Abstract
Malvidin-3-glucoside (M3G), an anthocyanin found in blueberries and grapes, shows promise as a natural anti-diabetic agent. However, its effect on insulin secretion and its underlying mechanisms remains unclear. This study investigated the impact of M3G on β-cells (INS-1) through real-time Ca2+ imaging and insulin secretion assays. M3G increased intracellular Ca2+ levels in a concentration-dependent manner, specifically targeting β-cells without affecting other pancreatic cell types. It enhanced insulin secretion under both basal (4 mM) and stimulatory (11 mM) glucose conditions while maintaining cell viability at concentrations up to 100 µM. Pharmacological inhibitors revealed that M3G-induced Ca2+ signals resulted from both Ca influx through L-type voltage-dependent calcium channels (L-type VDCCs) and Ca2+ release from the endoplasmic reticulum (ER) via the PLC/IP3 pathway. Nimodipine, an L-type VDCC blocker, inhibited M3G-induced Ca2+ influx, while U73122 (a PLC inhibitor) and 2-aminoethoxydiphenyl borate (2-APB), an IP3 receptor blocker, suppressed Ca2+ release from the ER. Additionally, M3G upregulated the expression of key glucose-stimulated insulin secretion (GSIS)-related genes, including Ins1 (insulin), Slc2a2 (GLUT2), and Gck (glucokinase). These findings suggest that M3G stimulates insulin secretion by promoting Ca2+ influx through L-type VDCCs, facilitating Ca2+ release from the ER, and upregulating GSIS-related genes. M3G holds promise as a natural anti-diabetic agent by enhancing insulin secretion and supporting β-cell function.
Keywords: Malvidin-3-glucoside, Pancreatic β-cells, Insulin secretion, Calcium signals, L-type voltage-dependent Ca2+ channels, PLC/IP3
Subject terms: Type 2 diabetes, Preventive medicine
Introduction
Inadequate insulin secretion is a hallmark of type 2 diabetes mellitus (T2DM). In healthy individuals, pancreatic β-cells maintain glucose homeostasis by secreting insulin through the activation of glucose-stimulated insulin secretion (GSIS). This process begins with glucose metabolism, generating ATP that closes ATP-sensitive K+ (KATP) channels and opens L-type voltage-dependent Ca2+ channels (L-type VDCCs). The influx of Ca2+ through L-type VDCCs increases intracellular Ca2+, triggering the exocytosis of insulin-containing granules1. However, chronic hyperglycemia impairs insulin secretion process, resulting in glucose homeostasis imbalance2. Prolonged exposure to high glucose induces excessive production of reactive oxygen species, which disrupts β-cell function through several damaging mechanisms3,4. Oxidative stress impairs mitochondria metabolism, reducing ATP production and insulin secretion5. It also downregulates key β-cell genes, including insulin, glucose transporter 2 (GLUT2), and glucokinase, further decreasing insulin synthesis and secretion6,7. Given the critical role of β-cell dysfunction in T2DM progression, developing strategies to enhance insulin secretion have become a major focus of diabetes research.
Several approaches aim to improve insulin secretion by targeting different steps of the secretion pathway. Modulating ion channels such as inhibiting KATP channels or activating L-type VDCCs can increase Ca2+ signals in β-cells, leading to insulin release8. Additionally, amplifying intracellular signaling pathways, including the phospholipase C (PLC)/inositol-1,4,5-trisphosphate (IP3), cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA), or phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathways, has been shown to enhance Ca2+ signaling and insulin granule exocytosis9–11. Improving the expression of key genes involved in insulin secretion also enhances β-cell function, as demonstrated in vitro using rodent pancreatic β-cells12,13.
Anthocyanins, a class of natural bioactive compounds, have drawn interest for their potential anti-diabetic effects due to their insulinotropic properties. Various types of anthocyanins have been reported to enhance insulin secretion through different mechanisms. For example, cyanidin stimulates insulin release by activating L-type VDCCs14. In contrast, its glycosylated form, cyanidin-3-rutinoside, demonstrates a broader range of activity by activating both L-type VDCCs and the phospholipase C/inositol trisphosphate (PLC/IP3) pathway15. Cyanidin 3-O-glucoside facilitates insulin secretion and upregulates the phosphorylation of the insulin receptor and insulin receptor substrate 1, while also promoting the expression of phosphoinositide 3-kinase (PI3K) proteins in INS-1 cells16. Other anthocyanins, such as delphinidin and pelargonidin, also stimulate insulin secretion in rodent pancreatic β-cells in vitro17.
In the study, we investigated the potential of glycosylated malvidin (M3G) to stimulate insulin secretion in rat INS-1 pancreatic β-cells. Real-time Ca2+ imaging was employed to explore its mechanism of action, insulin assays were used to quantify hormone secretion, and RT-qPCR was performed to assess the expression of GSIS-related genes.
Results
M3G increases intracellular Ca2+ and insulin secretion in pancreatic β-cells
Jayaprakasam and colleagues previously reported that the aglycone form of malvidin did not stimulate insulin secretion in INS-1 β-cells17. Based on this, we explored the potential of glycosylated malvidin (M3G) in promoting insulin secretion. Since an increase in intracellular Ca2+ is crucial for hormone secretion, we examined the effect of M3G on intracellular Ca2+ signaling across three primary types of pancreatic cells: α-cells (αTC1-6), β-cells (INS-1), and δ-cells (RIN-14B). Stimulation of cells with 100 μM M3G increased intracellular Ca2+ in insulin-secreting INS-1 cells but not in glucagon-secreting αTC1-6 cells or somatostatin-secreting RIN-14B cells (Fig. 1a and b). Among the three cell types, β-cells were the most responsive to cell depolarization caused by KCl treatment, while somatostatin-secreting δ-cells did not produce Ca2+ signals in response to KCl. Furthermore, stimulation of INS-1 cells with M3G (1–100 μM) led to a concentration-dependent increase in intracellular Ca2+, with the highest Ca2+ signals observed at 100 µM M3G (Fig. 1c and d). Since elevated intracellular Ca2+ is essential for insulin secretion, insulin levels were measured in response to M3G under basal glucose (4 mM) and stimulatory glucose (11 mM) conditions. Exposure of cells to 11 mM glucose alone increased insulin secretion 1.3-fold compared to 4 mM basal glucose. Under basal glucose conditions, 30-min exposure to 60 and 100 μM M3G significantly increased insulin secretion by 1.4- and 1.8-fold, respectively. Under stimulatory glucose conditions, 60 and 100 μM M3G enhanced insulin release by 1.3- and 1.8-fold, respectively (Fig. 1e). To assess whether the M3G concentrations used affected cell viability, an MTT assay was performed. Treatment of INS-1 cells with M3G (1–100 µM) for 24-h did not reduce cell viability compared to untreated control cells, while 300 μM M3G led to 14% decrease in cell survival (Fig. 1f).
Fig. 1.
M3G-induced intracellular Ca2+ signals and insulin secretion. (a) Average intracellular Ca2+ traces from α-cells (αTC1-6), β-cells (INS-1) and δ-cells (RIN-14B) following stimulation with 100 μM M3G. (b) Average peak Ca2+ signals from cells shown in panel a. (c) Average intracellular Ca2+ traces from INS-1 cells stimulated with M3G (1–100 μM). (d) Average peak Ca2+ signals from cells shown in panel c. (e) Dose–response of M3G on insulin secretion. (f) Cell viability after 24-h exposure to M3G. Results are presented as mean ± SEM from three independent experiments: n = 50–150 cells/group in real-time Ca2+ imaging experiments, n = 3 wells/group in insulin secretion experiments. Groups with different letters indicate statistical significance (P < 0.05).
Sources of Ca2+ signals for M3G
Intracellular Ca2+ signals can be caused by influx from the extracellular space and/or a release from internal stores (e.g., ER). To determine the Ca2+ sources contributing to the M3G-induced signals, Ca2+ measurements were conducted under extracellular Ca2+-free conditions and/or after depletion of ER Ca2+ stores using 1 μM thapsigargin. The absence of extracellular Ca2+ significantly reduced M3G-induced intracellular Ca2+ signals, and a decrease was also observed with ER Ca2+ depletion (Fig. 2a). When both extracellular Ca2+ was removed and ER stores were depleted, M3G-induced Ca2+ signals were completely abolished (Fig. 2a and b). The findings indicate that M3G-induced intracellular Ca2+ signals depend on both extracellular Ca2+ influx and the release of Ca2+ from ER stores.
Fig. 2.
Sources of Ca2+ signals in response to M3G. (a) Average intracellular Ca2+ traces from cells treated with thapsigargin (TG) and/or maintained in an extracellular Ca2+-free buffer. M3G-induced Ca2+ signals were partially inhibited by endoplasmic reticulum (ER) depletion with 1 μM thapsigargin or by removal of extracellular Ca2+. (b) Average peak Ca2+ signals from cells shown in panel a. Results are presented as mean ± SEM from three independent experiments: n = 180–250 cells/group. Groups with different letters indicate statistical significance (P < 0.05).
M3G induces Ca2+ influx by opening L-type VDCCs
Calcium influx into pancreatic β-cells primarily depends on the activation of L-type VDCCs. To examine the role of L-type VDCCs in M3G-induced Ca2+ signals, the L-type VDCC blocker nimodipine was used. Pretreatment with nimodipine (3–100 μM) inhibited intracellular Ca2+ signals to 100 μM M3G in a concentration-dependent manner, with complete inhibition at 30 and 100 μM nimodipine (Fig. 3a and b).
Fig. 3.
M3G-induced Ca2+ influx via L-type VDCCs. (a) Average intracellular Ca2+ traces from cells pretreated with 1–100 μM nimodipine following stimulation with 100 μM M3G. Treatment with nimodipine inhibited M3G-induced Ca2+ signals in a concentration-dependent manner. (b) Average peak Ca2+ signals from cells shown in panel a. Results are presented as mean ± SEM from three independent experiments: n = 200–250 cells/group. Groups with different letters indicate statistical significance (P < 0.05).
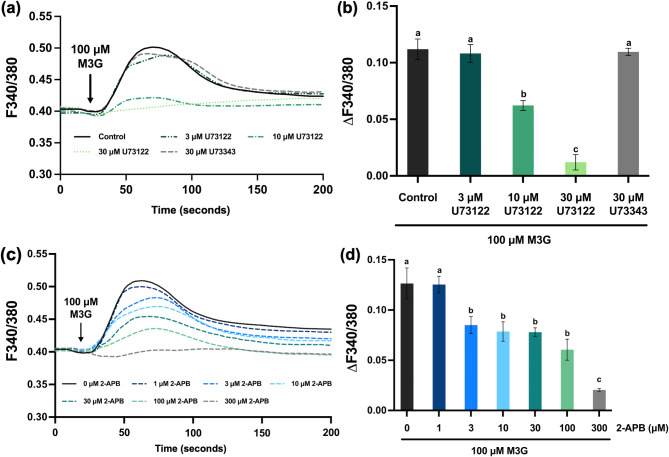
Activation of the PLC/IP3 pathway by M3G
Activation of the PLC/IP3 pathway leads to Ca2+ release from the ER, which increases intracellular Ca2+ signals. To determine if this pathway plays a role in M3G-induced intracellular Ca2+ signals, the PLC inhibitor U73122 was tested. Pretreatment with U73122 (3–30 μM) inhibited M3G-induced intracellular Ca2+ signals in a concentration-dependent manner compared to controls without U73122 or 30 μM U73343, an inactive analog of U73122 (Fig. 4a and b). Since activation of the PLC/IP3 pathway leads to IP3 production, which binds to its receptor in the ER to release Ca2+ into the cytosol, we also examined the effect of 2-aminoethoxydiphenyl borate (2-APB), an IP3 receptor blocker. Pretreatment with 2-APB (3–300 μM) inhibited M3G-induced Ca2+ signals in a concentration-dependent manner, with significant reduction observed at 300 μM 2-APB (Fig. 4c and d). These findings demonstrate the involvement of the PLC/IP3 pathway in M3G’s mechanism of action.
Fig. 4.
Involvement of the PLC/IP3 pathway on M3G-induced intracellular Ca2+ signals. (a) Average intracellular Ca2+ traces from cells pretreated with 3–30 μM U73122 following stimulation with 100 μM M3G. Increasing concentrations of U73122 (a PLC inhibitor) inhibited intracellular Ca2+ signals in a concentration-dependent manner. (b) Average peak Ca2+ signals from cells shown in panel a. (c) Average intracellular Ca2+ traces from cells pretreated with 1–300 μM 2-APB (an IP3 receptor blocker) following stimulation with 100 μM M3G. Treatment with 300 μM 2-APB completely abolished M3G-induced intracellular Ca2+ signals. (d) Average peak Ca2+ signals from cells shown in panel c. Results are presented as mean ± SEM from three independent experiments: n = 200–250 cells/group. Groups with different letters indicate statistical significance (P < 0.05).
M3G upregulates glucose-stimulated insulin secretion related genes
The results demonstrated that M3G effectively increases intracellular Ca2+ signals and stimulates insulin secretion from pancreatic β-cells. Given that glucose is the primary regulator of insulin secretion, the effect of M3G on the expression of key genes involved in GSIS was further examined. Cells were treated with 100 μM M3G for 24 h, and RNA collected at 2, 4, 6, 12, and 24 h for RT-qPCR analysis. After 24 h of M3G exposure, a significant upregulation in the expression of Ins, Slc2a2, and Gck gene, which encode insulin, GLUT2, and glucokinase, respectively, was observed (Fig. 5). Additionally, Kcnj11 expression, which encodes the Kir6.2 subunit of KATP channels, exhibited an upward trend after 24 h.
Fig. 5.
M3G upregulated genes involved in glucose-stimulated insulin secretion. Cells were maintained in 11 mM glucose and treated with 100 μM M3G. mRNA was collected at 0, 2, 4, 6, 12, and 24 h. The fold change in mRNA expression for the following genes was measured, including (a) Ins1 (insulin), (b) Slc2a2 (GLUT2), (c) Gck (glucokinase), (d) Cacna1c (Cav1.2), and (e) Kcnj11 (Kir6.2). Results are presented as mean ± SD from three independent experiments. * p < 0.05; ** p < 0.01.
Discussion
The results of this study demonstrate that M3G can stimulate insulin secretion from pancreatic β-cells under both basal glucose and glucose-stimulated insulin secretion (GSIS) conditions without affecting cellular viability. This stimulation is achieved by increasing intracellular Ca2+ signals through the activation of L-type VDCCs and the release of Ca2+ from the ER (Fig. 6). Notably, the addition of a glucose moiety to the malvidin structure (M3G) allowed the compound to stimulate insulin secretion in β-cells by elevating intracellular Ca2+ levels. This effect was specific to β-cells, with no similar response observed in α- or δ-cells. This specificity enhances the potential of M3G to promote insulin secretion efficiently while minimizing effects on other pancreatic cell types.
Fig. 6.
Proposed mechanism for insulin secretion by M3G. The mechanism involves the activation of L-type VDCCs, which promotes Ca2+ influx. Additionally, M3G activates the PLC/IP3 pathway, leading to the release of Ca2+ from the ER and insulin secretion. M3G also upregulated the expression of Ins1 (insulin), Slc2a2 (GLUT2), and Gck (glucokinase) genes. The image was created using Microsoft® PowerPoint for Mac (Version 16.94, available at: https://www.microsoft.com/powerpoint). The chemical structure of M3G was drawn using Marvin JS by ChemAxon (Version 24.3.187, available at: https://chemaxon.com/products/marvin).
Furthermore, M3G appears to exert its effects primarily through L-type VDCCs. Experiments with nimodipine demonstrated that these channels partially mediate M3G’s mechanism of action in β-cells, as nimodipine effectively inhibited the intracellular Ca2+ signals by M3G. This finding is consistent with previous studies on quercetin and cyanidin, which have shown that flavonoids activate L-type VDCCs in β-cells, leading to Ca2+ influx and subsequent insulin secretion14,18. Although L-type VDCCs are key regulators of Ca2+ influx, other type of calcium channels also modulate intracellular Ca2+ dynamics and insulin secretion19. Further studies on their involvement in M3G-induced insulin secretion would provide valuable mechanistic insights.
The increase in intracellular Ca2+ may also be associated with the activation of amplifying pathways in β-cells. Specifically, activation of the PLC/IP3 pathway generates IP3, which elevates intracellular Ca2+ by promoting its release from the endoplasmic reticulum (ER)20. The role of the PLC/IP3 pathway in the effect of M3G is supported by the observed inhibition of Ca2+ signals when cells were treated with U73122 (a PLC inhibitor) and 2-APB (an IP3 receptor blocker). These results indicate that M3G induces Ca2+ release from the ER through the activation of the PLC/IP3 pathway. Overall, M3G exhibits a dual mechanism that enhances intracellular Ca2+ signals, potentially facilitating insulin secretion. Glycosylated flavonoids, including rutin (quercetin-3-rutinoside) and cyanidin-3-rutinoside stimulate insulin secretion by elevating intracellular Ca2+ signals through the activation of L-type VDCCs. Additionally, these compounds promote the release of Ca2+ from the ER via the PLC/IP3 signaling pathway, a mechanism that parallels the effects observed with M3G15,21. In contrast, the aglycone forms, such as quercetin and cyanidin, primarily enhance insulin secretion by activating L-type VDCCs without facilitating ER Ca2+ release14,18. Furthermore, investigations into the aglycone malvidin indicate that the absence of a glycoside moiety may restrict its ability to stimulate insulin secretion17. This finding emphasizes the critical role of the glycoside attached to the phenyl ring of M3G in mediating its insulinotropic effects, highlighting its potential as a key factor in enhancing β-cell function. The glycoside moiety on M3G may improve its ability to interact with cellular receptors or proteins with higher affinity, thereby enhancing its biological efficacy22.
In type 2 diabetes mellitus (T2DM), β-cell dysfunction leads to reduced basal insulin secretion and impaired glucose-stimulated insulin secretion (GSIS), resulting in fasting and postprandial hyperglycemia23–25. In this study, M3G was shown to increase insulin secretion under both basal and GSIS conditions. Similar effects have been observed with other flavonoids, including epicatechin, quercetin, cyanidin-3-glucoside, and delphinidin-3-glucoside, which enhance insulin release in both settings16,17,26,27. The dual action of M3G suggests that it could restore basal insulin secretion and improve β-cell sensitivity to glucose, potentially offering therapeutic benefits for managing hyperglycemia in T2DM. Moreover, Grace et al.28 reported that M3G reduces fasting blood glucose levels in diabetic C57BL/6J mice within 6 h, suggesting hypoglycemic activity. While the precise mechanism of this effect remains unclear, it is likely related to M3G’s ability to stimulate insulin secretion. Further research is necessary to elucidate the effects of M3G on insulin release and glucose-induced insulin secretion in animal models. This would provide valuable insights into its potential as a therapeutic option for T2DM treatment.
The optimal concentration of M3G for promoting insulin secretion and increasing intracellular Ca2+ in pancreatic β-cells was found at 100 µM. The cell viability assays showed that a 24-hour treatment with M3G at concentrations up to 100 µM did not negatively affect cell viability. However, higher concentrations were cytotoxic to INS-1 cells, aligning with previous studies on human hepatoblastoma (HepG2) cells, which reported cytotoxicity at M3G concentrations above 100 µM29. These findings highlight the importance of using appropriate M3G concentrations, which effectively stimulate insulin secretion without harming β-cell viability, positioning M3G as a potential candidate for long-term therapeutic strategies to improve glucose control in T2DM.
One promising approach to enhancing insulin secretion is by boosting β-cell gene expression, as the downregulation of GSIS-related genes is associated with impaired insulin secretion30–33. Experiments examining the effect of M3G on gene expression revealed that a 24-hour exposure to M3G significantly upregulated the expression of a wide range of GSIS-related genes, including Ins1, Slc2a2, and Gck. Our results indicate that the insulinotropic effect of M3G is mediated by the activation of the PLC enzyme. The activation of PLC generates diacylglycerol (DAG), in addition to IP3, which directly activate protein kinase C (PKC), leading to enhanced β-cell gene expression34,35. This mechanism likely explains the M3G-induced upregulation of β-cell gene expression, and differs from other anthocyanins, such as cyanidin and cyanidin-3-rutinoside, which selectively enhance Slc2a2 and Kcnj11 within 6 h in INS-1 cells14,15. The longer response time and broader gene upregulation suggest that M3G may provide sustained insulin secretion and protect β-cell function from chronic metabolic stress, potentially offering long-term therapeutic benefits.
Materials and methods
Reagents
All reagents were purchased from ThermoFisher Co. (Waltham, MA, USA). The fura-2 acetoxymethyl ester (Fura-2AM) was purchased from Cayman Chemical (Ann Arbor, MI, USA). Ultrasensitive rat insulin ELISA was purchased from Mercodia AB (Uppsala, Sweden). Malvidin-3-glucoside (M3G) was purchased from Extrasynthèse (Genay, France).
Cell culture
Mouse pancreatic α-cells (αTC1-6) were purchased from the American Type Culture Collection (Manassas, VA, USA), rat pancreatic δ-cells (RIN-14B) were purchased from AddexBio (San Diego, CA, USA), and rat pancreatic β-cells (INS-1) were purchased from Sigma Aldrich (St. Louis, MO, USA). αTC1-6 and RIN-14B cells were cultured in RPMI 1640 media supplemented with 10% (v/v) fetal bovine serum (FBS). INS-1 cells were cultured in RPMI 1640 media supplemented with 50 μM 2-mercaptoethanol, 1 mM sodium-pyruvate, 2 mM L-glutamine, and 10% (v/v) FBS. Cells were cultured under 5% CO2 at 37 °C. The experiments were performed using αTC1-6 cells from passages 35 to 40, INS-1 cells from passages 70 to 89, and RIN-14B cells from passages 15 to 20.
Cell viability
Cell viability was determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay. The MTT stock solution (5 mg/mL) was diluted with cell culture medium to obtain a final concentration of 0.5 mg/mL. INS-1 cells were plated into 96-well plates at 1  104 cells/well for 48 h. The cell culture media was replaced by cell culture media containing M3G (1–300 μM) and incubated at 37 °C for 24 h. 100 mL of MTT solution (0.5 mg/mL) was added to each well, followed by incubation at 37 °C for 3 h. After removing the MTT solution, cells were incubated with dimethyl sulfoxide (100 μL/well) for 15 min to dissolve formazan crystals. The absorbance was measured at 550 nm using a spectrophotometer (Perkin Elmer, Waltham, MA, USA).
104 cells/well for 48 h. The cell culture media was replaced by cell culture media containing M3G (1–300 μM) and incubated at 37 °C for 24 h. 100 mL of MTT solution (0.5 mg/mL) was added to each well, followed by incubation at 37 °C for 3 h. After removing the MTT solution, cells were incubated with dimethyl sulfoxide (100 μL/well) for 15 min to dissolve formazan crystals. The absorbance was measured at 550 nm using a spectrophotometer (Perkin Elmer, Waltham, MA, USA).
Quantification of insulin secretion
INS-1 cells were plated into 24-well plates at 5 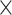 105 cells/well and grown for 48 h until ~ 90% confluent. The cell culture media was replaced by Krebs–Ringer bicarbonate buffer (KRB) containing 136 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5 mM NaHCO3, 10 mM HEPES, 4 mM Glucose, and 0.1% (w/v) BSA, at pH 7.4. After a 30 min equilibration period at 37 °C, the KRB was removed, and the KRB containing M3G (10–100 μM) in 4 mM or 11 mM glucose was added to stimulate insulin secretion for 30 min. At the end, the KRB was collected and stored at -80 °C for insulin measurement using ultrasensitive rat insulin ELISA kit following the manufacturer’s protocol. Experiments were performed in triplicate (3 wells/group) and repeated with three different cell passages.
105 cells/well and grown for 48 h until ~ 90% confluent. The cell culture media was replaced by Krebs–Ringer bicarbonate buffer (KRB) containing 136 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5 mM NaHCO3, 10 mM HEPES, 4 mM Glucose, and 0.1% (w/v) BSA, at pH 7.4. After a 30 min equilibration period at 37 °C, the KRB was removed, and the KRB containing M3G (10–100 μM) in 4 mM or 11 mM glucose was added to stimulate insulin secretion for 30 min. At the end, the KRB was collected and stored at -80 °C for insulin measurement using ultrasensitive rat insulin ELISA kit following the manufacturer’s protocol. Experiments were performed in triplicate (3 wells/group) and repeated with three different cell passages.
Real-time Ca2+ imaging analysis
INS-1 cells were plated into round glass coverslips and grown for 48 h. For experiments, cells were washed with a Ca2+ imaging buffer containing 136 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 10 mM HEPES, 4 mM Glucose, and 0.1% (w/v) BSA, at pH 7.4, and incubated with 2 μM Fura-2AM at 37 °C for 30 min. The Ca2+ imaging buffer was used throughout the experiments except in the extracellular Ca2+-free conditions where CaCl2 in the buffer was removed and 10 μM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) was added. In experiments utilizing pharmacological blockers (thapsigargin, nimodipine, U73312, U73343, and 2-APB), INS-1 cells were pretreated with the respective blocker for 20 min prior to M3G stimulation. Intracellular Ca2+ signals were recorded using a dual excitation fluorometric imaging system (Excelitas® Technologies, Waltham, MA) controlled by MetaFluor® software (Molecular Devices, San Jose, CA). Fura-2AM loaded cells were excited at wavelengths of 340 nm and 380 nm, with emission detected at 510 nm. Fluorescence emissions were collected and converted into F340/F380 ratio. Results were presented as average Ca2+ traces or peak Ca2+ signals from three different cell passages.
RT-qPCR
INS-1 cells were maintained in RPMI 1640 medium containing 11 mM glucose and treated with 100 µM M3G. Total RNA was extracted at 0, 2, 4, 6, 12, and 24 h with TRIzol reagent. Total RNA extraction was performed by bromochloropropane (BCP) separation and isopropanol precipitation method. To eliminate potential DNA contamination, the RNA was treated with Turbo™ DNase digestion. The quality and purity (OD260/280 > 1.8) of the extracted RNA were assessed using a Nanodrop spectrometer. Equal amounts of RNA from each treatment group were reverse transcribed into cDNA using M-MLV reverse transcriptase (Invitrogen, Waltham, MA, USA) following the manufacturer’s protocol. For real-time PCR analysis, the cDNA was combined with an iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and rat gene-specific primers for real-time PCR to obtain Ct values. The relative gene expression was calculated using the 2-∆∆Ct method, where ∆Ct = Ct (target gene) – Ct (reference gene) and ∆∆Ct = ∆Ct (M3G-treated sample at each time point)—∆Ct (time-matched untreated control)36. The experiments were repeated three times with different cell passages. Results were expressed as the relative mRNA expression of gene-specific rat primers: Ins1 (insulin), Slc2a2 (GLUT2), Gck (glucokinase), Cacna1c (Cav1.2), Kcnj11 (Kir6.2), and Actb (β-actin). Actb was used as the reference gene. The primers used were the same as those from the previous study37.
Statistical analysis
The results were presented as mean ± standard error of mean (SEM) or mean ± standard deviation (SD). The mean values were calculated based on data from three independent experiments. A one-way ANOVA was used to compare the difference between groups for cell viability, insulin release, and Ca2+ peak signals, followed by multiple comparisons of Duncan’s post hoc test. An unpaired Student’s t-test was used to compare the difference in relative gene expression between the control and M3G-treated groups at various time points. The data analysis and graph plotting were performed using GraphPad Prism version 10.0.3 (Boston, MA, USA) and SPSS Statistics version 29.0.2.0 (SPSS Inc., Chicago, IL, USA). The statistical significance was determined at P < 0.05.
Conclusion
This study demonstrated that M3G stimulates insulin secretion by increasing intracellular Ca2+ without compromising cell viability. This insulinotropic effect is mediated through Ca2+ influx via L-type VDCCs and the release of Ca2+ from the ER through activation of the PLC/IP3 pathway in pancreatic β-cells. Additionally, M3G upregulates genes associated with GSIS, including Ins1, Slc2a2, and Gck referring to insulin, GLUT2, and glucokinase, respectively. These findings suggest that M3G holds promise as a natural anti-diabetic agent by enhancing insulin secretion and improving β-cell function.
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- ER
Endoplasmic reticulum
- GK
Glucokinase
- GLUT2
Glucose transporter 2
- GSIS
Glucose-stimulated insulin secretion
- Ins
Insulin
- IP3
Inositol-1,4,5-trisphosphate
- KATP
ATP-sensitive K+ channel
- KRB
Krebs–Ringer bicarbonate buffer
- M3G
Malvidin-3-glucoside
- PLC
Phospholipase C
- VDCC
Voltage-dependent Ca2+ channel
Author contributions
S.Y., H.C., T.S., S.A.: Conceptualization, supervision and methodology; P.C., Y.Y.: Investigation; S.Y., F.V.B., H.C., S.A.: Resources; P.C. and S.Y.: Data curation; S.A., H.C.: Funding acquisition; P.C.: Writing—original draft; S.Y., F.V.B., H.C., T.S., S.A.: Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Council of Thailand (NRCT): NRCT5-RGJ63001-014 and N42A680622, and by an OSU-CVM RAC grant.
Data availability
The data generated or analysed during this study are available from the corresponding author upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Henrique Cheng, Email: henrique.cheng@okstate.edu.
Tanyawan Suantawee, Email: Tanyawan.S@chula.ac.th.
References
- 1.Kojima, I., Medina, J. & Nakagawa, Y. Role of the glucose-sensing receptor in insulin secretion. Diabetes. Obes. Metab.19(Suppl 1), 54–62. 10.1111/dom.13013 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Khin, P. P., Lee, J. H. & Jun, H.-S. Pancreatic beta-cell dysfunction in type 2 diabetes. Eur. J. Inflamm.21, 17217X27231154152. 10.1177/1721727X231154152 (2023). [Google Scholar]
- 3.Fu, J. et al. The impairment of glucose-stimulated insulin secretion in pancreatic β-cells caused by prolonged glucotoxicity and lipotoxicity is associated with elevated adaptive antioxidant response. Food. Chem. Toxicol.100, 161–167. 10.1016/j.fct.2016.12.016 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Robertson, R. P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem.279, 42351–42354. 10.1074/jbc.R400019200 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Rolo, A. P. & Palmeira, C. M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol.212, 167–178. 10.1016/j.taap.2006.01.003 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Eguchi, N., Vaziri, N. D., Dafoe, D. C. & Ichii, H. The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int. J. Mol. Sci.22, 1509. 10.3390/ijms22041509 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumel-Alterzon, S. & Scott, D. K. Regulation of Pdx1 by oxidative stress and Nrf2 in pancreatic beta-cells. Front Endocrinol.13, 1011187. 10.3389/fendo.2022.1011187 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velasco, M., Díaz-García, C. M., Larqué, C. & Hiriart, M. Modulation of ionic channels and insulin secretion by drugs and hormones in pancreatic beta cells. Mol. Pharmacol.90, 341. 10.1124/mol.116.103861 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Bisht, S. & Singh, M. F. The triggering pathway, the metabolic amplifying pathway, and cellular transduction in regulation of glucose-dependent biphasic insulin secretion. Arch. Physiol. Biochem.130, 854–865. 10.1080/13813455.2023.2299920 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Kalwat, M. A. & Cobb, M. H. Mechanisms of the amplifying pathway of insulin secretion in the β cell. Pharmacol. Ther.179, 17–30. 10.1016/j.pharmthera.2017.05.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oakie, A. & Wang, R. β-cell receptor tyrosine kinases in controlling insulin secretion and exocytotic machinery: C-kit and insulin receptor. Endocrinology159, 3813–3821. 10.1210/en.2018-00716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya, S., Oksbjerg, N., Young, J. F. & Jeppesen, P. B. Caffeic acid, naringenin and quercetin enhance glucose-stimulated insulin secretion and glucose sensitivity in INS-1E cells. Diabetes. Obes. Metab.16, 602–612. 10.1111/dom.12236 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Saji, N., Francis, N., Schwarz, L. J., Blanchard, C. L. & Santhakumar, A. B. Rice bran phenolic extracts modulate insulin secretion and gene expression associated with β-cell function. Nutrients12, 1889. 10.3390/nu12061889 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suantawee, T. et al. Cyanidin stimulates insulin secretion and pancreatic β-cell gene expression through activation of l-type voltage-dependent ca2+ channels. Nutrients9, 814. 10.3390/nu9080814 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kongthitilerd, P. et al. Cyanidin-3-rutinoside stimulated insulin secretion through activation of L-type voltage-dependent Ca2+ channels and the PLC-IP3 pathway in pancreatic β-cells. Biomed. Pharmacother.146, 112494. 10.1016/j.biopha.2021.112494 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Lee, D., Ham, J., Kang, K. S. & Lee, H.-J. Cyanidin 3-o-glucoside isolated from lonicera caerulea fruit improves glucose response in INS-1 cells by improving insulin secretion and signaling. Bull. Korean Chem. Soc.37, 2015–2018. 10.1002/bkcs.11017 (2016). [Google Scholar]
- 17.Jayaprakasam, B., Vareed, S. K., Olson, L. K. & Nair, M. G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food. Chem.53, 28–31. 10.1021/jf049018+ (2005). [DOI] [PubMed] [Google Scholar]
- 18.Bardy, G. et al. Quercetin induces insulin secretion by direct activation of L-type calcium channels in pancreatic beta cells. Br. J. Pharmacol.169, 1102–1113. 10.1111/bph.12194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson, B. & Satin, L. S. Beta-cell ion channels and their role in regulating insulin secretion. Compr. Physiol.11, 1–21. 10.1002/cphy.c210004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang, H. J., Jang, H. J., Cocco, L. & Suh, P. G. The regulation of insulin secretion via phosphoinositide-specific phospholipase Cβ signaling. Adv. Biol. Regul.71, 10–18. 10.1016/j.jbior.2018.09.011 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Kappel, V. D. et al. The role of calcium in intracellular pathways of rutin in rat pancreatic islets: potential insulin secretagogue effect. Eur. J. Pharmacol.702, 264–268. 10.1016/j.ejphar.2013.01.055 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Křen, V. in Glycoscience: Chemistry and chemical biology (eds Bertram O. Fraser-Reid, Kuniaki Tatsuta, & Joachim Thiem) 2589–2644 (Springer Berlin Heidelberg, 2008).
- 23.Oli, J. M. et al. Basal insulin resistance and secretion in Nigerians with type 2 diabetes mellitus. Metab. Syndr. Relat. Disord.7, 595–599. 10.1089/met.2009.0002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lupi, R. & Del Prato, S. β-cell apoptosis in type 2 diabetes: Quantitative and functional consequences. Diabetes. Metab.34, S56–S64. 10.1016/S1262-3636(08)73396-2 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Guillausseau, P. J. et al. Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes Metab.34(Suppl 2), S43-48. 10.1016/s1262-3636(08)73394-9 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Kittl, M. et al. Quercetin stimulates insulin secretion and reduces the viability of rat INS-1 beta-cells. Cell. Physiol. Biochem.39, 278–293. 10.1159/000445623 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Martín, M. Á., Fernández-Millán, E., Ramos, S., Bravo, L. & Goya, L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol. Nutr. Food. Res.58, 447–456. 10.1002/mnfr.201300291 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Grace, M. H. et al. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry. Vaccinium angustifolium Aiton. Phytomedicine.16, 406–415. 10.1016/j.phymed.2009.02.018 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, Y., Zhao, L., Wang, D., Huo, Y. & Ji, B. Anthocyanin-rich extracts from blackberry, wild blueberry, strawberry, and chokeberry: Antioxidant activity and inhibitory effect on oleic acid-induced hepatic steatosis in vitro. J. Sci. Food. Agric.96, 2494–2503. 10.1002/jsfa.7370 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Kajimoto, Y. et al. Induction of glycation suppresses glucokinase gene expression in HIT-T15 cells. Diabetologia42, 1417–1424. 10.1007/s001250051313 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Thorens, B., Wu, Y. J., Leahy, J. L. & Weir, G. C. The loss of GLUT2 expression by glucose-unresponsive beta cells of db/db mice is reversible and is induced by the diabetic environment. J. Clin. Invest.90, 77–85. 10.1172/jci115858 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laybutt, D. R. et al. Genetic regulation of metabolic pathways in β-cells disrupted by hyperglycemia. J. Biol. Chem.277, 10912–10921. 10.1074/jbc.M111751200 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Minami, K., Miki, T., Kadowaki, T. & Seino, S. Roles of ATP-sensitive K+ channels as metabolic sensors: Studies of Kir6.X null mice. Diabetes. Metab.53, S176–S180. 10.2337/diabetes.53.suppl_3.S176 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Kim, M. J., Kim, E., Ryu, S. H. & Suh, P. G. The mechanism of phospholipase C-gamma1 regulation. Exp. Mol. Med.32, 101–109. 10.1038/emm.2000.18 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto, N. et al. PKClambda regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic beta cells. J. Clin. Invest.115, 138–145. 10.1172/jci22232 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao, X., Huang, X., Zhou, Z. & Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath.3, 71–85 (2013). [PMC free article] [PubMed] [Google Scholar]
- 37.Channuwong, P. et al. Hyperglycemia from diabetes potentiates uncarboxylated osteocalcin-stimulated insulin secretion in rat INS-1 pancreatic β-cells. Nutrients16, 2384. 10.3390/nu16152384 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated or analysed during this study are available from the corresponding author upon request.