Abstract
This review explores the pathophysiology, clinical implications, and management of double diabetes. The increasing prevalence of obesity, sedentary lifestyles, and genetic predisposition has blurred the difference between type 1 and type 2 diabetes, leading to diagnostic and therapeutic challenges. Double diabetes presents with overlapping symptoms from both diabetes types, making accurate diagnosis crucial. Biomarkers, such as C-peptide levels, autoantibody testing, and insulin resistance markers, help differentiate double diabetes from classic diabetes subtypes. Early intervention is necessary because of the condition's elevated risk of microvascular and macrovascular consequences, such as retinopathy, nephropathy, and cardiovascular disease. Effective management integrates pharmacological and lifestyle approaches. Metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and insulin therapy adjustments all boost glycemic control and metabolic results. Additionally, structured exercise, dietary modifications, and weight management are essential for reducing insulin resistance and preserving beta-cell activity. The potential of precision medicine, artificial intelligence (AI)-driven healthcare, and continuous glucose monitoring (CGM) offers promising advancements for personalized treatment strategies. Future research should focus on targeted immunotherapies, genetic profiling, and refined clinical guidelines to improve early detection and individualized treatment, with long-term outcomes. The review emphasizes the need for a multidisciplinary approach in managing double diabetes, ensuring early diagnosis, optimized treatment, and improved metabolic health to mitigate long-term complications.
Keywords: autoimmune dysfunction, beta-cell dysfunction, double diabetes, insulin resistance, metabolic syndrome, precision medicine
Introduction and background
Double diabetes occurs when type 1 and type 2 diabetes share characteristics that represent a complex metabolic health condition. The combination of both types leads to double diabetes when insulin-resistant type 1 patients show type 2 diabetes symptoms, or when type 2 patients demonstrate type 1 diabetes markers [1]. Traditional diabetes classifications become insufficient when treating double diabetes because it requires both insulin deficiency therapy and insulin resistance management. Scientific research demonstrates that double diabetes exists as a unique medical condition, because patients frequently exhibit combined metabolic and autoimmune processes within their bodies [2].
The current rise of obesity, together with inactive lifestyle patterns, complicates the medical classification of diabetes between type 1 and type 2 [3]. Healthcare professionals detected double diabetes when type 1 diabetic patients developed symptoms of type 2 diabetes, including obesity, hypertension, and dyslipidemia [4]. Health studies demonstrate that double diabetes is becoming increasingly prevalent among type 1 diabetic children who face weight gain in addition to metabolic syndrome [5]. The worldwide increase in childhood obesity indicates that diabetes progression may change as people grow older. Research indicates that insulin resistance happens in 25%-30% of type 1 diabetic patients, causing cardiovascular complications that typically affect type 2 diabetic patients [6].
The diagnostic category of double diabetes produces extensive clinical challenges since it creates specific barriers to treatment management. Medical staff needs to detect double diabetes because individual treatments for type 1 or type 2 diabetes might not work effectively in these patients. The improper diagnosis or late identification of double diabetes results in inadequate treatment outcomes that heighten the probability of developing nephropathy, together with retinopathy and cardiovascular disease [7]. Double diabetic patients face major challenges with controlling their blood glucose, which requires comprehensive healthcare treatment by several professionals, combining lifestyle changes with both insulin therapy and insulin sensitizers [8]. The worldwide increase in diabetes cases requires a deep comprehension of double diabetes to create better patient outcomes and develop optimal healthcare systems.
The review investigates double diabetes through an evaluation of the pathophysiology, epidemiological data, clinical expressions, and emerging treatment approaches. The review explores current research on double diabetes, focusing on its metabolic and autoimmune components, challenges in diagnosis, and the need for personalized treatment. It highlights the role of precision medicine, artificial intelligence (AI), and pharmacological advancements in improving patient outcomes. Addressing these complexities is crucial for improving early detection, individualized treatment strategies, and long-term metabolic health in double diabetes management.
Review
Convergence of type 1 and type 2 diabetes leading to double diabetes
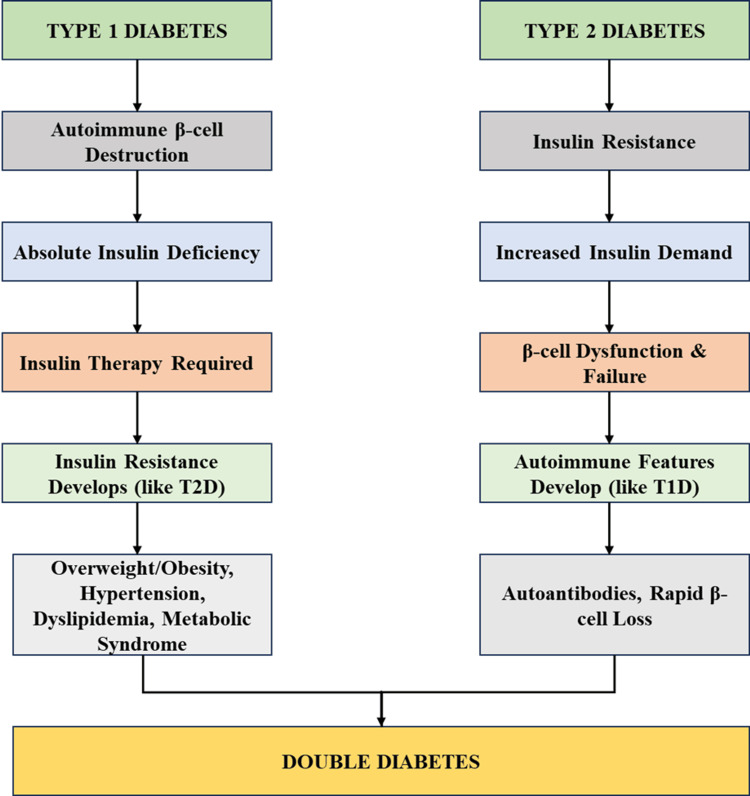
The combination of type 1 and type 2 diabetes characteristics produces double diabetes, which forms an intricate autoimmune and metabolic condition. The two fundamental processes through which double diabetes occurs are illustrated in Figure 1. Type 1 diabetic patients experience insulin resistance through a combination of obesity, sedentary habits, and genetic factors that delay their diabetes management, requiring extra medical support. As β-cell dysfunction worsens and autoimmune markers emerge, patients with type 2 diabetes become insulin-dependent. Such development resembles the insulin dependence of type 1 diabetes. Treatment and diagnosis, as well as management of this condition, become complex due to the convergence of these disease mechanisms. Early intervention, treatment planning, and improvement in metabolic outcomes for double diabetic patients require understanding these shared disease mechanisms.
Figure 1. Convergence of Type 1 and Type 2 Diabetes Leading to Double Diabetes.
Image credit: The image has been created by the authors of this article
Genetic predisposition and epigenetic influences
Double diabetes develops as a result of combined genetic elements and epigenetic regulatory mechanisms. Various genetic markers between type 1 and type 2 diabetes generate complex metabolic and autoimmune conditions that interact with insulin resistance. The chromosomal region 6p21 contains human leukocyte antigen (HLA)-DR/DQ, which enhances immune system regulation and raises susceptibility to beta-cell destruction caused by an autoimmune response. The HLA-DR/DQ gene expression shows changes through DNA methylation after environmental triggers, such as viral infections, activate the disease [8].
At chromosome 10q25, the transcription factor 7-like 2 (TCF7L2) type 2 diabetes risk gene controls beta-cell function as well as insulin secretion. Research shows TCF7L2 expression controls through histone adjustments and reacts to weight-related foods and obesity factors [9]. Another significant gene, INS (insulin gene) on chromosome 11p15, is crucial in insulin production. The INS gene expression receives control through epigenetic modifications, especially DNA methylation, that influence beta-cell function while affecting their response to metabolic stress [10].
The peroxisome proliferator-activated receptor gamma (PPARG) gene, located on the 3p25 chromosome in type 2 diabetes, controls lipid metabolism while simultaneously affecting insulin sensitivity. PPARG gene variants produce obesity-related insulin resistance because histone acetylation controls its functional activity [11]. DNA methylation and other epigenetic modifications influence the insulin secretion and potassium channel regulation functions of the type 2 diabetes gene potassium voltage-gated channel subfamily J member 11 (KCNJ11), which is located on chromosome 11p15.1 [12].
Type 1 diabetes risk and beta-cell destruction susceptibility depend on protein tyrosine phosphatase non-receptor type 22 (PTPN22), which serves as a primary immune regulatory gene found on chromosome 1p13. Studies suggest that the expression of PTPN22 becomes vulnerable to infections due to histone deacetylation effects. The combined genetic susceptibility and epigenetic control establish an individual's probability of developing double diabetes [13]. The key genetic markers, alongside their chromosomal positions, diabetes type, epigenetic modifications, and environmental triggers, are mentioned in Table 1.
Table 1. Genetic Predisposition and Epigenetic Influences in Double Diabetes.
HLA-DR/DQ: Human Leukocyte Antigen-DR/DQ; TCF7L2: Transcription Factor 7-Like 2; INS: Insulin Gene; PPARG: Peroxisome Proliferator-Activated Receptor Gamma; KCNJ11: Potassium Voltage-Gated Channel Subfamily J Member 11; PTPN22: Protein Tyrosine Phosphatase Non-Receptor Type 22
| Gene/Marker | Chromosome Location | Associated Diabetes Type | Mechanism | Epigenetic Influence | Environmental Trigger | Impact on Beta Cells | Effect on Insulin Sensitivity |
| HLA-DR/DQ | 6p21 | Type 1 | Autoimmune susceptibility | Methylation changes | Viral infections | Beta-cell destruction | Minimal |
| TCF7L2 | 10q25 | Type 2 | Beta-cell function | Histone modifications | Diet & Obesity | Reduced insulin secretion | High |
| INS | 11p15 | Type 1 | Insulin gene regulation | DNA methylation | Prenatal nutrition | Impaired insulin production | Moderate |
| PPARG | 3p25 | Type 2 | Lipid metabolism | Histone acetylation | High-fat diet | No direct impact | High |
| KCNJ11 | 11p15.1 | Type 2 | Potassium channel regulation | DNA methylation | Prenatal exposure to glucose | Impaired insulin release | Moderate |
| PTPN22 | 1p13 | Type 1 | Immune response modulation | Histone deacetylation | Infections | Autoimmune activation | Minimal |
Role of insulin resistance in type 1 diabetic patients
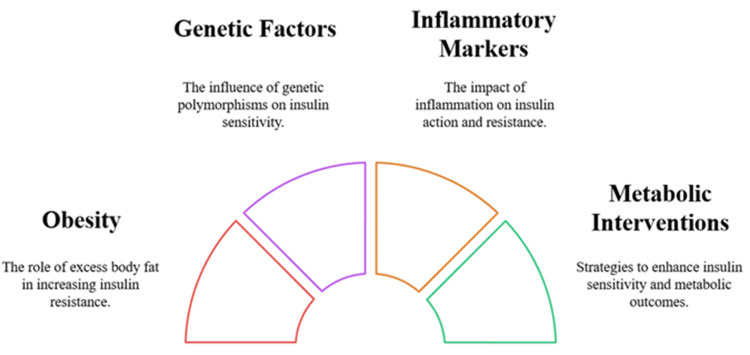
Insulin resistance is a major metabolic issue that individuals with type 1 diabetes face, despite its historical association with type 2 diabetes. Individuals with type 1 diabetes may develop insulin resistance even with absolute insulin deficiency, worsening glucose control and cardiovascular risk [2]. Multiple factors produce insulin resistance, such as the combination of excessive body fat, genetic components, long-term high blood sugar, and swelling mechanisms within the body.
The prevalence of obesity stands as a primary reason that causes insulin resistance for patients who have type 1 diabetes. Results from studies show that people who have a higher BMI need larger insulin doses because their insulin sensitivity decreases, which leads to complicated treatment, along with elevated metabolic syndrome and cardiovascular risk [14,15]. Glucotoxicity, together with oxidative stress and inflammation, causes type 1 diabetic patients to develop more insulin resistance through the reduction of insulin signaling and glucose uptake [16,17]. Type 1 diabetic patients with elevated tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) show worse insulin action due to their increased levels [18,19].
The insulin sensitivity levels of individuals with type 1 diabetes are influenced by the PPARG and TCF7L2 polymorphisms. Modifications of DNA structure, caused by dietary choices and life experiences, work to control metabolic responses [20,21]. The combination of metformin and sodium-glucose cotransporter 2 (SGLT2) inhibitors enhances both insulin sensitivity and metabolic results among patients who have double diabetes [22]. The early discovery of double diabetes, alongside specific intervention strategies, plays a vital role in preventing long-lasting medical complications and reaching optimal treatment results. Figure 2 shows the main parameters influencing insulin resistance in the management of type 1 diabetes.
Figure 2. Key Factors Influencing Insulin Resistance in Type 1 Diabetes Management.
Image credit: The image has been created by the authors of this article
Impact of autoimmune dysfunction on type 2 diabetes
Recent research findings demonstrate that autoimmune dysfunction is important for type 2 diabetes development, thus changing traditional disease classification methods. The diagnostic markers include insulin resistance, alongside beta-cell deterioration, but certain patients show evidence of autoimmune activity [23]. Pancreatic autoantibodies GADA (glutamic acid decarboxylase autoantibodies), IAA (insulin autoantibodies), and IA-2 (insulinoma-associated antigen-2) antibodies indicate that autoimmune destruction plays a role in the disease process [24].
Data shows that 10%-15% of type 2 diabetic patients carry pancreatic autoantibodies, which medical professionals diagnose as latent autoimmune diabetes in adults (LADAs) or double diabetes [25]. Type 1 diabetes differs from type 2 diabetes because autoimmunity in type 2 diabetes results in beta-cell decline, which increases insulin dependency progressively over time [26]. People with autoimmune markers experience quicker beta-cell deterioration, which leads them to need insulin therapy before antibody-negative patients [27].
The development of autoimmune dysfunction heavily depends on chronic inflammation. TNF-α, IL-6, and C-reactive protein (CRP) are pro-inflammatory cytokines that disrupt insulin signaling pathways while degrading beta-cell functioning abilities, disrupting normal blood glucose levels by permitting excessive hepatic glucose synthesis and impairing muscle glucose uptake [28,29]. The inflammatory process caused by autoimmunity leads to worsened insulin resistance and elevated cardiovascular dangers, which emerge as the main cause of death in these patients [30].
Genetic evaluations of HLA, PTPN22, and INS establish that the genes connect both autoimmune and metabolic diabetes types [31]. The disease progression of these conditions is influenced by environmental factors like diet and stress, together with infections, which modify epigenetic patterns [32].
The treatment of type 2 diabetes needs specific approaches when patients have autoimmune involvement. Patients with autoimmune markers show unresponsiveness to sulfonylureas and insulin secretagogues, which leads healthcare providers to consider glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, and insulin therapy as suitable alternatives [33].
Metabolic disturbances and beta-cell dysfunction
Metabolic disturbances give rise to both insulin resistance and impaired insulin secretion, together with beta-cell dysfunction, which advances diabetes progression. The combination of high blood sugar levels produces three detrimental effects on beta cells, which result in deteriorating blood glucose control. The prolonged elevation of blood glucose levels damages insulin signaling pathways, which causes insulin resistance to worsen [34].
Free fatty acids accumulating in the body through lipotoxicity reduce beta-cell function by generating problems with insulin granule release and raising both mitochondrial stress and inflammation levels, which drive insulin resistance. Reactive oxygen species produced by dysfunctional mitochondria cause beta-cell damage and DNA destruction, along with adenosine triphosphate (ATP) exhaustion, which speeds up metabolic decline. The unfolded protein response (UPR) is triggered by the endoplasmic reticulum's (ER) protein misfolding stress, which enhances both pro-inflammatory cytokine production and beta-cell death, thus accelerating diabetes development [35].
Chronic inflammation created by TNF-alpha, IL-6, and CRP interferes with insulin signaling and beta-cell functioning while raising cardiovascular danger levels. Type 2 diabetes advances faster when islet amyloid polypeptide (IAPP) builds up in the body because it damages beta cells and makes insulin resistance worse [36,37]. Initial treatment of glucotoxicity, lipotoxicity effects, and inflammatory processes, using blood sugar management solutions together with lifestyle changes, antioxidants, and anti-inflammatory drugs, helps delay disease progression and protect beta-cell functioning [38]. The influence of metabolic disturbances on beta-cell dysfunction appears in Table 2.
Table 2. Metabolic Disturbances and Their Impact on Beta-Cell Dysfunction.
ATP: Adenosine Triphosphate; ROS: Reactive Oxygen Species; TNF-α: Tumor Necrosis Factor-alpha; IL-6: Interleukin-6
| Metabolic Disturbance | Primary Cause | Impact on Beta Cells | Effect on Insulin Secretion | Role in Insulin Resistance | Inflammatory Contribution | Associated Complications | Reversibility Potential |
| Glucotoxicity | Chronic hyperglycemia | Beta-cell apoptosis | Reduced insulin secretion | Increases insulin resistance | High (via oxidative stress) | Microvascular complications | Moderate |
| Lipotoxicity | Elevated free fatty acids | Beta-cell dysfunction | Impaired insulin granule release | Aggravates insulin resistance | Moderate (via cytokine release) | Non-alcoholic fatty liver disease | Low |
| Mitochondrial Dysfunction | Oxidative stress | Reduced ATP production | Impaired insulin exocytosis | Promotes insulin resistance | High (due to ROS production) | Neurodegeneration | Low |
| Endoplasmic Reticulum Stress | Protein misfolding | Beta-cell stress and apoptosis | Decreased insulin synthesis | Triggers inflammatory pathways | High (via unfolded protein response) | Diabetic nephropathy | Moderate |
| Chronic Inflammation | Cytokine overproduction | Beta-cell impairment | Impaired glucose-stimulated insulin secretion | Major contributor | Very high (TNF-α, IL-6 involvement) | Cardiovascular disease | Moderate |
| Amyloid Deposition | Accumulation of islet amyloid polypeptide | Beta-cell toxicity | Inhibits insulin release | Exacerbates insulin resistance | Moderate | Type 2 diabetes progression | Low |
Epidemiological insights and risk factors in double diabetes
The increasing prevalence of double diabetes requires a thorough analysis of its risk factors, together with epidemiological data that involve insulin resistance alongside autoimmune dysfunction. The worldwide incidence of double diabetes continues to increase among youth because lifestyle changes and environmental elements affect their health. Scientific studies demonstrate that type 1 diabetic patients possess insulin resistance features in about 30% of cases, combined with obesity and metabolic syndrome, and type 2 diabetic patients show pancreatic autoantibodies that support an autoimmune origin [39]. The traditional separation between type 1 and type 2 diabetes recently became less evident because younger people face growing risks of obesity and inactivity, thus creating a new diabetes spectrum [40].
The pathophysiology and epidemiology of double diabetes strongly depend on lifestyle and environmental elements. Type 1 diabetic patients who are obese develop worse glycemic control and need more insulin because obesity acts as a major risk factor for insulin resistance [41]. Poor food choices, particularly processed carbohydrates, together with saturated fats and sugary beverages, create metabolic problems while causing insulin resistance in the body [42]. Early-life environmental exposures to maternal obesity and gestational diabetes put people at higher risk of developing beta-cell dysfunction and insulin resistance, which leads to a later-life double diabetes diagnosis [43].
The epidemiology of double diabetes is influenced by both genetic and ethnic variations of susceptibility. People who belong to the ethnic groups of Hispanic and African American people, along with those of South Asian and indigenous descent, exhibit natural tendencies toward insulin resistance and type 2 diabetes, which raises their chances of developing double diabetes when combined with autoimmune conditions [44]. The heterogeneity of double diabetes results from genetic variants that affect insulin resistance and autoimmune responses through HLA, TCF7L2, PTPN22, and INS genes [45]. The epigenetic modifications triggered by environmental elements, including diet, physical activity, and stress patterns, serve to control metabolic and immune functions, which affect the risk of double diabetes [46].
Clinical features and diagnosis
The diagnosis of double diabetes needs careful assessment because patients present symptoms that connect both type 1 and type 2 diabetes. The standard diagnosis indicators for type 2 diabetes, combined with type 1 diabetes features, include polyuria, polydipsia, and polyphagia (three Ps), alongside possible weight loss from type 1 diabetes [47,48].
The multiple ways through which double diabetes presents create difficulties in diagnosing the condition. The combination of insulin resistance in obese patients with type 1 diabetes can lead to a misdiagnosis of type 2 diabetes, yet type 2 diabetic patients with autoimmune markers require early insulin therapy because their beta-cell function declines rapidly [49]. The diagnostic challenges are summarized in Table 3, which presents clinical features and biomarkers, as well as laboratory criteria.
Table 3. Clinical Features and Diagnostic Markers of Double Diabetes.
DKA: Diabetic Ketoacidosis; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; IA-2: Islet Antigen-2 Autoantibodies; ZnT8: Zinc Transporter 8 Autoantibodies; GADA: Glutamic Acid Decarboxylase Autoantibodies; LADA: Latent Autoimmune Diabetes in Adults; HbA1c: Hemoglobin A1c (Glycated Hemoglobin); LDL: Low-Density Lipoprotein (Bad Cholesterol); TG: Triglycerides; HDL: High-Density Lipoprotein (Good Cholesterol); CGM: Continuous Glucose Monitoring
| Clinical Feature | Overlapping Symptoms | Diagnostic Challenge | Diagnostic Criteria | Biomarkers | Laboratory Findings |
| Polyuria & Polydipsia | In both | It can be misattributed to either type | Frequent thirst & urination | C-peptide levels | Low in Type 1, Normal/High in Type 2 |
| Unintended Weight Loss | More common in Type 1 but can occur in Type 2 | Obesity in Type 2 may mask weight loss | Unexplained weight loss | Insulin autoantibodies | Positive in autoimmune involvement |
| Hyperglycemia | In both | Severity varies with insulin resistance | Fasting glucose >126 mg/dL or HbA1c >6.5% | HbA1c | Elevated in both |
| Ketoacidosis | More common in Type 1 but can occur in insulin-deficient Type 2 | DKA risk in misdiagnosed Type 2 patients | Elevated ketones & acidosis | Beta-hydroxybutyrate | High in ketoacidosis |
| Insulin Resistance | Present in Type 2, emerging in Type 1 with obesity | Difficult to detect in early stages | HOMA-IR score & fasting insulin | Fasting insulin levels | High in Type 2, Normal/Low in Type 1 |
| Dyslipidemia | Common in Type 2, emerging in Type 1 with metabolic syndrome | Often underdiagnosed in Type 1 | Elevated LDL & triglycerides | Lipid profile | High LDL & TG, Low HDL |
| Autoimmune Markers | Absent in classic Type 2, but present in LADA/Double Diabetes | Overlap with late-onset Type 1 | GADA, IA-2, ZnT8 testing | Autoantibodies | Positive in autoimmune diabetes |
| Beta-Cell Function Decline | Occurs in both, more rapid in Type 1 | Difficult to differentiate in early stages | C-peptide testing | C-peptide levels | Low in Type 1, Declining in LADA |
| Glycemic Variability | Fluctuations in glucose levels | More in Type 1 but can occur in Type 2 | Continuous glucose monitoring (CGM) | Time-in-range analysis | Highly variable in Type 1, More stable in Type 2 |
A correct diagnosis depends on C-peptide testing because it establishes the functional state of beta cells. The detection of type 1 diabetes appears through C-peptide test results below the threshold, yet insulin resistance typically shows normal-to-high levels, although double diabetes features declining C-peptide levels [50]. The assessment process benefits from additional markers of insulin resistance, which include HOMA-IR, fasting insulin, low-density lipoprotein (LDL) cholesterol, triglycerides, and inflammatory cytokines (TNF-α and IL-6) [51,52]. The continuous glucose monitoring (CGM) system helps patients reveal information about glycemic variability, since this data aids doctors in identifying insulin-deficient patients [52].
Health risks and associated complications in double diabetes
The combination of diabetes mellitus types 1 and 2 creates serious cardiovascular dangers because metabolic syndrome and cardiovascular diseases cause greater death rates, along with complications. Those who suffer from insulin resistance, together with autoimmunity, develop higher probabilities of coronary artery disease, stroke, heart failure, atherosclerosis, hypertension, and dyslipidemia [53]. Urgent medical intervention becomes necessary because chronic low-grade inflammation, together with endothelial dysfunction, speeds up cardiovascular breakdown. Metabolic syndrome components, such as central obesity and hypertension, together with dyslipidemia and insulin resistance, degrade both blood glucose management and heart health [54].
Patients with double diabetes also experience higher rates of microvascular and macrovascular complications. Progressive small blood vessel damage occurs from diabetic retinopathy, nephropathy, and neuropathy because of chronic hyperglycemia, together with oxidative stress and inflammatory responses [55]. The fast deterioration of renal function in diabetic nephropathy happens through two mechanisms, including beta-cell autoimmune damage and insulin-resistant hyperfiltration [56]. Additionally, peripheral artery disease and stroke severity increase due to arterial stiffening, platelet dysfunction, and pro-inflammatory cytokine activity [57]. Medical teams need to implement aggressive cardiovascular risk management because these complications lead to a significant reduction in patient life expectancy.
Mental health and the overall quality of life represent significant problems for these individuals. Patients who have double diabetes face more severe complications of diabetes distress, anxiety, and depression because they must manage insulin resistance, together with autoimmune dysfunction [58]. Those patients who experience burnout and treatment fatigue, together with complications-related anxiety, demonstrate lower rates of medical advice and lifestyle intervention compliance [59]. The extensive nature of diabetes care, with its ongoing observations and long-term medical complications, leads to emotional distress, which diminishes patients' quality of life [60]. The successful treatment of patients depends on psychological assistance, along with structured counseling and self-care programs, to achieve better patient results and improved well-being.
Pharmacological approaches in double diabetes management
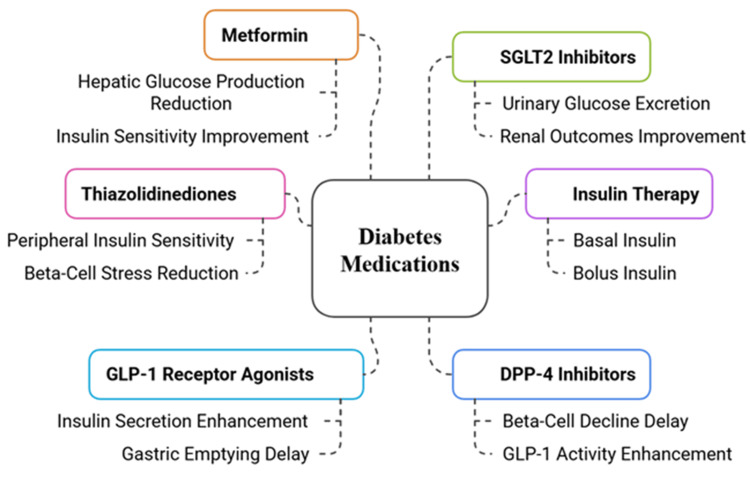
Patients with double diabetes need insulin therapy combined with additional medications to treat the deficiency and resistance of insulin in their bodies. The main insulin therapy for significant beta-cell dysfunction is basal-bolus insulin therapy, yet each patient requires separate dose adjustments to manage hypoglycemic episodes and weight gain risks [61].
The administration of metformin produces beneficial effects for patients with type 1 diabetes features and insulin resistance because it boosts glucose uptake, along with decreased hepatic glucose production, which helps lower insulin needs and enhances metabolic measurements [62]. The medication thiazolidinediones (e.g., pioglitazone) boosts peripheral insulin sensitivity, but doctors need to exercise caution because these drugs can cause fluid retention, together with cardiovascular complications [63].
Liraglutide and semaglutide, among GLP-1 receptor agonists, help release insulin and restrain glucagon, while assisting in weight reduction and delivering heart protection for insulin-resistant and overweight patients [64]. Empagliflozin and dapagliflozin, among SGLT2 inhibitors, decrease blood glucose through kidney-based glucose elimination and simultaneously protect heart and kidney health. Euglycemic ketoacidosis poses a risk for patients who lack insulin, so they require special attention [65]. The DPP-4 inhibitors sitagliptin and linagliptin improve GLP-1 function to control blood glucose levels without causing hypoglycemia events and maintain beta-cell functionality [66]. Double diabetes treatment needs customized methods that combine insulin therapy with pharmaceutical medications chosen based on metabolic characteristics, heart health risks, and pancreatic cell functions. The pharmacological approaches with their clinical implications are presented in Figure 3.
Figure 3. Pharmacological Approaches in Double Diabetes Management.
Image credit: The image has been created by the authors of this article
SGLT2: Sodium-Glucose Cotransporter 2; GLP-1: Glucagon-Like Peptide-1; DPP-4: Dipeptidyl Peptidase-4
Lifestyle modifications for improved metabolic health
Lifestyle modifications function as the essential approach for double diabetes management by treating both insulin resistance and beta-cell dysfunction. Strategic physical exercise, along with nutritional adjustments, leads to powerful enhancements of diabetic blood glucose management, together with better insulin response and better heart health. The medical treatment of diabetes should combine nutritional foods with monitored carbohydrate intake to achieve metabolic equilibrium. Studies prove that the Mediterranean and low-GI dietary patterns improve insulin sensitivity, decrease inflammation, and lower cardiovascular risk [67].
Nutritious foods containing fiber, combined with lean proteins and healthy fats, stop rapid glucose changes while stabilizing metabolism. The removal of processed carbohydrates, together with trans fats and unnecessary saturated fats, supports decreased insulin resistance and prevents weight gain [68]. Patients with double diabetes and hypertension should follow the DASH (Dietary Approaches to Stop Hypertension) dietary plan because it leads to a better lipid profile, along with blood pressure enhancement [69]. Engaging in routine physical exercise leads to better insulin response and a reduction in abdominal body fat, while improving heart health. Glucose metabolism improves, while insulin requirements decrease, when patients perform aerobic exercises, such as walking, cycling, and swimming, alongside resistance training methods, like weightlifting or bodyweight activities [70].
Two sessions of resistance training and 150 minutes of moderate aerobic activity per week are the bare minimum needed for metabolic health [71]. Weight management stands as an essential requirement for people who have obesity, together with insulin resistance. Weight reduction between 5% and 10% leads to substantial improvements in glucose control, together with better cardiovascular health. The combination of controlled intermittent fasting and calorie restriction has been proven to decrease insulin resistance and beta-cell stress levels, according to research [72]. Cost-control management plans that unite lifestyle interventions, CGM-feedback systems, and digital health platforms lead patients to better adhere and succeed over the long term with dual-diabetes management.
Personalized medicine approach in double diabetes
Through personalized medicine approaches, the management of double diabetes has evolved through individual-specific genetic profiles and metabolic characteristics, as well as phenotypic features. Early case identification happens through precision medicine and genetic profiling, while personalized treatment plans are created through understanding genetic aspects of insulin resistance and autoimmune dysfunction [73]. The development of double diabetes depends heavily on genetic markers, which include HLA variants, together with TCF7L2 polymorphisms and PTPN22 mutations. Genetic testing allows medical professionals to sort patients so they can start insulin treatment immediately for people with beta-cell deterioration and enhance insulin-sensitizing medications for those with insulin resistance [74].
The rising presence of AI and machine learning (ML) applications helps doctors with diabetes management by predicting glycemic variations, treatment optimization, and clinical decisions. AI algorithms process insulin pump data and lifestyle patterns, with CGM information, to provide dynamic adjustments for adjusting insulin doses and dietary suggestions that occur in real time [71]. ML enables the detection of early complications through its detection of diabetic nephropathy, together with diabetic retinopathy, thus facilitating preventive measures. Through risk stratification tools powered by AI, it becomes possible to establish which high-risk patients will gain the most benefit from GLP-1 receptor agonists or SGLT2 inhibitors, through the assessment of cardiovascular and metabolic data points [73].
The use of personalized medicine in double diabetes includes metabolite detection with AI and microbiome assessment, in addition to treatment algorithms that focus on individual lifestyle needs to build total patient care approaches. The advancement of precision medicine through genomics, digital health technologies, and AI analytics systems will boost early detection and optimized treatment and reduce complications in managing double diabetic patients.
Conclusions
This review analyzed double diabetes pathophysiology, epidemiological data, clinical outcomes, and treatment strategies. The condition is becoming more prevalent due to obesity, sedentary lifestyles, and genetic factors. Early detection and targeted treatment approaches are essential. Double diabetes presents diagnostic challenges. Biomarker analysis, C-peptide levels, and autoantibody testing help differentiate it from standard diabetes types. Patients face a higher risk of both microvascular and macrovascular complications. Effective treatment requires a combination of insulin therapy, metformin, GLP-1 receptor agonists, or SGLT2 inhibitors. Lifestyle modifications, including structured exercise and dietary adjustments, are also necessary. Future advancements in AI-driven healthcare and CGM may enhance treatment options. Research should focus on refining diagnostic methods, exploring immunotherapies, and utilizing genetic testing for early detection. Sustained metabolic health in double diabetic patients remains a key priority.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Raj K. Chaudhary, Obaid Ali, Amrendra Kumar, Abilesh Kumar, Anjum Pervez
Acquisition, analysis, or interpretation of data: Raj K. Chaudhary, Obaid Ali, Amrendra Kumar, Abilesh Kumar, Anjum Pervez
Drafting of the manuscript: Raj K. Chaudhary, Obaid Ali, Amrendra Kumar, Abilesh Kumar, Anjum Pervez
Critical review of the manuscript for important intellectual content: Raj K. Chaudhary, Obaid Ali, Amrendra Kumar, Abilesh Kumar, Anjum Pervez
Supervision: Raj K. Chaudhary
References
- 1.Double diabetes-when type 1 diabetes meets type 2 diabetes: definition, pathogenesis and recognition. Bielka W, Przezak A, Molęda P, Pius-Sadowska E, Machaliński B. Cardiovasc Diabetol. 2024;23:62. doi: 10.1186/s12933-024-02145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obesity and diabetes - not only a simple link between two epidemics. Chobot A, Górowska-Kowolik K, Sokołowska M, Jarosz-Chobot P. Diabetes Metab Res Rev. 2018;34:0. doi: 10.1002/dmrr.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. American Diabetes Association Professional Practice Committee. Diabetes Care. 2022;45:0–38. [Google Scholar]
- 4.Type 1 diabetes. Atkinson MA, Eisenbarth GS, Michels AW. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obesity and type 2 diabetes in children: epidemiology and treatment. Pulgaron ER, Delamater AM. Curr Diab Rep. 2014;14:508. doi: 10.1007/s11892-014-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rising tide: the global surge of type 2 diabetes in children and adolescents demands action now. Pappachan JM, Fernandez CJ, Ashraf AP. World J Diabetes. 2024;15:797–809. doi: 10.4239/wjd.v15.i5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Samsu N. Biomed Res Int. 2021;2021:1497449. doi: 10.1155/2021/1497449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Double diabetes: a distinct high-risk group? Kietsiriroje N, Pearson S, Campbell M, Ariëns RA, Ajjan RA. Diabetes Obes Metab. 2019;21:2609–2618. doi: 10.1111/dom.13848. [DOI] [PubMed] [Google Scholar]
- 9.The role of TCF7L2 in type 2 diabetes. Del Bosque-Plata L, Martínez-Martínez E, Espinoza-Camacho MÁ, Gragnoli C. Diabetes. 2021;70:1220–1228. doi: 10.2337/db20-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epigenetic regulation in etiology of type 1 diabetes mellitus. Cerna M. Int J Mol Sci. 2019;21:36. doi: 10.3390/ijms21010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gene-gene and gene-environment interactions in lipodystrophy: lessons learned from natural PPARγ mutants. Broekema MF, Savage DB, Monajemi H, Kalkhoven E. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:715–732. doi: 10.1016/j.bbalip.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 12.KCNJ11: genetic polymorphisms and risk of diabetes mellitus. Haghvirdizadeh P, Mohamed Z, Abdullah NA, Haghvirdizadeh P, Haerian MS, Haerian BS. J Diabetes Res. 2015;2015:908152. doi: 10.1155/2015/908152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genetic polymorphism of PTPN22 in autoimmune diseases: a comprehensive review. Tizaoui K, Shin JI, Jeong GH, et al. Medicina (Kaunas) 2022;58:1034. doi: 10.3390/medicina58081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM, Mayer-Davis EJ. Endocr Rev. 2018;39:629–663. doi: 10.1210/er.2017-00191. [DOI] [PubMed] [Google Scholar]
- 15.The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Holt RI, DeVries JH, Hess-Fischl A, et al. Diabetes Care. 2021;44:2589–2625. doi: 10.2337/dci21-0043. [DOI] [PubMed] [Google Scholar]
- 16.Insulin resistance and associated factors in patients with type 1 diabetes. Teixeira MM, Diniz Mde F, Reis JS, et al. Diabetol Metab Syndr. 2014;6:131. doi: 10.1186/1758-5996-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Front Endocrinol (Lausanne) 2018;9:2. doi: 10.3389/fendo.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prevalence of positive diabetes-associated autoantibodies among type 2 diabetes and related metabolic and inflammatory differences in a sample of the Bulgarian population. Zaharieva ET, Velikova TV, Tsakova AD, Kamenov ZA. J Diabetes Res. 2017;2017:9016148. doi: 10.1155/2017/9016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insulin resistance in type 1 diabetes mellitus. Kaul K, Apostolopoulou M, Roden M. Metabolism. 2015;64:1629–1639. doi: 10.1016/j.metabol.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 20.The evolving landscape of autoantigen discovery and characterization in type 1 diabetes. Purcell AW, Sechi S, DiLorenzo TP. Diabetes. 2019;68:879–886. doi: 10.2337/dbi18-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetes management in chronic kidney disease: synopsis of the 2020 KDIGO clinical practice guideline. Navaneethan SD, Zoungas S, Caramori ML, et al. Ann Intern Med. 2021;174:385–394. doi: 10.7326/M20-5938. [DOI] [PubMed] [Google Scholar]
- 22.Mechanisms of insulin resistance in type 1 diabetes mellitus: a case of glucolipotoxicity in skeletal muscle. Sammut MJ, Dotzert MS, Melling CW. J Cell Physiol. 2024;239:0. doi: 10.1002/jcp.31419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The complex interplay between imbalanced mitochondrial dynamics and metabolic disorders in type 2 diabetes. Van Huynh T, Rethi L, Rethi L, Chen CH, Chen YJ, Kao YH. Cells. 2023;12:1223. doi: 10.3390/cells12091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prevalence of diabetes-associated autoantibodies among patients presenting with type 2 diabetes and related metabolic differences. Moosaie F, Meftah N, Deravi N, et al. Prim Care Diabetes. 2021;15:169–174. doi: 10.1016/j.pcd.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Diabetes trends in youth. Bloomgarden Z, Rapaport R. J Diabetes. 2023;15:286–288. doi: 10.1111/1753-0407.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The causes of insulin resistance in type 1 diabetes mellitus: is there a place for quaternary prevention? Wolosowicz M, Lukaszuk B, Chabowski A. Int J Environ Res Public Health. 2020;17:8651. doi: 10.3390/ijerph17228651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Insulin sensitivity and complications in type 1 diabetes: new insights. Bjornstad P, Snell-Bergeon JK, Nadeau KJ, Maahs DM. World J Diabetes. 2015;6:8–16. doi: 10.4239/wjd.v6.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The evolving landscape of type 1 diabetes management. Ebekozien O. Endocrinol Metab Clin North Am. 2024;53:17–19. doi: 10.1016/j.ecl.2023.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Long-term glycemic variability and vascular complications in type 2 diabetes: post hoc analysis of the FIELD study. Scott ES, Januszewski AS, O'Connell R, et al. J Clin Endocrinol Metab. 2020;105:3638–3649. doi: 10.1210/clinem/dgaa361. [DOI] [PubMed] [Google Scholar]
- 30.Insulin resistance and risk of major vascular events and all-cause mortality in type 1 diabetes: a 10-year follow-up study. Garofolo M, Gualdani E, Scarale MG, et al. Diabetes Care. 2020;43:0–41. doi: 10.2337/dc20-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population-based cohort study. Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Ann Rheum Dis. 2015;74:1968–1975. doi: 10.1136/annrheumdis-2014-205216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipotoxicity and β-cell failure in type 2 diabetes: oxidative stress linked to NADPH oxidase and ER stress. Vilas-Boas EA, Almeida DC, Roma LP, Ortis F, Carpinelli AR. Cells. 2021;10:3328. doi: 10.3390/cells10123328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endoplasmic reticulum stress in beta-cells and development of diabetes. Fonseca SG, Burcin M, Gromada J, Urano F. Curr Opin Pharmacol. 2009;9:763–770. doi: 10.1016/j.coph.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The role of inflammation in diabetes: current concepts and future perspectives. Tsalamandris S, Antonopoulos AS, Oikonomou E, et al. Eur Cardiol. 2019;14:50–59. doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islet amyloid polypeptide: structure, function, and pathophysiology. Akter R, Cao P, Noor H, et al. J Diabetes Res. 2016;2016:2798269. doi: 10.1155/2016/2798269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Dinić S, Arambašić Jovanović J, Uskoković A, et al. Front Endocrinol (Lausanne) 2022;13:1006376. doi: 10.3389/fendo.2022.1006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Type 1 diabetes mellitus and autoimmune diseases: a critical review of the Association and the application of personalized medicine. Popoviciu MS, Kaka N, Sethi Y, Patel N, Chopra H, Cavalu S. J Pers Med. 2023;13:422. doi: 10.3390/jpm13030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evaluation and management of youth-onset type 2 diabetes: a position statement by the American Diabetes Association. Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Diabetes Care. 2018;41:2648–2668. doi: 10.2337/dci18-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dynamics of diabetes and obesity: epidemiological perspective. Boles A, Kandimalla R, Reddy PH. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1026–1036. doi: 10.1016/j.bbadis.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Effect of diet on type 2 diabetes mellitus: a review. Sami W, Ansari T, Butt NS, Hamid MR. https://pubmed.ncbi.nlm.nih.gov/28539866/ Int J Health Sci (Qassim) 2017;11:65–71. [PMC free article] [PubMed] [Google Scholar]
- 41.The impact of gestational diabetes and maternal obesity on the mother and her offspring. Catalano PM. J Dev Orig Health Dis. 2010;1:208–215. doi: 10.1017/S2040174410000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Racial/ethnic differences in the burden of type 2 diabetes over the life course: a focus on the USA and India. Golden SH, Yajnik C, Phatak S, Hanson RL, Knowler WC. Diabetologia. 2019;62:1751–1760. doi: 10.1007/s00125-019-4968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.TCF7L2 genetic variants contribute to phenotypic heterogeneity of type 1 diabetes. Redondo MJ, Geyer S, Steck AK, et al. Diabetes Care. 2018;41:311–317. doi: 10.2337/dc17-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epigenetics in human obesity and type 2 diabetes. Ling C, Rönn T. Cell Metab. 2019;29:1028–1044. doi: 10.1016/j.cmet.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A modern overview on diabetes mellitus: a chronic endocrine disorder. Mukhtar Y, Galalain A, Yunusa U. http://dx.doi.org/10.47672/ejb.409 Eur J Biol. 2020;5:1–4. [Google Scholar]
- 46.Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?) Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Nat Rev Endocrinol. 2021;17:150–161. doi: 10.1038/s41574-020-00443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Wondmkun YT. Diabetes Metab Syndr Obes. 2020;13:3611–3616. doi: 10.2147/DMSO.S275898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.C-peptide determination in the diagnosis of type of diabetes and its management: a clinical perspective. Maddaloni E, Bolli GB, Frier BM, Little RR, Leslie RD, Pozzilli P, Buzzetti R. Diabetes Obes Metab. 2022;24:1912–1926. doi: 10.1111/dom.14785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glutamic acid decarboxylase and tyrosine phosphatase-related islet antigen-2 positivity among children and adolescents with diabetes in Korea. Kim KY, Kim MS, Lee YJ, Lee YA, Lee SY, Shin CH, Kim JH. Diabetes Metab J. 2022;46:948–952. doi: 10.4093/dmj.2021.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Homeostasis model assessment for insulin resistance mediates the positive association of triglycerides with diabetes. Wang Y, Fang Y, Vrablik M. Diagnostics (Basel) 2024;14:733. doi: 10.3390/diagnostics14070733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Petrie JR, Guzik TJ, Touyz RM. Can J Cardiol. 2018;34:575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obesity and diabetes. Leong KS, Wilding JP. https://doi.org/10.1053/beem.1999.0017. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:221–237. doi: 10.1053/beem.1999.0017. [DOI] [PubMed] [Google Scholar]
- 53.Understanding the clinical relationship between diabetic retinopathy, nephropathy, and neuropathy: a comprehensive review. Kulkarni A, Thool AR, Daigavane S. Cureus. 2024;16:0. doi: 10.7759/cureus.56674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cardiovascular disease in diabetes, beyond glucose. Eckel RH, Bornfeldt KE, Goldberg IJ. https://doi.org/10.1016/j.cmet.2021.07.001. Cell Metab. 2021;33:1519–1545. doi: 10.1016/j.cmet.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diabetes and peripheral artery disease: a review. Soyoye DO, Abiodun OO, Ikem RT, Kolawole BA, Akintomide AO. World J Diabetes. 2021;12:827–838. doi: 10.4239/wjd.v12.i6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The association between diabetes mellitus and depression. Bădescu SV, Tătaru C, Kobylinska L, Georgescu EL, Zahiu DM, Zăgrean AM, Zăgrean L. https://pubmed.ncbi.nlm.nih.gov/27453739/ J Med Life. 2016;9:120–125. [PMC free article] [PubMed] [Google Scholar]
- 57.Burnout related to diabetes mellitus: a critical analysis. Kontoangelos K, Raptis A, Lambadiari V, et al. Clin Pract Epidemiol Ment Health. 2022;18:0. doi: 10.2174/17450179-v18-e2209010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Body weight considerations in the management of type 2 diabetes. Apovian CM, Okemah J, O'Neil PM. Adv Ther. 2019;36:44–58. doi: 10.1007/s12325-018-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The effects of metformin in type 1 diabetes mellitus. Beysel S, Unsal IO, Kizilgul M, Caliskan M, Ucan B, Cakal E. BMC Endocr Disord. 2018;18:1. doi: 10.1186/s12902-017-0228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Are thiazolidinediones a preferred drug treatment for type 2 diabetes? Hurren KM, Dunham MW. https://doi.org/10.1080/14656566.2020.1853100. Expert Opin Pharmacother. 2021;22:131–133. doi: 10.1080/14656566.2020.1853100. [DOI] [PubMed] [Google Scholar]
- 61.GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Nauck MA, Quast DR, Wefers J, Meier JJ. Mol Metab. 2021;46:101102. doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Exploring the cardiovascular benefits of sodium-glucose cotransporter-2 (SGLT2) inhibitors: expanding horizons beyond diabetes management. Fatima A, Rasool S, Devi S, et al. Cureus. 2023;15:0. doi: 10.7759/cureus.46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DPP-4 inhibitors for treating T2DM - hype or hope? An analysis based on the current literature. Saini K, Sharma S, Khan Y. Front Mol Biosci. 2023;10:1130625. doi: 10.3389/fmolb.2023.1130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. A review. Martín-Peláez S, Fito M, Castaner O. Nutrients. 2020;12:2236. doi: 10.3390/nu12082236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Evert AB, Dennison M, Gardner CD, et al. Diabetes Care. 2019;42:731–754. doi: 10.2337/dci19-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The Dietary Approaches to Stop Hypertension (DASH) eating pattern in special populations. Tyson CC, Nwankwo C, Lin PH, Svetkey LP. Curr Hypertens Rep. 2012;14:388–396. doi: 10.1007/s11906-012-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.The essential role of exercise in the management of type 2 diabetes. Kirwan JP, Sacks J, Nieuwoudt S. Cleve Clin J Med. 2017;84:0–21. doi: 10.3949/ccjm.84.s1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The importance of exercise for glycemic control in type 2 diabetes. Syeda US, Battillo D, Visaria A, Malin SK. Am J Med Open. 2023;9:100031. doi: 10.1016/j.ajmo.2023.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Davies MJ, Aroda VR, Collins BS, et al. Diabetes Care. 2022;45:2753–2786. doi: 10.2337/dci22-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Chung WK, Erion K, Florez JC, et al. Diabetes Care. 2020;43:1617–1635. doi: 10.2337/dci20-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Study of transcription factor 7-like 2 (TCF7L2) gene polymorphism in cirrhotic patients with diabetes. Hassouna MM, Moustafa MS, Hamdy M, Abdelsameea E, Abbasy M, Naguib M. Egypt Liver J. 2023;5:54. [Google Scholar]
- 72.Artificial intelligence in diabetes management: advancements, opportunities, and challenges. Guan Z, Li H, Liu R, et al. Cell Rep Med. 2023;4:101213. doi: 10.1016/j.xcrm.2023.101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Artificial intelligence powered glucose monitoring and controlling system: pumping module. Medanki S, Dommati N, Bodapati HH, et al. World J Exp Med. 2024;14:87916. doi: 10.5493/wjem.v14.i1.87916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Artificial intelligence-driven transformations in diabetes care: a comprehensive literature review. Iftikhar M, Saqib M, Qayyum SN, et al. Ann Med Surg (Lond) 2024;86:5334–5342. doi: 10.1097/MS9.0000000000002369. [DOI] [PMC free article] [PubMed] [Google Scholar]