies were treated with 10 µg/mL Lipopolysaccharide A (Lipopolysaccharides from Escherichia coli O111:B4 Cat. N° L2630 Sigma Aldrich SRL, Milan, IT) diluted in culture medium. Biopsies and culture medium were collected 2, 24 and 48 h post-treatment. The biopsies were fixed in neutral buffered formalin for morphometric evaluation of vasodilation, while the culture media were maintained at -80 °C for subsequent analyses of oxylipins content. Blank samples (culture media with and without LPS) were included to validate experimental results.
Canine plasma samples
Dogs referred to the Veterinary Teaching Hospital at our institution were enrolled in this study. Animal ethics approval (authorisation.no.24/2020) and informed client consent was obtained for all participants. Blood samples were collected in EDTA tubes from 10 healthy dogs and 12 septic dogs. After centrifugation, plasma samples were stored at − 80 °C for later analyses. To preserve polyunsaturated fatty acids from in vitro lipid peroxidation, the antioxidant butylhydroxytoluene (BHT, 15 mg/mL in methanol) was added before storage at a ratio of 1:100 (BHT: sample volume).
Healthy dogs were identified based on clinical examinations and standard blood test results. Septic dogs, admitted to the Intensive Care Unit (ICU) for emergency surgery due to septic peritonitis, were prospectively enrolled. Diagnosis of sepsis was based on a positive culture and/or the presence of intracellular bacteria, combined with at least two of the five Systemic Inflammatory Response Syndrome (SIRS) criteria25. Upon ICU admission, comprehensive recording of clinical parameters as heart rate and blood pressure, a complete blood count, biochemistry panel, and arterial blood gas analysis were done to evaluate SIRS score, the shock index and Sequential Organ Failure Assessment (SOFA) score to assess the severity of the condition.
Evaluation of vasodilation in ex vivo samples
Fixed skin samples were embedded in JB-4 resin (cat N. EM0100, Sigma Aldrich), sectioned into 1 μm-thick slices, and stained with 0.1% acetate thionine in distilled water. After washing, and the slides were mounted with a xylene-based mounting medium. Initial slides analysis focused on identifying morphological changes associated with the treatments. The lumen of superficial plexus capillaries was measured using NIS-Elements Br Microscope Imaging Software (Nikon). Capillary dimensions were manually traced and assessed, identifying capillaries by their walls composed of single endothelial cells, occasionally accompanied by pericytes, with red blood cells visible inside the lumen. Measurements were taken from capillaries located within 100 μm of the epidermal basal layer. Capillaries were classified into three categories based on their equivalent diameter, as previously described11.
Quantification of Oxylipins and PUFAs
The MS-based targeted profiling of 52 oxylipins (e.g., isoprostanoids, prostanoids, hydroxy- and epoxy-fatty acids, lipoxins, resolvins, protectins, and maresins)26–28 and 4 PUFAs (AA, EPA, DHA, and DPAn−3) was performed on skin organ culture media and canine plasma by using the Micro-Extraction by Packed Sorbent (MEPS) technique coupled to ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS)29–31.
Both plasma and culture media were treated as follows: after thawing, an aliquot (500 µL) of the samples was added with 20 µL of Internal Standard (IS) mixture (20 ng/mL). Proteins were precipitated by the sequential addition of salts (i.e., 250 µL of CuSO4·5H2O 10% w/v and 250 µL of Na2WO4·2H2O 12% w/v) and acetonitrile (500 µL). Samples were vortex-mixed (2000 rpm for 1 min), centrifuged (7000 rpm for 5 min), and filtered at 0.2 μm. The supernatant was then diluted (1:5 v/v) with water and purified by means of the Micro-Extraction by Packed Sorbent (MEPS) technique30,31. The methanolic extract (30 µL) was directly injected into the Agilent 1290 Infinity II LC system − 6495 Triple Quadrupole Mass Spectrometer (UHPLC-QqQ), which was equipped with a Jet Stream electrospray (ESI) ionization source (Agilent Technologies, USA). For the chromatographic separation we employed a Polaris 3C18-A column (50 × 4.6 mm, 3 μm, Agilent Technologies, USA) and a gradient elution with a mobile phase consisting of 0.1% aqueous formic acid and 50:50 v/v methanol: acetonitrile. Mass spectrometer control, data acquisition and data analysis were performed with the MassHunter Workstation software (B.07.00). The mass spectrometer operated in ESI negative ionization mode and performed multiple reaction monitoring (MRM) with unit mass resolution. Detailed chromatographic parameters, ESI and MRM operating conditions are shown in the Supporting Information. Analytes were quantified by calibration curves plotting the analyte to an internal standard peak area ratio (Quantifier transition) versus the corresponding concentration ratio. The calibration curve parameters, as well as the main analytical figures of merit of the method developed in culture medium are listed in Table S1.
Statistical analysis
Measurements of the equivalent capillary diameter were analysed using the non-parametric Mann-Whitney test at each time point. The significance level was set at p < 0.05. Data normality was tested with the Shapiro-Wilk test. The chi-square test was applied in the post hoc analysis to compare the distribution of equivalent diameter categories between controls and treated subjects at each time point. Statistical analysis was performed with GraphPad Prism version 9.4.1 for Windows, GraphPad Software, San Diego, California USA.
The levels of 52 oxylipins and 4 PUFAs were measured in both culture media and plasma. Analytes that showed concentrations below the limits of detection for more than 50% of samples were excluded from the dataset. Missing data were replaced by a random value ranging between 0 and the minimum value observed for each variable. Normality was tested using the Shapiro–Wilk test. A decimal logarithmic transform of all the variables was used to correct for asymmetry32.
Data were analysed by parametric and non-parametric univariate tests as well as unsupervised multivariate analysis (cluster analysis, PCA)33. A two-tailed p-value of < 0.05 was considered statistically significant. Data were analysed by R software (RStudio, USA) using the ggplot2 and ggpubr packages.
Results
LPS treatment induces vasodilation on ex-vivo cultured skin biopsies
Microscopic analysis revealed no treatment-related adverse effects in the biopsies. The skin in both control and treated samples was stratified into 2–3 layers, with the dermis displaying well-organized collagen fibres, clearly defined fibroblasts, and other resident cells. Skin appendages remained in a physiological condition throughout the experiment in both groups.
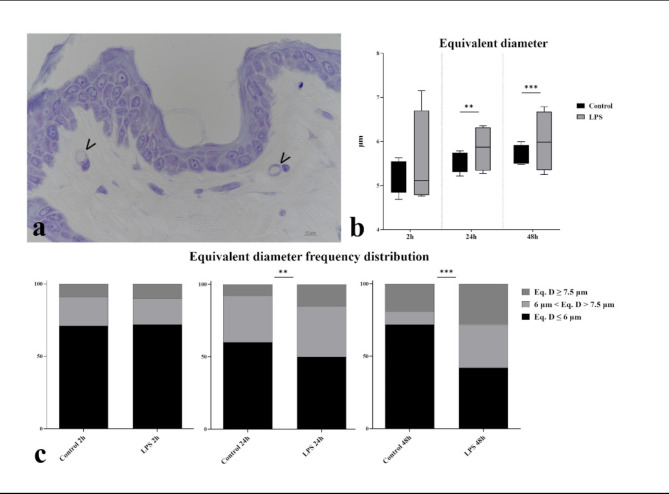
Analysis of the equivalent diameter of the superficial plexus capillaries (Fig. 1) showed a significant increase (p < 0.01) at 24 and 48 h in the equivalent diameter in LPS treated samples compared to controls. Frequency distribution analysis further confirmed an increased number of capillaries with an equivalent diameter exceeding 7.5 μm, with statistically significant differences observed at 24 h (p = 0.0048) and 48 h (p < 0.0001).
Fig. 1.
Superficial plexus capillary size. (a) Photomicrograph of a resin embedded sample of cultured skin treated with LPS for 48 h. Arrowheads indicate superficial plexus blood capillaries. (b) Median equivalent diameters of capillaries show statistically significant differences in LPS treated samples at 24 and 48 h compared to controls. (c) Frequency distribution of equivalent capillary diameters highlights statistically significant differences in LPS treated samples at 24 and 48 h compared to controls.
LPS treatment increases 15-F2t-IsoP, TXB2, 15-E2t-IsoP, Lipoxin B4, PGE2 levels in ex-vivo SOCs
Among the 52 molecules tested, only 8 were detectable in at least 50% of samples, with 5 showing a time-dependent increase in response to LPS treatment. Specifically, samples treated with 10 µg/mL LPS showed significantly higher levels of TXB2 and 15-F2t-IsoP compared to the control at 2 h (p = 0.028) and 24 h (p = 0.029) post-treatment, respectively (Fig. 2A and B). PGE2, 15-E2t-IsoP and lipoxin B4 levels were significantly elevated (p ≤ 0.004) in treated samples relative to controls, but only at 48 h post-treatment (Fig. 2C, D and E). Although 20-HETE values in LPS treated samples were consistently higher than in controls, statistical significance only achieved at 2 h post-treatment (p < 0.05, Fig. 2F). No significant differences were observed between control and LPS-treated samples for 5-F2t-IsoP and 5-epi-5-F2t-IsoP (p > 0.05).
Fig. 2.
– Temporal variation in 15-F2t-Isop (A), TX-B2 (B), 15-E2t-Isop (C), Lipoxin-B4 (D), PGE2 (E), and 20-HETE (F) levels in Control (black box) and LPS treated samples (grey box). The boxplots illustrate the minimum, 25th percentile, median, 75th percentile, and maximum value for each variable. Black dots indicate outliers. Data were Log10-transformed for analysis.
Overall, a significant time-dependent increase (p < 0.003) in 15-F2t-IsoP, PGE2, 15-E2t-IsoP, and lipoxin B4 was observed in treated samples at both 24 and 48 h. Notably, the increase of PGE2 was significant only at 48 h (p = 0.042).
Clinical parameters of the septic dogs
Of the 12 septic dogs, 4 were females and 8 were males and 7 survived and 5 did not. The causes for laparotomic surgeries were pyometra (3 cases), intestinal occlusion for foreign body ingestion (4 cases) and volvulus (2 cases), prostatic abscesses (3 cases). The bacterial agents revealed from the culture were: E. Coli, Staphylococcus pseudointermedius, Bacteroides pyogenes and Serratia marcescens. The parameters recorded at the arrival are summarized in Table 1.
Table 1.
Mean values and standard deviation or median and range of the parameters recorded at arrival of the septic dogs.
| Parameters | Weight (kg) | Age (years) | Shock index | SIRS score | SOFA |
|---|---|---|---|---|---|
| values | 23 ± 9 | 10 ± 3 | 1.7 ± 0.6 | 5 (4–5) | 5.5 (1–8) |
Fourteen oxylipins showed variations in septic vs. healthy dogs
Among the 52 tested molecules, 14 were detectable in at least 50% of canine plasma samples, all of which exhibited significant differences between healthy and septic dogs. Notably, PGE2 was the only molecule significantly increased (p = 0.008) in septic dogs, consistent with observations in SOCs. In contrast, all other detectable molecules - AA, 15-E2t-IsoP, 14,15-EET, 11,12-EET, 8,9-EET, 5-HETE, 15-HETE, 8,9-DiHETE, 11,12-DiHETE, 14,15-DiHETE, 13-HODE, Lipoxin B4 and diH-AdA - were significantly higher (p < 0.05) in healthy dogs. Representative examples are shown in Fig. 3.
Fig. 3.
Levels of 15-E2t-Isop (A), Lipoxin-B4 (B), PGE2 (C), 15-HETE (D), 14,15-EET (E), and AA (F) in Control (black box) and septic dogs (grey box). Box-plot shows: The boxplot illustrates the minimum, 25th percentile, median, 75th percentile, and maximum value for each variable. Black dots indicate outliers. Data were Log10-transformed for analysis.
Discussion
This study demonstrated that LPS induces both morphological and biochemical changes in canine skin organ cultures, evidenced by increased blood capillary size and elevated levels of specific oxylipins. These findings validate the use of LPS in this model to mimic the inflammatory process. Similar effects have already been reported in human skin organ cultures, where LPS upregulated pro-inflammatory cytokines such as IL-6, IL-8, TNFα and IL-1β, as well as the anti-inflammatory cytokine IL-1015,34. In septic dogs, a different oxylipin modulation pattern was observed compared to controls. Among the molecules analysed, lipoxin B4, 15-E2t-IsoP and PGE2 were modulated in both the skin organ culture model and in vivo under septic conditions. However, only PGE2 showed a consistent trend in both systems. This discrepancy suggests that septic dogs may already be in the late phase of inflammation upon hospital admission, as they are typically referred only when their condition becomes critical. By contrast, the organ culture model is designed to capture early hyper-inflammatory stage of sepsis, which induced by LPS stimulation, highlighting its utility in studying the initial phases of the inflammatory response. The late immunosuppressive phase of sepsis is still not fully understood, particularly regarding the mechanisms driving immune dysfunction. Three key hallmarks have been identified, as monocyte and macrophage exhaustion, widespread lymphocyte apoptosis, and the migration of myeloid-derived suppressor cells. Low levels of anti-inflammatory mediators and specialized pro-resolving mediators (SPMs) can be interpreted in this context as suggesting uncoupled inflammation resolution pathways in various immune cells.
Treatment with LPS induced a significant increase (p < 0.05) in TXB2 and isoprostane 15-F2t-IsoP, a specific marker of oxidative stress, compared to controls, observed as early as 2 h and 24 h, respectively. PGE2, a well-known marker of inflammation, along with isoprostane 15-F2t-IsoP and lipoxin B4, a mediator involved in the inflammation resolution, exhibited a significant increase (p < 0.05) compared to the control only at 48 h, indicating a delayed response compared to the earlier markers.
In contrast, no significant differences were observed between treated and untreated skin samples for the F2-isoprostanes of the 5-series or for 20-HETE, excluding these compounds from further discussion. Temporal analysis of TXB2, 15-F2t-IsoP, 15-E2t-IsoP, PGE2, and lipoxin B4— compounds which displayed significant differences between LPS-treated and control samples— revealed distinct patterns. Except for PGE2, all compounds showed significant differences (p < 0.05) between 2 and 24 or 48 h, with lipoxin B4 showing highly significant changes. However, no further increase was observed between 24 and 48 h. For PGE2, concentration levels differ significantly only between 2 and 48 h, highlighting a unique temporal response.
In the organ culture model, LPS stimulation activates the arachidonic acid (AA) cascade. AA, an n-6 essential fatty acid, is a major component of the mammalian cell phospholipid membranes. Upon release by phospholipase A2, AA can be oxidized into biologically active eicosanoids - including prostaglandins (PGs), thromoboxanes (TXs), leukotrienes (LTs), hydroxyeicosatetraenoic acids (HETEs), and epoxyeicosatrienoic acids (EETs) - through enzymatic pathways such as cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450) pathway35. Eicosanoids play crucial role in regulating cell fate processes, including proliferation, differentiation, apoptosis, fibrosis, and inflammation36. Our results demonstrate that COX-induced PGs and TXs are increased ex vivo as part of the pro-inflammatory response triggered by LPS-stimulation. TXB2, the biological inactive metabolite of TXA2, is known to activate leukocytes and platelets, contributing to microcirculatory alterations37. PGE2, a key regulator of neutrophils, macrophages, and mast cell functions38, is a well-established vasoactive agent capable of modulating vascular tone through four EP receptors, each with distinct vasoactive properties39. These vascular changes were confirmed histologically in the cultured samples.
15-E2t-isoprostane, a PGE2-like compound generated from the non-enzymatic oxidation of AA, exhibits a significant increase upon LPS-treatment. This mediator exerts various bioactive roles in vivo, including promoting vasoactivity, stimulating mitogenesis and contributing to atherogenesis40. Notably, 15-E2t-isoprostane has been found to be more potent than 15-F2t-Isoprostane in inducing vasodilation in systemic and pulmonary vessels, acting through EP-receptors41. The increased production of these vasodilator mediators fully agrees with the observed increase in the equivalent diameter of superficial plexus capillaries in the skin biopsies.
PGE2 also initiates a lipid mediator switch, promoting the production of anti-inflammatory and pro-resolving mediators, such as CYP450-derived EETs and 12/15-LOX-derived SPMs, including lipoxin B4. Intermediate products of 5-LOX and 15-LOX activities, such as HETEs, serve as precursors for lipoxin biosynthesis. These molecules play a pivotal role in resolving inflammation by inhibiting neutrophil recruitment, stimulating vasodilation, and promoting efferocytosis42,43. Consistent with ex vivo experiments, PGE2 levels were significantly elevated in septic dogs in ivo, reflecting a pronounced inflammatory response. In contrast, plasma from septic dogs showed lower levels of anti-inflammatory HETEs (5-HETE, 15-HETE), EETs (8,9-EET, 11,12-EET, 14,15-EET), and SPMs (lipoxin -B4 and diH-AdA) compared to controls. This probably indicates an impaired resolution of inflammation, contributing to a sustained and unresolved pro-inflammatory state in septic dogs. Regarding sepsis severity, all dogs presented with a SIRS score higher than 3 and a high shock index, indicating a critical condition upon admission. Conversely, the SOFA score ranged from 1 to 8, with higher values observed in non-survivors. This score reflects the progression of sepsis, as an increasing SOFA score correlates with both the number of affected organ systems and the severity of organ dysfunction. Progression to severe septic conditions, and in some cases death, may be associated with the failure to effectively activate resolution pathways and restore homeostasis43,44. Jundi et al. reported similar findings, demonstrating reduced bioavailability of endogenously produced SPMs in septic patients. This deficiency ultimately resulted in impaired resolution pathways of inflammation across various immune cells. Notably, ex vivo treatment of these septic patients with RvD1 and RvD2 restored the normal function of their inflammation resolution pathways45. The use of SPMs as therapeutic agents holds significant promise in protecting patients from secondary infections and reducing mortality resulting from sepsis-induced immunosuppression.
This study has some limitations, particularly concerning the in vivo component. First, the small number of septic patients enrolled did not allow for a comparison between survivors and non-survivors, which could have been valuable in assessing whether the oxylipin profile correlates with sepsis severity. Second, the heterogeneity of sepsis etiology resulted in varying clinical presentations, potentially influencing the findings. Further insights could be gained by studying dogs with infectious inflammatory conditions that do not progress to sepsis. These animals would likely exhibit a less severe inflammatory profile, offering an opportunity to observe the dynamics of oxylipins in milder inflammatory states. Such investigation could clarify how oxylipin expression changes across different stages of inflammation, providing crucial information on whether these changes occur progressively with severity or are regulated by distinct mechanisms.
Conclusions
This study demonstrates the potential of oxylipins as biomarkers for sepsis in dogs, utilizing both in vivo plasma analysis and an ex vivo skin organ culture model. LPS stimulation effectively mimicked the inflammatory response observed in sepsis, leading to capillary vasodilation and elevated levels of key oxylipins such as PGE2, isoprostanes, and lipoxin B4. Notably, PGE2 emerged as a promising candidate for early diagnostic and therapeutic monitoring, showing consistent trends across both models.
The findings emphasize the relevance of the canine skin organ culture as a versatile and clinically significant model for studying sepsis-related inflammation. It captures early-phase molecular and morphological changes that are often inaccessible in critically ill patients. Furthermore, the study highlights the imbalance between pro-inflammatory and anti-inflammatory mediators in septic dogs, suggesting that impaired resolution mechanisms may contribute to the progression of severe sepsis.
Future research should focus on validating these findings in larger cohorts and exploring oxylipin dynamics in less severe inflammatory conditions. Such investigations could refine the understanding of the inflammatory cascade, enabling the development of targeted interventions to improve sepsis outcomes in veterinary and human medicine.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors extend their gratitude to Dr. Thierry Durand and the Bioactive Lipids Team of the Institute of Biomolécules Max Mousseron for their work on the total synthesis of some pure standards of oxylipins, which were utilized in the LC-MS/MS analysis.
Author contributions
D.B. and T.L performed oxylipin quantification, drafted the manuscript and figures; C.d.F. sampled in vivo material and selected cases with A.B.; V.M. drafted the manuscript; G.L. performed ex-vivo experiments and drafted manuscript histology figures; F.d.F., VM and A.B. conceived the work, made the final revision of the manuscript and supervised junior staff.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Declarations
Competing interests
The authors declare no competing interests.
Disclaimer
This document has been edited and refined for fluency, clarity, and coherence with the assistance of AI language processing tools (ChatGPT 4o, OpenAI). The content, scientific accuracy, and conclusions of the paper remain entirely the responsibility of the authors. The AI was used solely to enhance the readability and presentation of the manuscript without altering the underlying meaning or intent of the original text.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Denise Biagini, Chiara di Franco, Fabio Di Francesco and Angela Briganti have equally contributed to this work.
References
- 1.Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama315, 801–810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd, K. E. et al. Global, regional, and National sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet395, 200–211 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar, S., Tripathy, S., Jyoti, A. & Singh, S. G. Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review. Biosens. Bioelectron.124, 205–215 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Quenot, J. P., Pavon, A., Fournel, I., Barbar, S. D. & Bruyère, R. Le choc septique de L’adulte En France: Vingt Ans de Données épidémiologiques. Réanimation24, 303–309 (2015). [Google Scholar]
- 5.Monneret, G., Gossez, M., Aghaeepour, N., Gaudilliere, B. & Venet, F. How clinical flow cytometry rebooted sepsis immunology. Cytom Part. A. 95, 431–441 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischmann, C. et al. Assessment of global incidence and mortality of Hospital-treated sepsis. Curr. Estimates Limitations Am. J. Respir Crit. Care Med.193, 259–272 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Bentley, A. M., Otto, C. M. & Shofer, F. S. Comparison of dogs with septic peritonitis: 1988–1993 versus 1999–2003. J. Vet. Emerg. Crit. Care. 17, 391–398 (2007). [Google Scholar]
- 8.Winkler, K. P. & Greenfield, C. L. Potential prognostic indicators in diffuse peritonitis treated with open peritoneal drainage in the canine patient. J. Vet. Emerg. Crit. Care. 10, 259–265 (2000). [Google Scholar]
- 9.Troìa, R. et al. Multiorgan dysfunction syndrome in feline sepsis: Prevalence and prognostic implication. J. Feline Med. Surg.21, 559–565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, M., Cai, S. & Su, J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci.20, 5376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abramo, F. et al. Ultramicronized palmitoylethanolamide counteracts the effects of compound 48/80 in a canine skin organ culture model. Vet. Dermatol.28, 456–e104 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Pasparakis, M., Haase, I. & Nestle, F. O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol.14, 289–301 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Jatana, S. & DeLouise, L. A. Understanding engineered nanomaterial skin interactions and the modulatory effects of ultraviolet radiation skin exposure. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol.6, 61–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facchin, B. et al. Anti-inflammatory screening biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and Meta-analysis. (2024). 10.22541/au.170668302.25187803/v1 [DOI] [PubMed]
- 15.Gvirtz, R., Ogen-Shtern, N. & Cohen, G. Kinetic cytokine secretion profile of LPS-Induced inflammation in the human skin organ culture. Pharmaceutics12, 299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaven, E. P. & Cox, A. J. Organ culture of human skin. J. Investig. Dermatol.44, 151–156 (1965). [PubMed] [Google Scholar]
- 17.Kondo, S., Hozumi, Y. & Aso, K. Long-Term organ culture of rabbit skin: Effect of EGF on epidermal structure in vitro. J. Investig. Dermatol.95, 397–402 (1990). [DOI] [PubMed] [Google Scholar]
- 18.Flaxman, B. A. & Harper, R. A. Organ culture of human skin in chemically defined medium. J. Investig. Dermatol.64, 96–99 (1975). [DOI] [PubMed] [Google Scholar]
- 19.Gherardini, J. et al. Tissue-resident macrophages can be generated de Novo in adult human skin from resident progenitor cells during substance P-mediated neurogenic inflammation ex vivo. PLoS ONE15, e0227817 (2020). [DOI] [PMC free article] [PubMed]
- 20.Lu, Z. et al. Towards the development of a simplified long-term organ culture method for human scalp skin and its appendages under serum‐free conditions. Exp. Dermatol.16, 37–44 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Knuever, J. et al. Thyrotropin-releasing hormone controls mitochondrial biology in human epidermis. J. Clin. Endocrinol. Metab.97, 978–986 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Ferwerda, B. et al. Functional consequences of Toll-like receptor 4 polymorphisms. Mol. Med.14, 346–352 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nedeva, C., Menassa, J., Puthalakath, H. & Sepsis Inflammation is a necessary evil. Front. Cell. Dev. Biol.7, 108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das, U. N. & Infection inflammation, and immunity in sepsis. Biomolecules13, 1332 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauptman, J. G., Walshaw, R. & Olivier, N. B. Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Vet. Surg.26, 393–397 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Balas, L., Durand, T. & Dihydroxylated E E,Z-docosatrienes. An overview of their synthesis and biological significance. Prog. Lipid Res.61, 1–18 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Balas, L. et al. Rapid metabolization of protectin D1 by Β oxidation of its Polar head chain. J. Med. Chem.62, 9961–9975 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Oger, C., Brinkmann, Y., Bouazzaoui, S., Durand, T. & Galano, J. M. Stereocontrolled access to isoprostanes via a Bicyclo[3.3.0]octene framework. Org. Lett.10, 5087–5090 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Ghimenti, S. et al. Salivary lactate and 8-isoprostaglandin F2α as potential non-invasive biomarkers for monitoring heart failure: A pilot study. Sci. Rep.10, 7441 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biagini, D. et al. Saliva as a non-invasive tool for monitoring oxidative stress in swimmers athletes performing a VO2max cycle ergometer test. Talanta216, 120979 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Biagini, D. et al. Micro-extraction by packed sorbent combined with UHPLC-ESI-MS/MS for the determination of prostanoids and isoprostanoids in dried blood spots. Talanta206, 120236 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Oliveri, P., Malegori, C., Simonetti, R. & Casale, M. The impact of signal pre-processing on the final interpretation of analytical outcomes—A tutorial. Anal. Chim. Acta. 1058, 9–17 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Pirro, V. et al. Multivariate strategies for screening evaluation of harmful drinking. Bioanalysis5, 687–699 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Quílez, C. et al. Targeting the complexity of in vitro skin models: A review of Cutting-Edge developments. J. Invest. Dermatol. 144 (12), 2650–2670 (2024). [DOI] [PubMed] [Google Scholar]
- 35.Padovani, C. M. & Yin K.Immunosuppression in sepsis: Biomarkers and specialized pro-resolving mediators. Biomedicines12 (1), 175 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, Y. et al. Arachidonic acid metabolism in health and disease. MedComm. 4 (5), e363 (2023). [DOI] [PMC free article] [PubMed]
- 37.Johnson, G. J. Handbook of platelet physiology and pharmacology. 38–79 (1999).
- 38.Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol.188, 21–28 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, T. Role of PGE2 in blood pressure regulation. Curr. Hypertens. Rev.6, 199–209 (2010). [Google Scholar]
- 40.Cracowski, J. L., Durand, T. & Bessard, G. Isoprostanes as a biomarker of lipid peroxidation in humans: Physiology, pharmacology and clinical implications. Trends Pharmacol. Sci.23 (8), 360–366 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Catalli, A., Zhang, D. & Janssen, L. J. Receptors and signaling pathway underlying relaxations to isoprostanes in canine and Porcine airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol.283, L1151–L1159 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Serhan, C. N., Chiang, N. & Van Dyke, T. E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol.8 (5), 349–361 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalli, J., Gomez, E. A. & Jouvene, C. C. Utility of the specialized pro-resolving mediators as diagnostic and prognostic biomarkers in disease. Biomolecules12 (3), 353 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serhan, C. N., Chiang, N. & Nshimiyimana, R. Low-dose pro-resolving mediators temporally reset the resolution response to microbial inflammation. Mol. Med. (Camb. Mass). 30 (1), 153 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jundi, B. et al. Inflammation resolution circuits are uncoupled in acute sepsis and correlate with clinical severity. JCI Insight. 6 (15), e148866 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).