Abstract
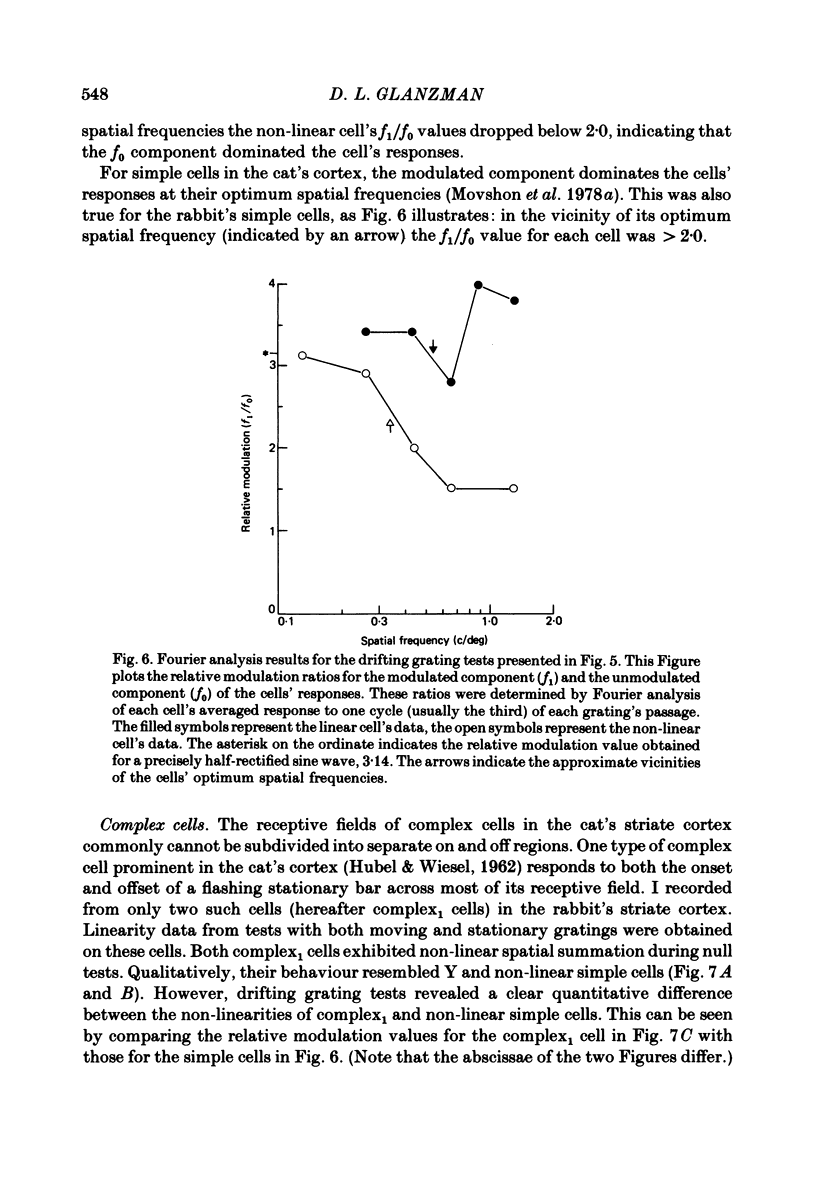
The rabbit's striate cortex contains a variety of receptive-field types, including concentric, uniform, simple and complex types. The spatial summation of these receptive fields was studied. Two types of linearity tests were performed: null tests and drifting grating tests. For the null tests, stimuli were sine-wave gratings, temporally modulated by either a 1 Hz sine-wave or by a 1 Hz on-off square wave. For the drifting grating tests, the stimuli were sine-wave gratings moved across the cells' receptive fields at 1-3 Hz. Fourier analyses were performed on the averaged data from both the null tests (sine-wave modulation) and the drifting grating tests. Thirty-one concentric cells were studied. Of these, nine were classified as X and fourteen as Y based upon their responses to ratings. The X cells exhibited linear spatial summation; their responses were mainly at the fundamental modulation frequency. The Y cells exhibited non-linear spatial summation. Their responses contained, in addition to a fundamental component, a second harmonic component whose relative strength increased as the spatial frequency of the display was raised. Eight other concentric cells responded poorly to gratings and were classified as sluggish. Other properties of concentric cells were examined, particularly their responses to standing contrast. The X cells' responses were generally sustained, the Y cells' responses generally transient. Sluggish cells were either sustained or transient, but their responses to standing contrast could frequently be distinguished from those of X and Y cells. All five uniform cells studied exhibited non-linear spatial summation. Their behaviour on the linearity tests was indistinguishable from that of Y cells. Of the seventeen simple cells studied, seven behaved like X cells (linear simple) and ten like Y cells (non-linear simple). However, near their optimum spatial frequencies the responses of both linear and non-linear simple cells were dominated by the fundamental component. Two complex cells behaved like complex cells in the cat's striate cortex (Movshon, Thompson & Tolhurst, 1978b). Their responses were qualitatively like those of non-linear simple cells, but were quantitatively more non-linear. Near their optimum spatial frequencies, harmonic components dominated their responses. The behaviour of nine other complex cells was difficult to describe. Generally, they were unresponsive to gratings. When they could be made to respond to gratings, they gave phase-sensitive responses like those of simple cells.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. New properties of rabbit retinal ganglion cells. J Physiol. 1978 Mar;276:257–276. doi: 10.1113/jphysiol.1978.sp012232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K. L., Lindsley D. F. Influences of residual eye movements in single-unit studies of the visual system. Brain Res. 1968 May;8(2):385–388. doi: 10.1016/0006-8993(68)90060-7. [DOI] [PubMed] [Google Scholar]
- Chow K. L., Masland R. H., Stewart D. L. Receptive field characteristics of striate cortical neurons in the rabbit. Brain Res. 1971 Oct 29;33(2):337–352. doi: 10.1016/0006-8993(71)90107-7. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. Topographical relationships between the anatomy and physiology of the rabbit visual system. Doc Ophthalmol. 1971 Sep 12;30:33–159. doi: 10.1007/BF00142518. [DOI] [PubMed] [Google Scholar]
- Kato H., Bishop P. O., Orban G. A. Hypercomplex and simple/complex cell classifications in cat striate cortex. J Neurophysiol. 1978 Sep;41(5):1071–1095. doi: 10.1152/jn.1978.41.5.1071. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J Physiol. 1967 Feb;188(3):285–307. doi: 10.1113/jphysiol.1967.sp008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Receptive field organization of complex cells in the cat's striate cortex. J Physiol. 1978 Oct;283:79–99. doi: 10.1113/jphysiol.1978.sp012489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol. 1978 Oct;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. H., Berman N. The rabbit and the cat: a comparison of some features of response properties of single cells in the primary visual cortex. J Comp Neurol. 1979 Dec 1;188(3):401–427. doi: 10.1002/cne.901880305. [DOI] [PubMed] [Google Scholar]
- Oyster C. W., Takahashi E., Levick W. R. Information processing in the rabbit visual system. Doc Ophthalmol. 1971 Sep 12;30:161–204. doi: 10.1007/BF00142519. [DOI] [PubMed] [Google Scholar]
- Rose J. E., Malis L. I. Geniculo-striate connections in the rabbit. II. Cytoarchitectonic structure of the striate region and of the dorsal lateral geniculate body; organization of the geniculo-striate projections. J Comp Neurol. 1965 Aug;125(1):121–137. doi: 10.1002/cne.901250109. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Finlay B. L., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. I. Spatiotemporal organization of receptive fields. J Neurophysiol. 1976 Nov;39(6):1288–1319. doi: 10.1152/jn.1976.39.6.1288. [DOI] [PubMed] [Google Scholar]
- Shapley R., Hochstein S. Visual spatial summation in two classes of geniculate cells. Nature. 1975 Jul 31;256(5516):411–413. doi: 10.1038/256411a0. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. Organization of cat striate cortex: a correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol. 1975 Sep;38(5):1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- So Y. T., Shapley R. Spatial properties of X and Y cells in the lateral geniculate nucleus of the cat and conduction veolcities of their inputs. Exp Brain Res. 1979 Aug 1;36(3):533–550. doi: 10.1007/BF00238521. [DOI] [PubMed] [Google Scholar]
- Stewart D. L., Chow K. L., Masland R. H. Receptive-field characteristics of lateral geniculate neurons in the rabbit. J Neurophysiol. 1971 Jan;34(1):139–147. doi: 10.1152/jn.1971.34.1.139. [DOI] [PubMed] [Google Scholar]
- Sur M., Sherman S. M. Linear and nonlinear W-cells in C-laminae of the cat's lateral geniculate nucleus. J Neurophysiol. 1982 May;47(5):869–884. doi: 10.1152/jn.1982.47.5.869. [DOI] [PubMed] [Google Scholar]
- Toyoda J. Frequency characteristics of retinal neurons in the carp. J Gen Physiol. 1974 Feb;63(2):214–234. doi: 10.1085/jgp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]


