Abstract
A simple immunocytochemical method was standardized for the direct demonstration of mycobacterial antigen in cerebrospinal fluid (CSF) specimens of patients with tuberculous meningitis (TBM). CSF-cytospin smears were prepared from 22 patients with a clinical diagnosis of TBM and also from an equal number of patients with nontuberculous neurological diseases (disease control). Immunocytological demonstration of mycobacterial antigens in the cytoplasm of monocytoid cells was attempted, by using rabbit immunoglobulin G to Mycobacterium tuberculosis as the primary antibody. Of the 22 CSF-cytospin smears from TBM patients, 16 showed positive immunostaining, while all of the CSF-cytospin smears from the disease control showed negative immunostaining for mycobacterial antigen. The technical aspects of this immunocytological method for the demonstration of mycobacterial antigens are simple, rapid, and reproducible, as well as specific, and therefore can be applied for the early diagnosis of TBM, particularly in patients in whom bacteriological methods did not demonstrate the presence of M. tuberculosis in the CSF.
A confirmatory (“gold standard”) laboratory diagnosis of tuberculous meningitis (TBM) depends upon the demonstration of the causative agent of the disease, i.e., Mycobacterium tuberculosis, in the cerebrospinal fluid (CSF) specimens by bacteriological methods such as Ziehl-Neelsen staining and cultures. The acid-fast bacilli are seldom demonstrated in CSF smears. The culture methods are not only time-consuming but also less sensitive. In an earlier study we reported that lumbar CSF samples contain fewer M. tuberculosis bacilli than does cisternal and ventricular CSF in patients with TBM (9). However, for the routine bacteriological studies, cisternal and ventricular CSF cannot be obtained since these procedures can sometimes lead to fatal consequences; thus; CSF samples are usually collected from the lumbar region in patients with TBM. Because of the catastrophic nature of the disease and because effective as well as specific chemotherapy is available for this potentially curable infectious disease, clinicians cannot delay antituberculosis treatment (ATT) while waiting for a confirmatory bacteriological diagnosis of TBM. It is also well recognized that early diagnosis and institution of appropriate treatment in a patient with TBM will lead to complete neurological recovery, whereas a delay in diagnosis and treatment often leads to irreversible neurological sequelae.
During the past two decades several biochemical (3, 6, 7), immunological (1, 2, 5, 8, 11), and molecular biological methods (4) have been described as an adjunct for the laboratory diagnosis of TBM. All of these studies are aimed at the analysis of CSF and measure either the host response to infection or the breakdown products of M. tuberculosis bacilli. In the present study, a simple immunocytochemical method was devised to demonstrate mycobacterial antigens in the cytoplasm of monocytoid cells (macrophages) in the CSF of patients with TBM. This was attempted by using rabbit immunoglobulin G (IgG) to M. tuberculosis as the primary antibody. The specificity of this immunocytochemical method was critically assessed in the CSF-cytospin smears of patients with nontuberculous neurological diseases and the sensitivity of the assay has been evaluated in CSF culture-proven patients with TBM. We consider this newer approach for the laboratory diagnosis of TBM to be unique; it can be easily performed in any routine clinical laboratory and therefore is best suited to the laboratories in developing countries where laboratory resources are limited.
MATERIALS AND METHODS
The Sree Chitra Tirunal Institute for Medical Sciences and Technology (referred to here as “the Insitute”), located in the Kerala State, is the major tertiary referral center for neurological diseases in Thiruvananthapuram, India. In 2000, 22 patients with a clinical diagnosis of TBM were referred to the Insitute from several outlying hospitals in the Kerala State. Prior to admission, most of these patients (20 of 22) had received ATT for periods ranging between 2 and 7 weeks (rifampin at 450 mg, isoniazid at 300 mg, streptomycin at 500 mg, and ethambutol at 50 mg daily). At the Insitute, the diagnosis of TBM in these patients is based on relevant clinical features such as neck rigidity, positive Kernig's sign, and compatible neuroradiological evidences of basal exudates in magnetic resonance image scans. None of these patients had clinical or radiological evidence of tuberculosis in the lungs. At the Insitute, CSF analysis is one of the laboratory investigations performed in the management of patients with meningitis. CSF obtained from the lumbar region was collected from all of these patients and was analyzed by routine cytological, biochemical, microbiological, and immunological methods.
The biochemical parameters in all of the CSF samples showed elevated protein (70 to 900 mg/100 ml) and reduced glucose concentration (10 to 30 mg/100 ml). A cytospin (Cytopro; Wescor, USA) was used for the CSF cytological studies. Three cytospin smears from each CSF sample were prepared. One smear was stained with hematoxylin and eosin and then examined by microscopy. Of 22 CSF smears from TBM patients, 16 showed a mixture of lymphocytes, plasmacytoid lymphocytes, and monocytoid cells. The number of monocytoid cells ranged between 10 to 65 in individual CSF-cytospin smears. In six TBM patients, the CSF-cytospin smear showed only occasional lymphocytes and monocytoid cells were not present. The second cytospin smear from each of the 22 TBM patients was fixed in cold acetone and used for immunocytological studies. M. tuberculosis was isolated in the CSF samples from 3 of 22 TBM patients by culture and, in 19 patients, the CSF cultures were repeated twice but they did not grow M. tuberculosis. Acid-fast bacilli were not demonstrated in any of the 22 CSF smears by the Ziehl-Neelsen staining method. CSF specimens from 22 patients with nontuberculous neurological diseases were selected as disease controls. The 22 patients in the disease control group were grouped as follows: 5 had bacterial meningitis due to Haemophilus influenzae (n = 3) or Nisseria meningitidis (n = 2), 5 had partially treated pyogenic meningitis, 2 had cryptococcal meningitis, and 10 had chronic meningitis. The CSF samples from the disease control were similarly subjected to cytological, biochemical, microbiological, and immunocytological analyses. The CSF samples from the disease control group also showed elevated proteins (60 to 750 mg/100 ml) and reduced glucose concentrations (5 to 30 mg/100 ml). The CSF-cytospin smears in the disease control group showed a mixture of neutrophils, lymphocytes, and monocytoid cells in 18 of 22 CSF-cytospin smears. The number of monocytoid cells in the patients ranged between 0 to 8 cells. In four patients, CSF-cytospin smears showed only a few lymphocytes (<10/mm3) and no monocytoid cells.
Immunocytochemical method for the demonstration of mycobacterial antigens.
The cytospin smears from the TBM and disease control groups were simultaneously stained by the immunocytological method to demonstrate the presence of mycobacterial antigens. Briefly, the acetone-fixed CSF smears were washed several times with 0.05 M Tris-buffered saline with Tween 20 (pH 7.6) (TBS-T). CSF-cytospin smears were then treated with 3% H2O2 for 5 min and washed thrice in 0.05 M TBS-T. Smears were then incubated with primary antibody (20 μg of polyvalent rabbit IgG to M. tuberculosis/ml [10]) for 1 h at 37°C. Subsequently, the smears were incubated with the anti-rabbit IgG-biotin conjugate and streptavidin horseradish peroxidase (Dako LSAB2 System) for 45 min each at room temperature. After that, the smears were washed thoroughly with TBS-T. Smears were then incubated for 10 min at room temperature in a substrate, consisting of diaminobenzidine tetrachloride (10 mg dissolved in 5 ml of 0.05 M TBS-T and 5 ml of 3% H2O2). Finally, the smears were counterstained with Harris hematoxylin, dehydrated, cleared in xylene, mounted in Permount (Sigma Chemical Co.), and visualized under a microscope.
RESULTS
Of 22 CSF-cytospin smears from TBM patients, 16 showed a mixture of lymphocytes and monocytoid cells (Fig. 1). Approximately, 15% monocytoid cells and lymphocytes showed degenerative changes in their cytoplasm. All of the well-preserved monocytes in the smear showed positive immunostaining for mycobacterial antigens in the form of brownish red granules in the cytoplasm (Fig. 2). About 70 to 80% of monocytoid cells in the smears showed positive immunostaining or mycobacterial antigens. Besides this, aggregates of immunostained extracellular brownish material was also seen in the smears. In six CSF smears of TBM patients, the immunostaining was negative because in these cases the smears showed only a few lymphocytes. Positive immunobinding in the monocytoid cells was also seen in the three TBM CSF samples in which M. tuberculosis was isolated by culture. In order to evaluate the reproducibility of the assay, immunostaining was repeated on the third CSF-cytospin smear in the same patient. There was no variation in the immunostaining pattern. All of the 22 patients received ATT based on the results of the immunocytochemical staining. A total of 16 patients had optimal neurological recovery, and in 6 patients the neurological recovery was suboptimal. None of these CSF-cytospin smears from the patients in the disease control group showed positive immunostaining, indicating that nonspecific immunostaining did not occur by this technique.
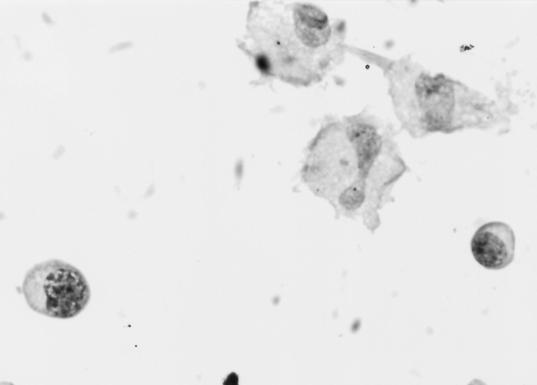
FIG. 1.
CSF-cytospin smears from TBM patients showing a mixture of monocytoid cells and lymphocytes (hematoxylin and eosin staining; magnification, ×200).
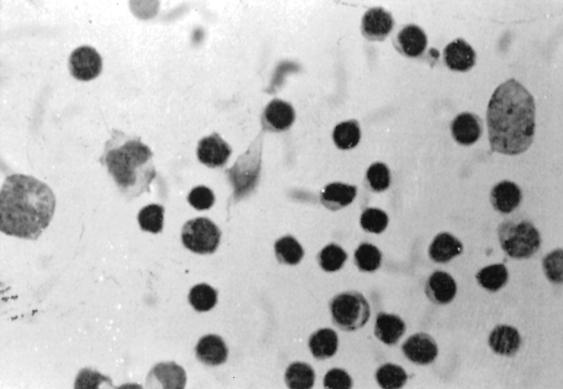
FIG. 2.
Positive immunostaining in the cytoplasm of monocytoid cells in TBM patients by the ABC method (magnification, ×200).
DISCUSSION
TBM is a potentially curable infectious disease of the CNS, and thus there is a need to design an alternative diagnostic method to the conventional microbiological method for the early laboratory diagnosis of TBM so that an effective therapeutic modality can be instituted quickly in patients with TBM. To meet the above objective, we devised a simple immunocytological method. The fundamental principle of this assay is that the CSF in patients with TBM during active stages of the disease contains monocytoid cells (macrophages) and lymphocytes. The function of these monocytes is to phagocytose the tubercle bacilli and process the antigenic component of the bacilli. Thus, the cytoplasm of the monocytes in patients with TBM during the active stages contain mycobacterial antigen. The presence of mycobacterial antigens in these monocytes has been demonstrated by an immunocytochemical method in this study. We used rabbit IgG to M. tuberculosis as the primary antibody to demonstrate mycobacterial antigens in the CSF smears.Of 22 CSF-cytospin smears from patients with TBM, 16 showed positive immunostaining, while cytospin smears from 6 TBM patients yielded negative immunostaining because there was a paucity of monocytoid cells in these CSF smears. There was no false-positive immunostaining in the CSF smears from the disease control group.
Earlier immunoassays described in the literature for the detection of mycobacterial antigen in the CSF of patients with TBM include the latex agglutination test with anti-plasma membrane antibody (5), a sandwich enzyme-linked immunosorbent assay (ELISA) with anti-BCG antibody (12), and an inhibition ELISA with polyvalent antibody against M. tuberculosis (2). In our earlier study, we also used a Dot-Iba to detect a 14-kDa mycobacterial antigen in the CSF of patients with TBM (13). The goal of these earlier studies was to detect the circulating mycobacterial antigens in the CSF of patients with TBM. In the present study, however, we have demonstrated the presence of mycobacterial antigens in the monocytoid cells instead of the CSF. This method carries a sensitivity of 72.5% (16 of 22) and a specificity of 100%. The technical part of the assay is much more simple than the methodology described in earlier studies. The result of this assay can be easily visualized under the microscope and can be obtained within 5 h of the receipt of CSF samples in the laboratory. The presence of an adequate number of monocytoid cells (>5/high-power field) in the CSF is essential for immunostaining, and this should be ascertained in the intimal hematoxylin-and-eosin-stained smear. A positive result obtained by this immunocytological method has potential diagnostic application in patients with TBM. Hitherto, a similar study has not been described in the literature. We therefore consider this newer diagnostic approach to have potential application for the laboratory diagnosis of TBM, particularly in patients for whom bacteriological methods did not confirm the diagnosis.
Acknowledgments
We thank the Director of the Insitute for the kind permission to publish the article.
This study received support from the Department of Science and Technology, New Delhi, India (SP/SO/B-94), and the Council for Scientific and Industrial Research, New Delhi, India.
REFERENCES
- 1.Annamma, M., V. V. Radhakrishnan, and S. Sehgal. 1990. Diagnosis of tuberculous meningitis by enzyme-linked immunosorbant assays to detect mycobacterial antigen and antibody in cerebrospinal fluid of patients with tuberculous meningitis. Med. Microbiol. Immunol. 179:281-288. [DOI] [PubMed] [Google Scholar]
- 2.Bal, V. R., J. Kamat, J. Kamath, and P. Kadoth. 1983. Enzyme linked immunosorbent assay for mycobacterial antigens. Ind. J. Med. Res. 78:477-483. [PubMed] [Google Scholar]
- 3.Brooks, I. B., D. C. Edman, C. C. Alley, R. B. Craven, and N. I. Girgis. 1980. Frequency-pulsed electron capture gas-liquid chromatography and the tryptophan color test for rapid diagnosis of tuberculous and other forms of lymphocytic meningitis. J. Clin. Microbiol. 12:208-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kox, L. F. F., S. Kuijper, and A. H. J. Kolk. 1995. Early diagnosis of tuberculous meningitis by polymerase chain reaction. Neurology 45:2228-2232. [DOI] [PubMed] [Google Scholar]
- 5.Krambovitis, E., M. B. McIllmurray, P. E. Lock, W. Hendrickse, and H. Holzel. 1984. Rapid diagnosis of tuberculous meningitis by latex particle agglutination. Lancet ii:1229-1231. [DOI] [PubMed] [Google Scholar]
- 6.Mandal, B. K., D. I. K. Evans, A. G. Ironside, and B. R. Pullan. 1972. Radioactive bromide partition test in differential diagnosis of tuberculous meningitis. Br. Med. J. 4:413-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mardh, P. A., L. Larson, N. Hoiboy, H. C. Engback, and G. Odham. 1983. Tuberculostearic acid as a diagnostic marker in tuberculous meningitis. Lancet ii:367-369. [DOI] [PubMed] [Google Scholar]
- 8.Mastroianni, C. M., V. Vullo, F. Paoletti, A. P. Massetti, F. Sorice, and S. Delia. 1991. Detection of mycobacterial antigen by dot-blot assay in the cerebrospinal fluid of patients with TBM. J. Infect. 22:106-107. [DOI] [PubMed] [Google Scholar]
- 9.Radhakrishnan, V. V., A. Mathai, and M. Thomas. 1991. Correlation between culture of Mycobacterium tuberculosis and antimycobacterial antibody in lumbar, ventricular, and cisternal cerebrospinal fluids of patients with tuberculous meningitis. Ind. J. Exp. Biol. 29:845-848. [PubMed] [Google Scholar]
- 10.Radhakrishnan, V. V., A. Mathai, S. B. Rao, and S. Sehgal. 1990. Immunoelectrophoresis for mycobacterial antigens. Ind. J. Exp. Biol. 28:812-815. [PubMed] [Google Scholar]
- 11.Ramkisson, A., Y. M. Coovadia, and H. M. Coovadia. 1988. A competition ELISA for the detection of mycobacterial antigen in tuberculous exudate. Tubercle 69:209-212. [DOI] [PubMed] [Google Scholar]
- 12.Sada, E., G. M. Ruiz-Palacios, Y. Lopez-Vidal, and S. Ponce de Leon. 1983. Detection of mycobacterial antigens in cerebrospinal fluid of patients with tuberculous meningitis by enzyme-linked immunosorbent assay. Lancet ii:651-652. [DOI] [PubMed] [Google Scholar]
- 13.Sumi, M. G., M. Annamma, C. Sarada, and V. V. Radhakrishnan. 1999. Rapid diagnosis of tuberculous meningitis by a dot immunobinding assay to detect mycobacterial antigen in cerebrospinal fluid specimens. J. Clin. Microbiol. 37:3925-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]