Abstract
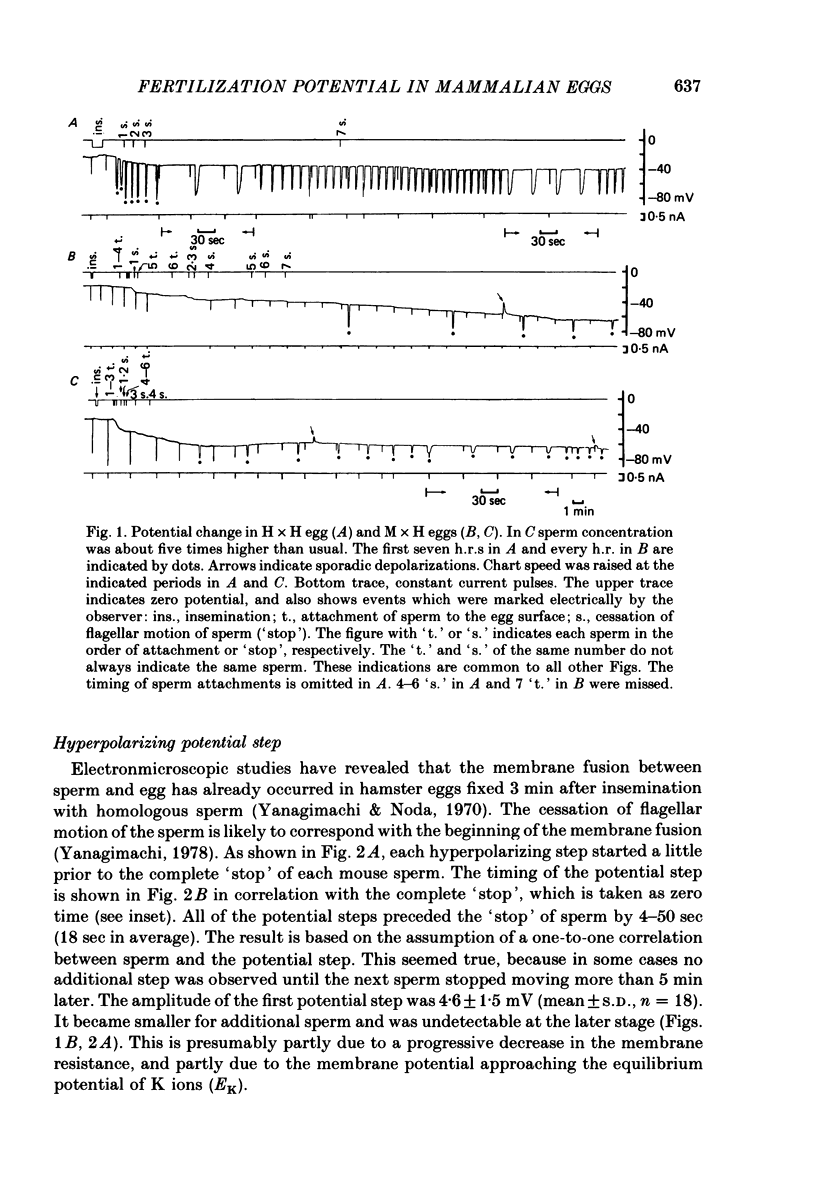
1. The zona-free hamster egg allows multiple entries of heterologous as well as homologous sperm. The hamster egg inseminated with mouse sperm (M × H egg) showed recurring, transient hyperpolarizing responses (h.r.s) with the peak of -70 to -80 mV. They were superimposed on a hyperpolarizing shift of the resting potential (h.s.) which gradually reached -60 mV in 50 min after insemination.
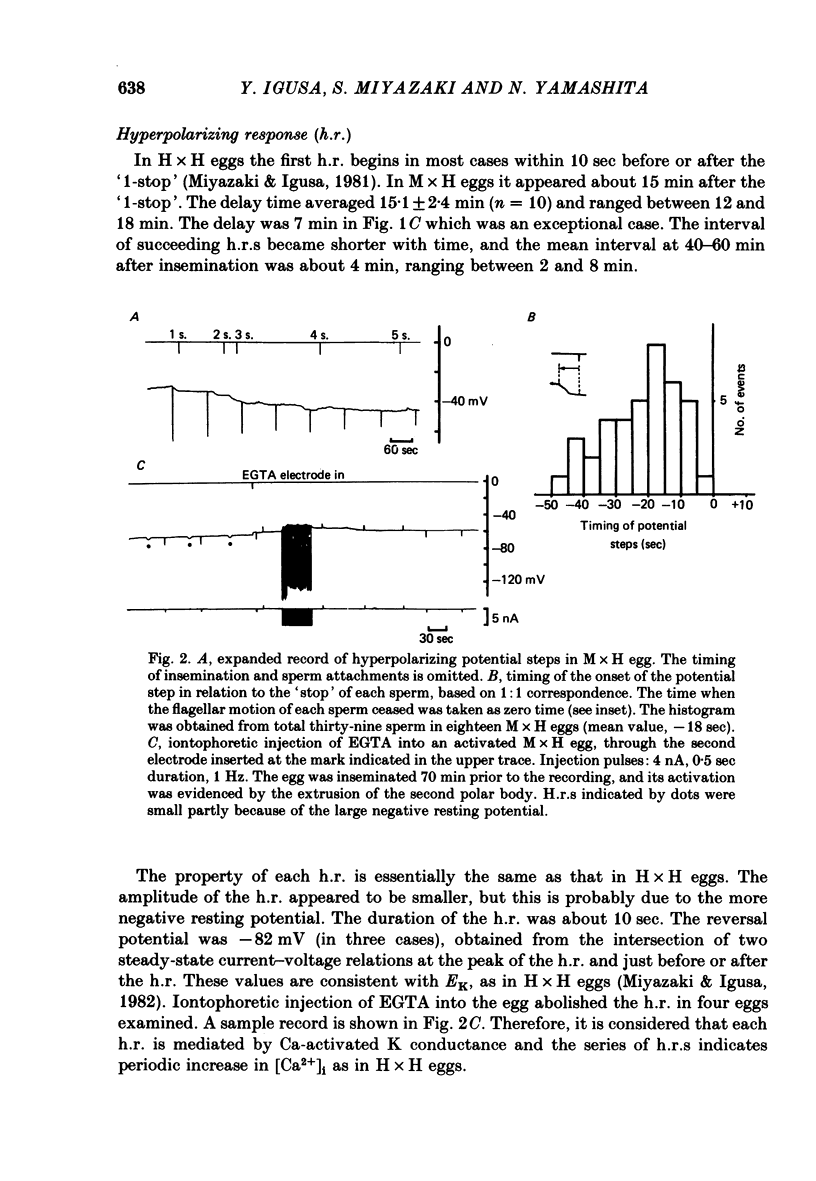
2. Unlike the hamster sperm, the cessation of flagellar motion of the first mouse sperm (`1-stop') failed to induce the first h.r. but produced only a small hyperpolarizing `step' of 3-7 mV. Similar steps occurred for each of additional sperm with a one-to-one correspondence, 4-50 sec ahead of the cessation of sperm motion.
3. In M × H eggs, the h.r. first appeared about 15 min after the `1-stop'. The intervals of the h.r.s thereafter were in the range between 2-10 min, in contrast to 30-45 sec in hamster eggs inseminated with hamster sperm (H × H eggs).
4. The h.r.s in M × H eggs were abolished by intracellular injection of EGTA, suggesting that they were caused by periodic increase in the intracellular Ca2+ concentration ([Ca2+]i) as in H × H eggs.
5. The gradual h.s. in M × H eggs was considered to be due mainly to an increase in Ca-independent K permeability, since the resting potential beyond -60 mV at 50-70 min after insemination was changed by only 3-5 mV on the removal of Cl ions and on EGTA injection.
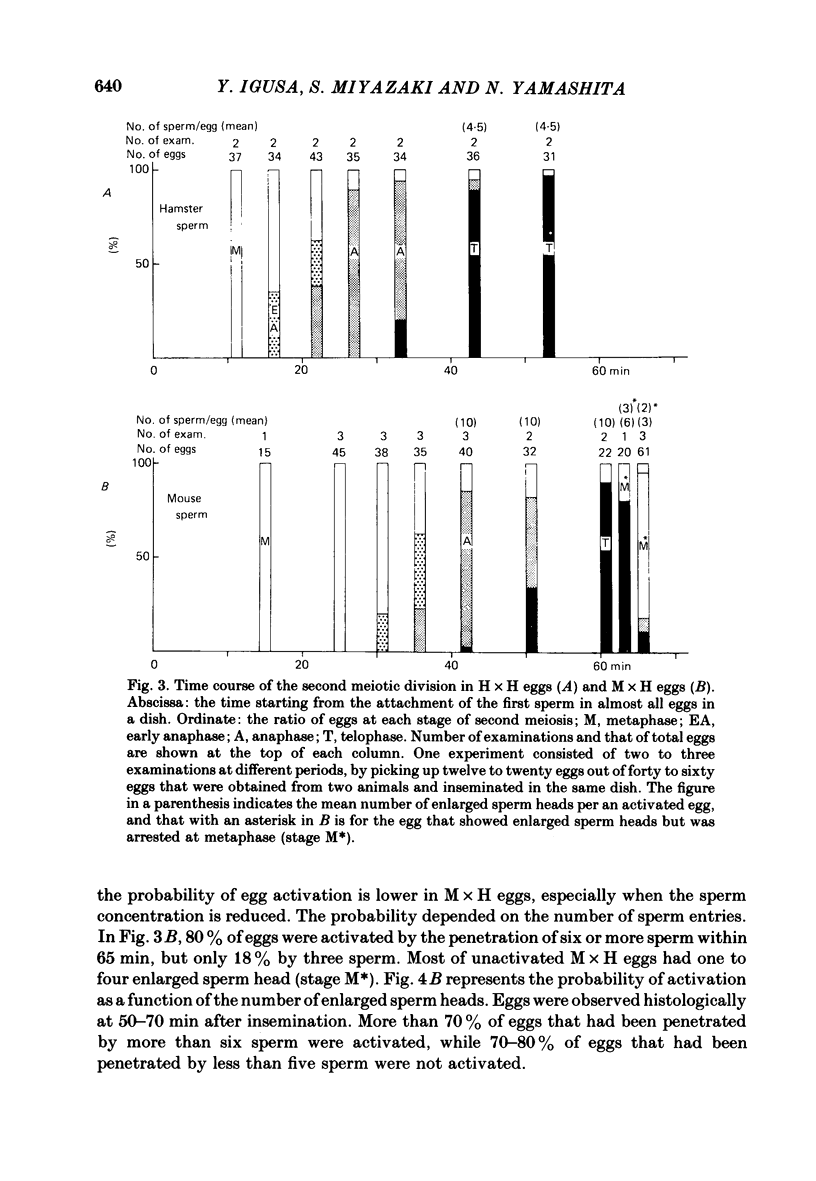
6. Histological observations revealed that the resumption of the second meiosis, the indication of egg activation, is delayed in M × H eggs by about 15 min, compared with that in H × H eggs. There was a good correlation between the delay of activation and that of the occurrence of the first h.r.
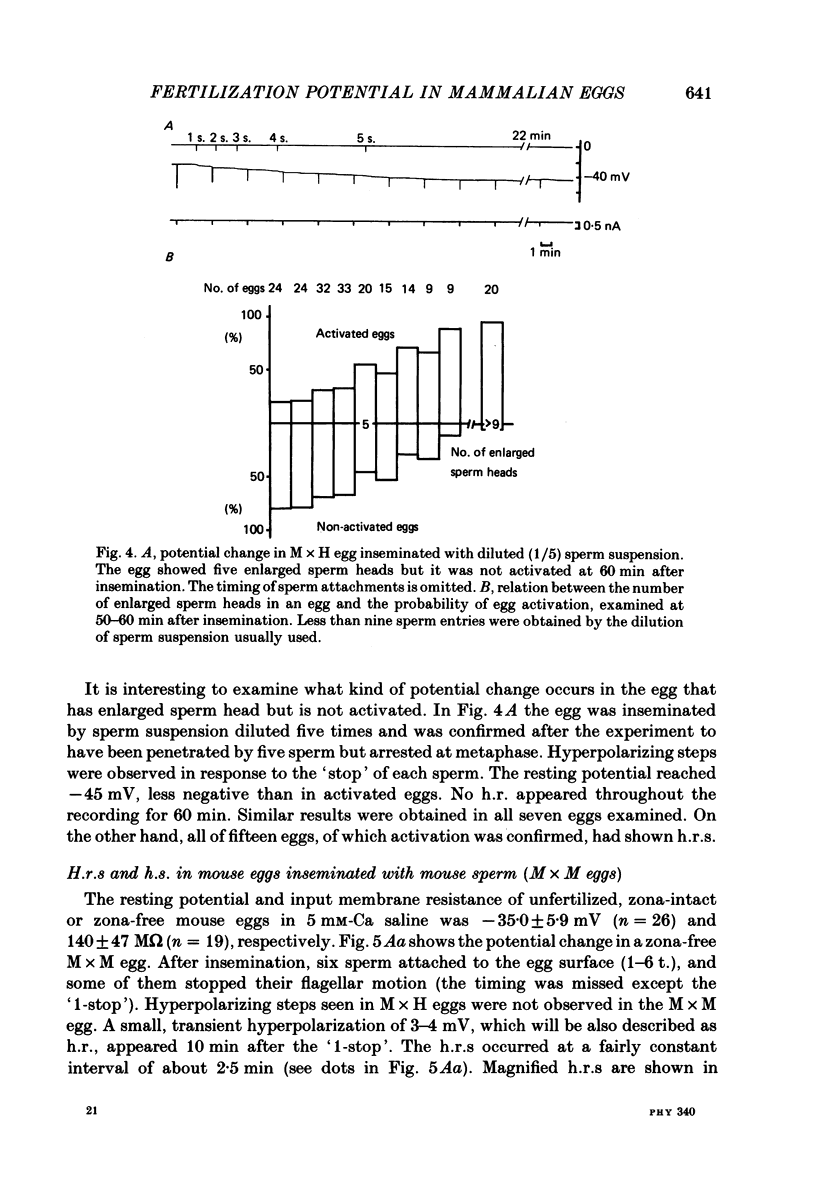
7. In M × H eggs, the probability of egg activation within 70 min was dependent on the number of sperm penetrations: 90% for more than ten sperm while 20-30% for less than five sperm. Eggs in which sperm penetration was not followed by activation showed no h.r.s.
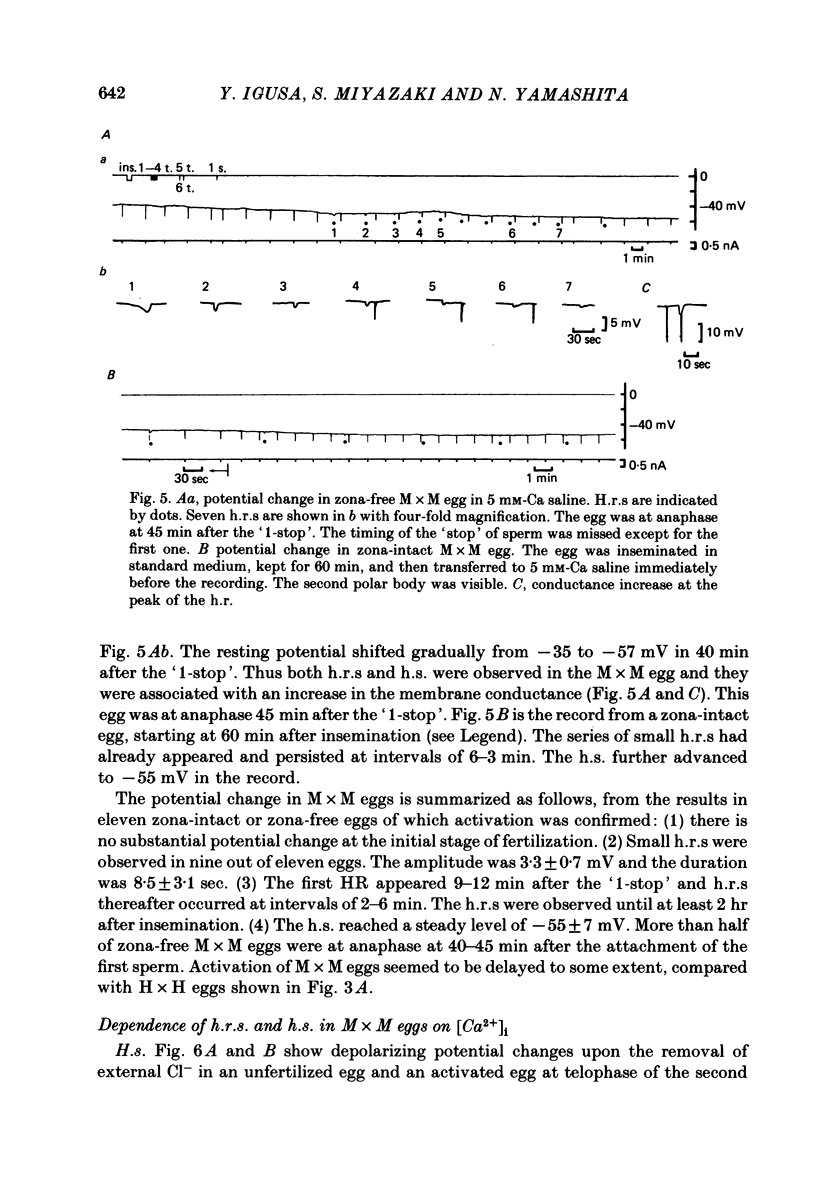
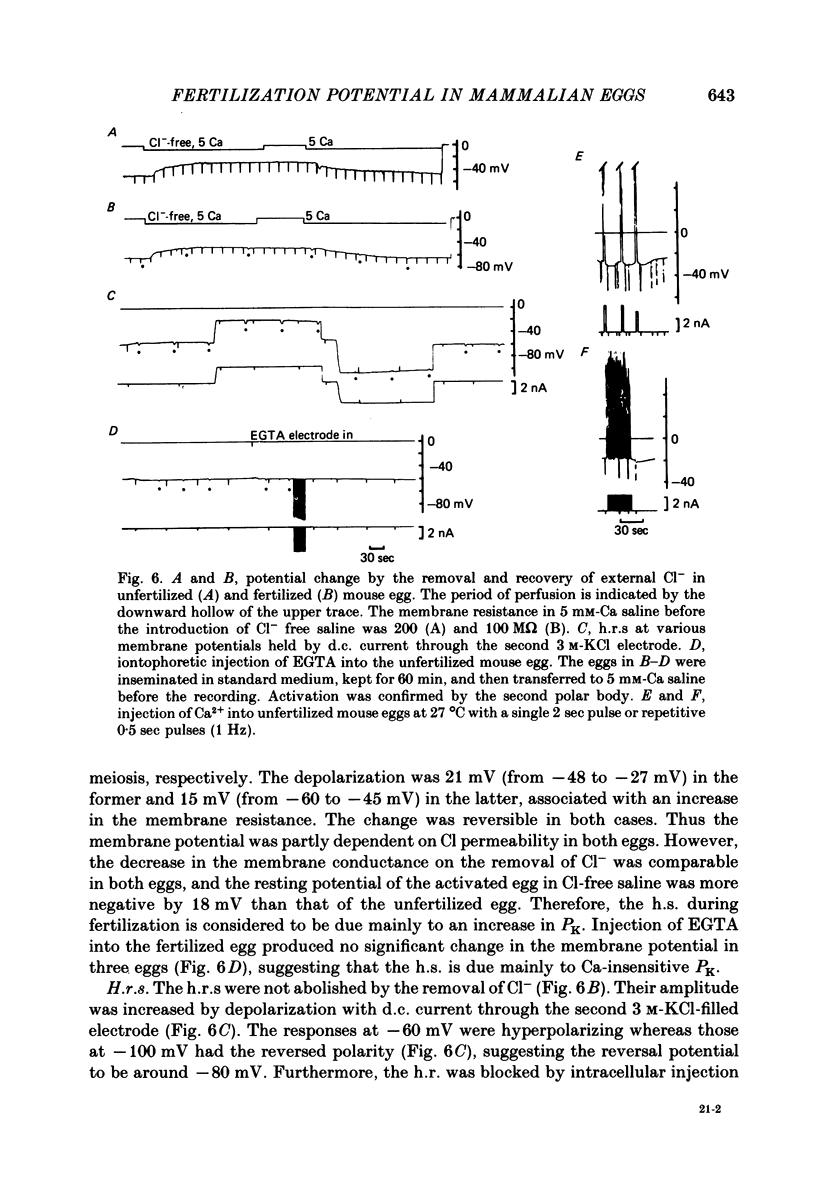
8. The mouse egg inseminated with mouse sperm showed small h.r.s (3-4 mV) superimposed on the h.s. from -35 to -55 mV in 50 min after insemination. Both h.r.s and h.s. were associated with an increase in the membrane conductance. The h.s. was considered to be due mainly to a Ca-independent increase in K permeability.
9. Iontophoretic injection of Ca2+ into the unfertilized mouse egg could not increase the K conductance with injection currents up to 4 nA. However, the h.r.s were suggested to be resulted from a periodic increase in [Ca2+]i, since they were abolished by injection of EGTA.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN C. R. Fertilization of mammalian eggs in vitro. Int Rev Cytol. 1961;12:337–359. [PubMed] [Google Scholar]
- Cuthbertson K. S., Whittingham D. G., Cobbold P. H. Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature. 1981 Dec 24;294(5843):754–757. doi: 10.1038/294754a0. [DOI] [PubMed] [Google Scholar]
- DeFelice L. J., Dale B. Voltage response to fertilization and polyspermy in sea urchin eggs and oocytes. Dev Biol. 1979 Oct;72(2):327–341. doi: 10.1016/0012-1606(79)90122-2. [DOI] [PubMed] [Google Scholar]
- Deguchi T. Rhodopsin-like photosensitivity of isolated chicken pineal gland. Nature. 1981 Apr 23;290(5808):706–707. doi: 10.1038/290706a0. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Chang M. C. The time of cortical granule breakdown and sperm penetration in mouse and hamster eggs inseminated in vitro. Biol Reprod. 1978 Sep;19(2):261–266. doi: 10.1095/biolreprod19.2.261. [DOI] [PubMed] [Google Scholar]
- Fulton B. P., Whittingham D. G. Activation of mammalian oocytes by intracellular injection of calcium. Nature. 1978 May 11;273(5658):149–151. doi: 10.1038/273149a0. [DOI] [PubMed] [Google Scholar]
- Gallin E. K., Gallin J. I. Interaction of chemotactic factors with human macrophages. Induction of transmembrane potential changes. J Cell Biol. 1977 Oct;75(1):277–289. doi: 10.1083/jcb.75.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkey J. C., Jaffe L. F., Ridgway E. B., Reynolds G. T. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J Cell Biol. 1978 Feb;76(2):448–466. doi: 10.1083/jcb.76.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada A., Chang M. C. Penetration of zone-free eggs by spermatozoa of different species. Biol Reprod. 1972 Apr;6(2):300–309. doi: 10.1093/biolreprod/6.2.300. [DOI] [PubMed] [Google Scholar]
- Igusa Y., Miyazaki S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol. 1983 Jul;340:611–632. doi: 10.1113/jphysiol.1983.sp014783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. A. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature. 1976 May 6;261(5555):68–71. doi: 10.1038/261068a0. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Hirai S. Fast polyspermy block and activation potential. Correlated changes during oocyte maturation of a starfish. Dev Biol. 1979 Jun;70(2):327–340. doi: 10.1016/0012-1606(79)90031-9. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Igusa Y. Ca-mediated activation of a K current at fertilization of golden hamster eggs. Proc Natl Acad Sci U S A. 1982 Feb;79(3):931–935. doi: 10.1073/pnas.79.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya W., Yada T., Yano J., Yawo H. Phagocytic activity and hyperpolarizing responses in L-strain mouse fibroblasts. J Physiol. 1981;313:101–119. doi: 10.1113/jphysiol.1981.sp013653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yamashita N. Ionic currents through the membrane of the mammalian oocyte and their comparison with those in the tunicate and sea urchin. J Physiol. 1977 May;267(2):465–495. doi: 10.1113/jphysiol.1977.sp011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D., Carroll E. J., Jr, Yanagimachi R. Is calcium ionophore a universal activator for unfertilised eggs? Nature. 1974 Nov 1;252(5478):41–43. doi: 10.1038/252041a0. [DOI] [PubMed] [Google Scholar]
- Steinhardt R., Zucker R., Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977 Jul 1;58(1):185–196. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. P., Inoue M., Stark R. A. Penetration of zona-free mouse ova. Biol Reprod. 1976 Sep;15(2):213–221. doi: 10.1095/biolreprod15.2.213. [DOI] [PubMed] [Google Scholar]
- Yamashita N. Enhancement of ionic currents through voltage-gated channels in the mouse oocyte after fertilization. J Physiol. 1982 Aug;329:263–280. doi: 10.1113/jphysiol.1982.sp014302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R., Noda Y. D. Ultrastructural changes in the hamster sperm head during fertilization. J Ultrastruct Res. 1970 Jun;31(5-6):465–485. doi: 10.1016/s0022-5320(70)90163-2. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Penetration of guinea-pig spermatozoa into hamster eggs in vitro. J Reprod Fertil. 1972 Mar;28(3):477–480. doi: 10.1530/jrf.0.0280477. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Sperm-egg association in animals. Curr Top Dev Biol. 1978;12:83–105. [PubMed] [Google Scholar]