Abstract
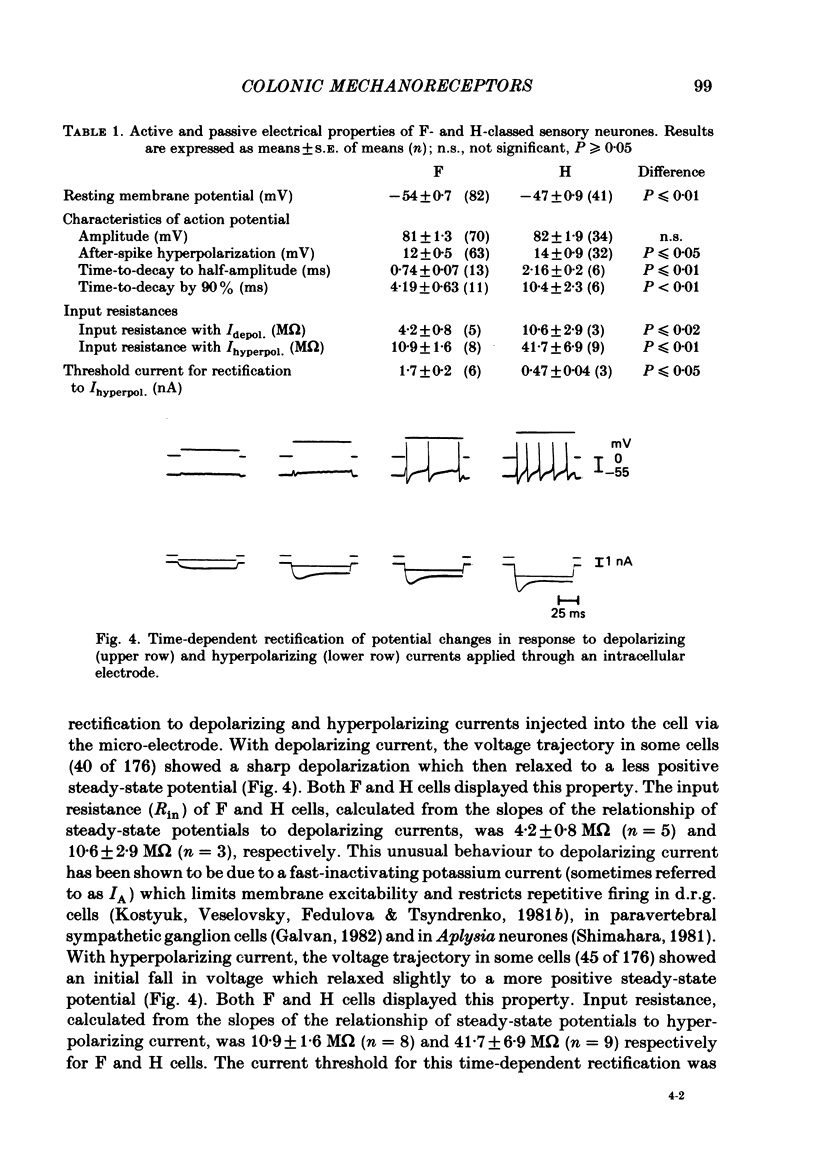
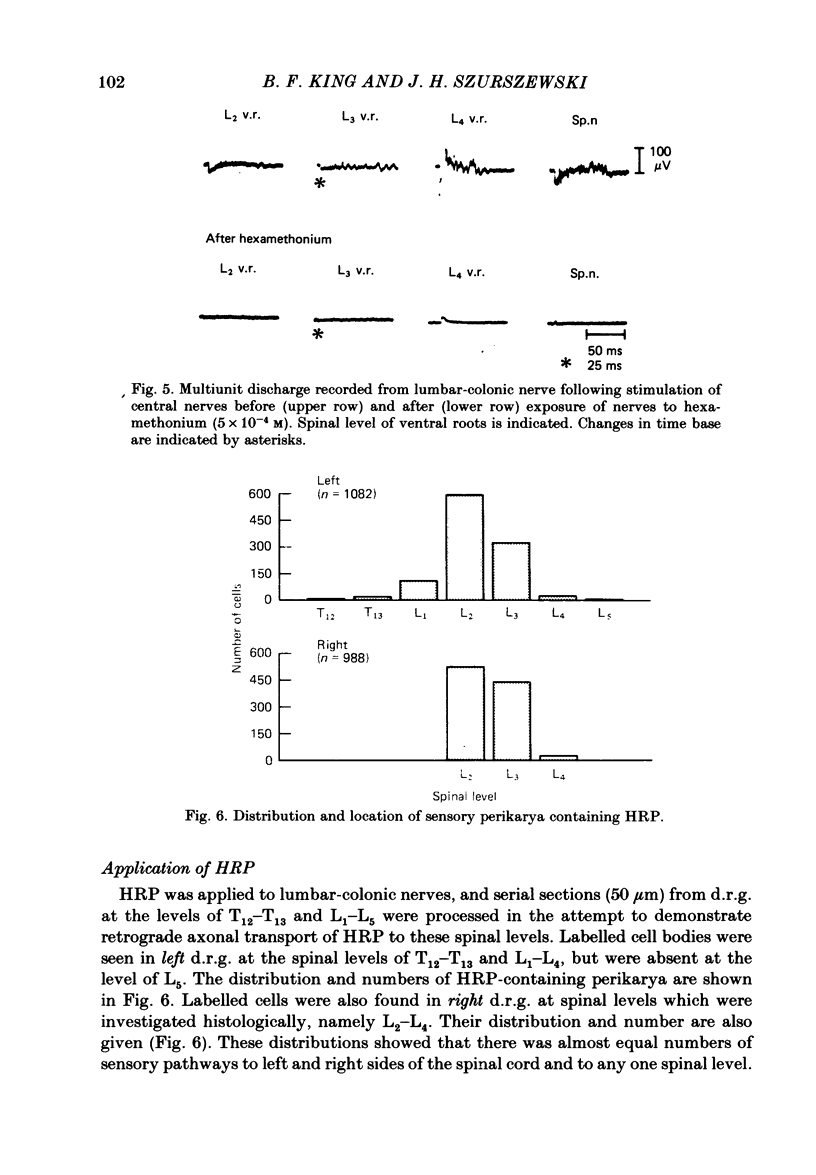
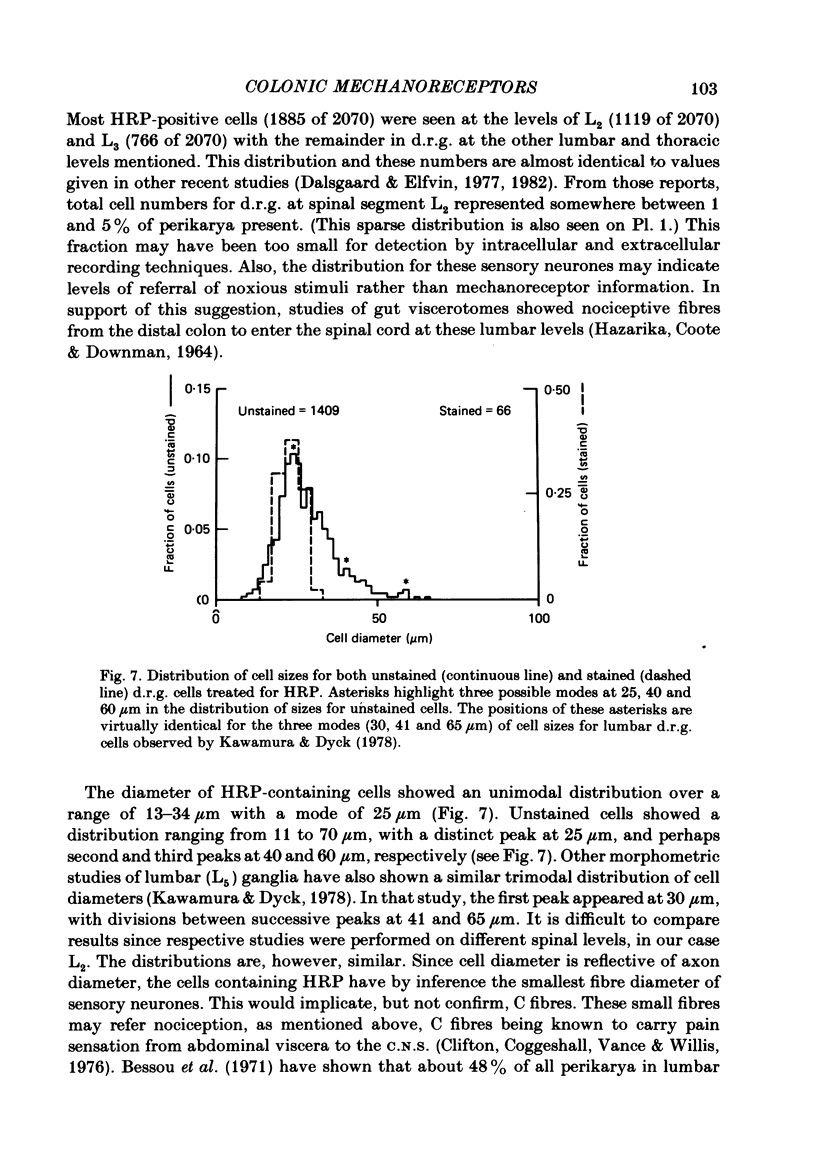
Electrophysiological and histological techniques were used to trace sensory pathways for stretch mechanoreceptor fibres from the distal colon to dorsal root ganglia. Extracellular and intracellular recording techniques revealed sensory pathways for mechanoreceptors to the prevertebral sympathetic ganglia but no further centrally. Histological studies involving the retrograde transport of horseradish peroxidase revealed sensory pathways from the distal colon to the spinal cord, mainly to the level of the second lumbar vertebra. Few (less than 2000) fibres were involved; their perikarya were small (ca. 25 micron). Sensory perikarya in spinal ganglia in the guinea-pig could be categorized into two populations, F and H cells, after a previously defined nomenclature for murine spinal ganglion cells. F and H cells were distinguished initially by their times to decay by 50% of the action potential. H cells took three times as long to repolarize. F and H cells were distinguished further by their electrical properties including membrane potential, input resistance and amplitude and duration of the after-potential following the action potential. Both F and H cells showed unusual time-dependent rectification following either depolarizing or hyperpolarizing current pulses. Threshold currents to show rectification were different for F and H cells. When taken in conjunction with conduction velocities, the electrophysiological evidence may assist in identifying sensory neurones. For example, H cells appeared to have slow conducting (C fibre) axons. From the lack of electrophysiological evidence and limited histological support for major central sensory pathways, it is concluded that stretch mechanoreceptor information from the colon of the guinea-pig is referred mainly to the prevertebral ganglia with minimal involvement of the spinal cord.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2(1):141–145. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- Archakova L. I., Bulygin I. A., Netukova N. I. The ultrastructural organization of sympathetic ganglia of the cat. J Auton Nerv Syst. 1982 Jul;6(1):83–93. doi: 10.1016/0165-1838(82)90025-x. [DOI] [PubMed] [Google Scholar]
- BROWN G. L., PASCOE J. W. Conduction through the inferior mesenteric ganglion of the rabbit. J Physiol. 1952 Sep;118(1):113–123. doi: 10.1113/jphysiol.1952.sp004777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Burgess P. R., Perl E. R., Taylor C. B. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol. 1971 Jan;34(1):116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- Clifton G. L., Coggeshall R. E., Vance W. H., Willis W. D. Receptive fields of unmyelinated ventral root afferent fibres in the cat. J Physiol. 1976 Apr;256(3):573–600. doi: 10.1113/jphysiol.1976.sp011340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowcroft P. J., Holman M. E., Szurszewski J. H. Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J Physiol. 1971 Dec;219(2):443–461. doi: 10.1113/jphysiol.1971.sp009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh G., Kudo N., Kuno M. Membrane properties and conduction velocity in sensory neurones following central or peripheral axotomy. J Physiol. 1977 Aug;270(1):165–180. doi: 10.1113/jphysiol.1977.sp011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard C. J., Elfvin L. G. Spinal origin of preganglionic fibers projecting onto the superior cervical ganglion and inferior mesenteric ganglion of the guinea pig, as demonstrated by the horseradish peroxidase technique. Brain Res. 1979 Aug 17;172(1):139–143. doi: 10.1016/0006-8993(79)90901-6. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Elfvin L. G. Structural studies on the connectivity of the inferior mesenteric ganglion of the guinea pig. J Auton Nerv Syst. 1982 May;5(3):265–278. doi: 10.1016/0165-1838(82)90070-4. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Krier J. The central control of the lumbar sympathetic pathway to the large intestine of the cat. J Physiol. 1979 Apr;289:449–468. doi: 10.1113/jphysiol.1979.sp012746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Jiang Z. G. Non-cholinergic excitatory transmission in inferior mesenteric ganglia of the guinea-pig: possible mediation by substance P. J Physiol. 1982 Apr;325:145–159. doi: 10.1113/jphysiol.1982.sp014141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfvin L. G., Dalsgaard C. J. Retrograde axonal transport of horseradish perioxidase in afferent fibers of the inferior mesenteric ganglion of the guinea pig. Identification of the cells of origin in dorsal root ganglia. Brain Res. 1977 Apr 22;126(1):149–153. doi: 10.1016/0006-8993(77)90221-9. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Vogt M. Acetylcholine synthesis in different regions of the central nervous system. J Physiol. 1948 Jun 25;107(3):372–381. doi: 10.1113/jphysiol.1948.sp004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M. A transient outward current in rat sympathetic neurones. Neurosci Lett. 1982 Aug 31;31(3):295–300. doi: 10.1016/0304-3940(82)90036-2. [DOI] [PubMed] [Google Scholar]
- Garry R. C. The nervous control of the caudal region of the large bowel in the cat. J Physiol. 1933 Mar 15;77(4):422–431. doi: 10.1113/jphysiol.1933.sp002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAZARIKA N. H., COOTE J., DOWNMAN C. B. GASTROINTESTINAL DORSAL ROOT VISCEROTOMES IN THE CAT. J Neurophysiol. 1964 Mar;27:107–116. doi: 10.1152/jn.1964.27.2.107. [DOI] [PubMed] [Google Scholar]
- HEBB C. O., SILVER A. Choline acetylase in the central nervous system of man and some other mammals. J Physiol. 1956 Dec 28;134(3):718–728. doi: 10.1113/jphysiol.1956.sp005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOB C., LUNDBERG A. Reflex excitation of cells in the inferior mesenteric ganglion on stimulation of the hypogastric nerve. Acta Physiol Scand. 1952;26(4):366–382. doi: 10.1111/j.1748-1716.1952.tb00918.x. [DOI] [PubMed] [Google Scholar]
- Kawamura Y., Dyck P. J. Evidence for three populations by size in L5 spinal ganglion in man. J Neuropathol Exp Neurol. 1978 May;37(3):269–272. doi: 10.1097/00005072-197805000-00005. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6(12):2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-III. Potassium currents. Neuroscience. 1981;6(12):2439–2444. doi: 10.1016/0306-4522(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Kreulen D. L., Szurszewski J. H. Reflex pathways in the abdominal prevertebral ganglia: evidence for a colo-colonic inhibitory reflex. J Physiol. 1979 Oct;295:21–32. doi: 10.1113/jphysiol.1979.sp012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krier J., Schmalz P. F., Szurszewski J. H. Central innervation of neurones in the inferior mesenteric ganglion and of the large intestine of the cat. J Physiol. 1982 Nov;332:125–138. doi: 10.1113/jphysiol.1982.sp014405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. On Reflex Action from Sympathetic Ganglia. J Physiol. 1894 May 29;16(5-6):410–440. doi: 10.1113/jphysiol.1894.sp000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specific innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Jan;264(2):565–583. doi: 10.1113/jphysiol.1977.sp011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord K., Ota M., Oenning R. F., Dyck P. J. Method of morphometric evaluation of spinal and autonomic ganglia. J Neurol Sci. 1974 May;22(1):65–71. doi: 10.1016/0022-510x(74)90054-9. [DOI] [PubMed] [Google Scholar]
- ROSS J. G. On the presence of centripetal fibres in the superior mesenteric nerves of the rabbit. J Anat. 1958 Apr;92(2):189–197. [PMC free article] [PubMed] [Google Scholar]
- Shimahara T. Modulation of synaptic output by the transient outward potassium current in aplysia. Neurosci Lett. 1981 Jul 2;24(2):139–142. doi: 10.1016/0304-3940(81)90237-8. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H., Weems W. A. A study of peripheral input to and its control by post-ganglionic neurones of the inferior mesenteric ganglion. J Physiol. 1976 Apr;256(3):541–556. doi: 10.1113/jphysiol.1976.sp011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y., Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J Neurophysiol. 1978 Sep;41(5):1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]