Abstract
To investigate the in vivo role of CD4+ T lymphocytes during acute anaplasmosis, thymectomized calves were selectively depleted of CD4+ T lymphocytes by treatment with anti-CD4 monoclonal antibody (MAb) and were then infected with the Florida strain of Anaplasma marginale in two sequential experiments (experiments 1 and 2). Treatment of thymectomized calves with a total of 5.0 mg of anti-CD4 MAb/kg of body weight during the 1st week followed by 0.3-mg/kg doses administered twice weekly for 7 weeks resulted in significant depletion of CD3+ CD4+ and CD4+ CD45R+ (naive) T lymphocytes from blood, spleen, and peripheral lymph nodes for the duration of the 8-week study, compared to the results for thymectomized control calves treated with a subclass-matched MAb. All calves became parasitemic and pyretic following experimental infection with A. marginale, and decreases in packed cell volume (PCV) coincided with peak parasitemia. No significant differences in PCV or parasitemia were observed between treatment groups. Thymectomized calves treated with anti-CD4 MAb were able to mount an anti-A. marginale antibody response, although in experiment 2, anti-CD4 MAb-treated calves had four- to sixfold lower immunoglobulin G1 (IgG1) and no detectable IgG2 anti-A. marginale major surface protein 2-specific antibody titers compared to thymectomized control calves treated with a subclass-matched MAb. At the level of CD4+-T-lymphocyte depletion achieved and experimental anaplasmosis induced, thymectomized anti-CD4 MAb-treated calves were able to control acute anaplasmosis. This was in contrast to the prediction that significant depletion of CD4+ T lymphocytes would abrogate resistance to acute infection.
Anaplasmosis is one of the most prevalent tick-transmitted hemoparasitic diseases that continue to constrain the production, movement, and utilization of cattle worldwide (24). The causative parasite, Anaplasma marginale, is a member of a genetically defined cluster of bacteria (genogroup II) recently designated as ehrlichia on the basis of 16S rRNA sequence similarity (11, 44). During acute natural infection, greater than 50% of erythrocytes may be parasitized (38), resulting in severe hemolytic anemia (1, 2) with subsequent mortality rates greater than 50% in some herds of cattle (25). Despite extensive losses impacting the major cattle-producing regions of the world, immunization against A. marginale with a safe and effective vaccine has not yet been achieved. The development of an effective anaplasmosis vaccine has been impeded by the lack of knowledge of basic in vivo immune effector mechanisms that are required for development of protective immunity.
The present model of protective immunity in cattle during acute anaplasmosis hypothesizes that clearance of the hemoparasite requires induction of high titers of opsonizing immunoglobulin G2 (IgG2) antibody against surface-exposed epitopes concurrent with CD4+-T-lymphocyte-mediated macrophage activation for opsonization and microbial killing (35). The central component of this model is the CD4+ T lymphocyte that produces gamma interferon (IFN-γ). Recent studies have demonstrated that protection in outer membrane-immunized calves is characterized by A. marginale-specific CD4+ T lymphocytes that produce high levels of IFN-γ and a biased parasite-specific IgG2 antibody response (6). IFN-γ has also been shown to be produced by A. marginale-stimulated peripheral blood mononuclear cells (PBMC) obtained from calves during acute infection (18). IFN-γ activates bovine macrophages in vitro to produce nitric oxide (40), and bovine macrophages have been shown to upregulate expression of inducible nitric oxide synthetase when activated by IFN-γ in the presence of either tumor necrosis factor alpha or lipopolysaccharides (19). In cattle, immunization using interleukin 12 (IL-12) as an adjuvant was shown to significantly enhance IFN-γ production by lymph node mononuclear cells and lymph node-derived CD4+-T-lymphocyte clones following recall stimulation with A. marginale (41), suggesting that IL-12 may enhance a type 1 cytokine response through the induction of IFN-γ. The existing evidence regarding the likely effector mechanisms of protective immunity following protective immunization is supportive of a preferentially induced T-helper 1-like, IFN-γ-dominated response that may enhance production of opsonizing IgG2 antibody in cattle, activation of macrophages, and production of toxic metabolites that mediate parasite killing. Since cattle often recover spontaneously from acute infection, we hypothesized that a similar response would be required for resolution of acute anaplasmosis.
To directly assess the in vivo role of CD4+-T-lymphocyte-mediated immunity in cattle during acute anaplasmosis, we utilized a long-term in vivo CD4+-T-lymphocyte depletion model that was recently developed and validated in thymectomized calves for investigation of mechanisms of CD4+-T-lymphocyte-mediated immunity (42). We report here the effect of selective in vivo depletion of CD4+ T lymphocytes with high doses of anti-CD4 monoclonal antibody (MAb) from thymectomized calves before and during acute experimental infection with A. marginale.
MATERIALS AND METHODS
Animals and surgical procedures.
Holstein bull calves were purchased at birth, castrated, and thymectomized (9) at 2 months of age. The spleen of each animal was marsupialized (43) to permit acquisition of multiple splenic biopsy specimens. Calves were randomly allocated into one of four groups consisting of two calves per group: group 1 (calves 534 and 842), group 2 (calves 843 and 844), group 3 (537 and 865), and group 4 (535 and 860).
In vivo depletion of CD4+ T lymphocytes.
At 3 months of age, in two sequential studies (experiments 1 and 2), animals in group 2 (calves 843 and 844) and group 4 (calves 535 and 860) were treated with an anti-CD4 MAb (ILA-11A; IgG2a) (5) and animals in group 1 (calves 534 and 842) and group 3 (calves 537 and 865) were treated with a subclass-matched control MAb (ColiS205; IgG2a) (4). The doses of MAb required for selective long-term in vivo depletion of functional CD4+ T lymphocytes from thymectomized calves and the frequency of injection were established in a previous study in which significant depletion of CD4+ T lymphocytes from blood, spleen, and peripheral lymph nodes was sustained for the duration of an 8-week study in thymectomized calves compared to thymus-intact, anti-CD4 MAb-treated calves (42). In that study, the mean percentage of naive CD4+ T lymphocytes in thymectomized anti-CD4 MAb-treated calves remained below 1.0% from posttreatment day 7 to the end of the study (42). Briefly, calves treated with MAb were injected intravenously with a total of 540 mg of MAb (approximately 5.0 mg/kg of body weight) during the 1st week of the study. The injected MAb was administered on day 0 (60 mg), day 1 (120 mg), day 3 (240 mg), and day 4 (120 mg). All calves then received 30-mg doses of MAb (approximately 0.3 mg/kg) administered twice weekly for an additional 7 weeks.
A. marginale infection.
Erythrocytes used to experimentally infect all calves were obtained from splenectomized donor calves infected with the Florida strain of A. marginale (29). Splenectomized donor calves were infected with bovine erythrocytes parasitized with A. marginale maintained as a liquid nitrogen-cryopreserved stabilate in dimethyl sulfoxide-phosphate-buffered saline (PBS). Parameters of clinical disease monitored throughout the study included changes in prepatent period (day postinfection to 1% parasitemia), packed cell volume (PCV), and percentage of parasitized erythrocytes (PPE). Calves in each experiment were infected only once. In the first of the two sequential experiments (experiment 1), calves were infected on day 5 following the commencement of MAb treatment with 2 × 104 A. marginale parasitized erythrocytes. In the second of the two sequential experiments (experiment 2), calves were infected on day 12 following the commencement of MAb treatment with 4 × 104 A. marginale parasitized erythrocytes. The design of experiment 2 was based on the outcome of experiment 1. The purpose of delaying the timing by 1 week and doubling the infective dose of A. marginale in experiment 2 was twofold: (i) to prevent potential activation of not-yet-depleted CD4+ T lymphocytes by A. marginale antigen during early experimental infection, thus precluding subsequent resistance of activated CD4+ T lymphocytes to anti-CD4 MAb-mediated mechanisms of depletion (8), and (ii) to attempt to increase parameters of clinical disease (i.e., changes in PCV and PPE) observed in calves following experimental infection.
FC analysis.
Samples of blood and biopsy specimens from spleen and peripheral lymph nodes (superficial cervical or prefemoral) were collected weekly for flow cytometry (FC) analysis. PBMC and mononuclear cells isolated from spleen and lymph node biopsy specimens were prepared for FC analysis as previously described (14, 42). Differentiation markers on mononuclear cells were identified by FC analysis as described previously (14). The MAbs used were specific for bovine CD2 (BAQ95; IgG1) (13); CD3 (MM1A; IgG1) (15); CD4 (IL-A11A; IgG2a) (5); B lymphocytes (BAQ44A; IgM) (30); monocytes and macrophages (MM29A; IgM) (32); CD8 (7C2B; IgG2a) (C. MacKay, unpublished data); and γδ T lymphocytes (GB21A; IgG2b) (16). In order to ensure accurate assessment of numbers of CD4+ T lymphocytes following anti-CD4 MAb treatment with the anti-CD4 MAb IL-A11A (5), a second MAb of a different isotype but with the same specificity (GC50A; IgM) (26) was also used to quantitate CD4+ T lymphocytes by FC analysis. Naive CD4+ T lymphocytes were identified with MAb to CD45R (GS5A; IgG1) (31), and memory CD4+ T lymphocytes were identified with MAb to CD45RO (GC44A; IgG3) (W. C. Davis, unpublished data).
Immunoblots.
Samples of serum were collected weekly and stored at −20°C until used. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblots were performed with MAbs specific for bovine IgG1 and IgG2 (Serotec Ltd., Oxford, United Kingdom) to determine the subclass of the specific IgG response to A. marginale, as described previously (6), with slight modifications as follows. Immunoblots for all animals were individually completed using identical quantities of A. marginale initial body lysate-purified initial bodies from approximately 1.5 × 108 infected bovine erythrocytes as the antigen. Initial bodies of A. marginale were purified from bovine erythrocytes parasitized with the Florida strain of A. marginale (34). Purified initial bodies were applied in a single 8.0-cm lane of a 7.5 to 17.5% polyacrylamide gradient gel for electrophoresis. Control antigen, prepared from approximately 5 × 107 uninfected bovine erythrocytes, was applied to a single 2.5-cm lane on the same 7.5 to 17.5% polyacrylamide gradient gel. Following electrophoresis and transfer of protein to nitrocellulose membranes, the membranes were placed in a Miniblotter 25 apparatus (Immunetics, Cambridge, Mass.) to permit serial titering of individual serum samples from each animal and determination of anti-A. marginale IgG1 and IgG2 antibody titers, as described previously (6). All conditions (i.e., incubation times, washes, antibody dilutions, and exposure times) under which each individual immunoblot used to determine the titer of the specific IgG response to A. marginale was completed were unchanged from animal to animal and from immunoblot to immunoblot to ensure completion of each immunoblot under identical experimental conditions. The end point titer was defined as the reciprocal of the highest dilution with a positive anti-A. marginale major surface protein 2 (MSP-2) signal on the blots. MSP-2 was selected for end point titering since it is an immunodominant protein that has been shown to correlate with protection following homologous and heterologous A. marginale challenge (36, 37).
Statistical analyses.
Overall differences in CD3+ CD4+, CD4+ CD45R+ (naive), and CD4+ C45RO+ (memory) T lymphocytes between treatment groups were determined by repeated-measure analysis of variance (45) between days 7 and 56 following MAb treatment using a mixed linear model procedure and commercially available software (PROC MIXED, version 8.1; SAS Institute Inc., Cary, N.C.). The Tukey-Kramer method for multiple comparisons was used to identify differences between treatment groups (45). A P of <0.05 was considered statistically significant.
RESULTS
Depletion of CD4+ T lymphocytes from blood, spleen, and peripheral lymph nodes.
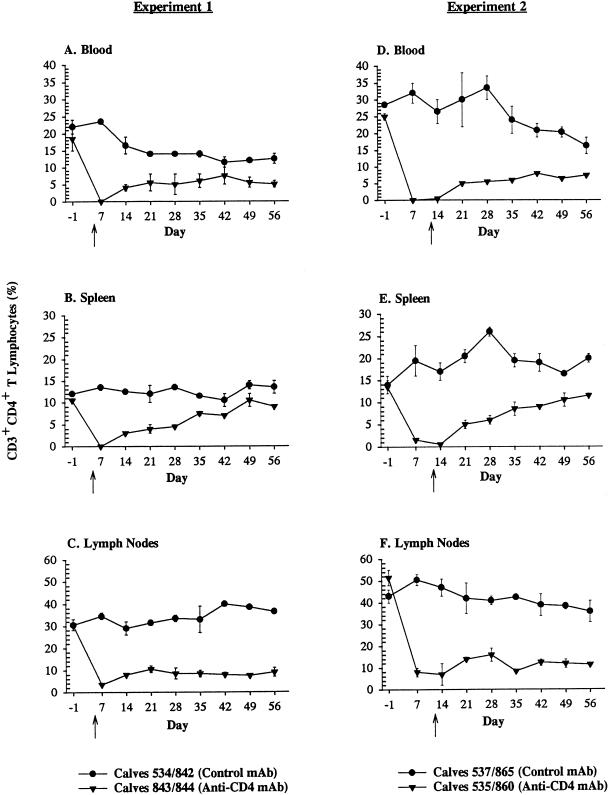
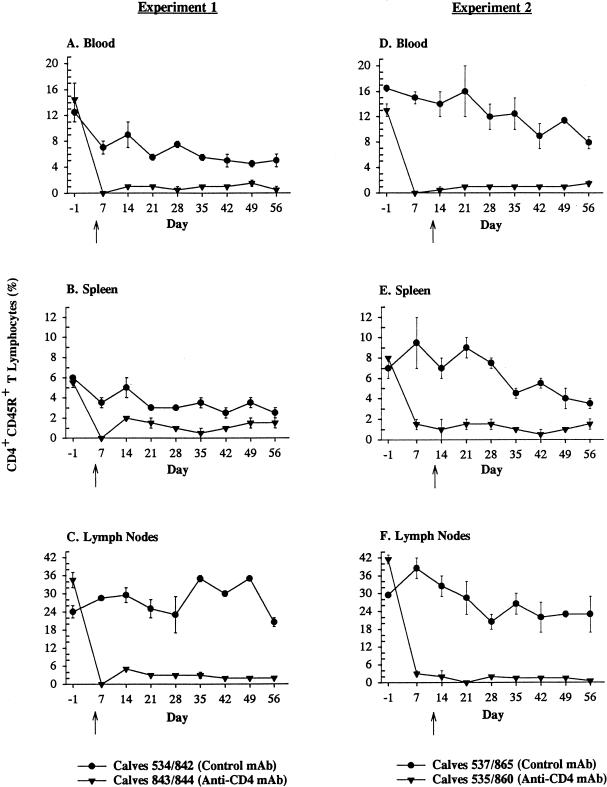
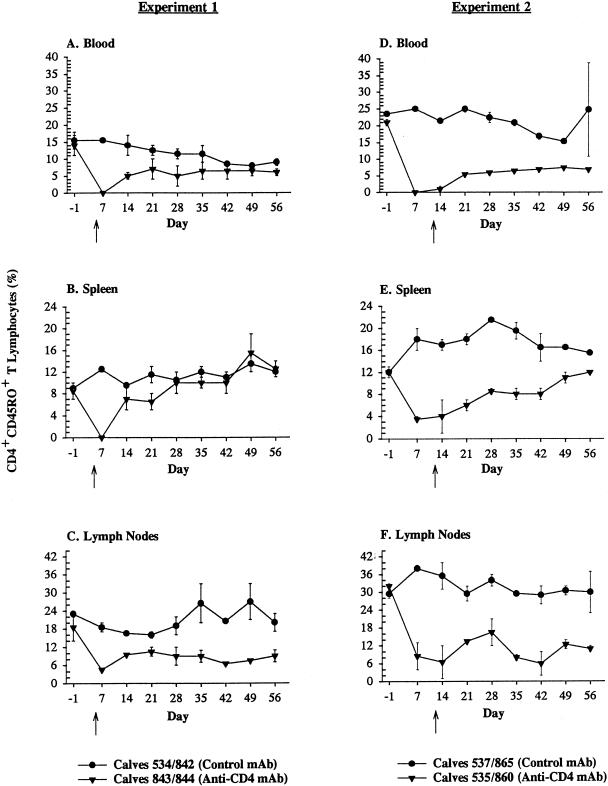
Treatment of thymectomized calves with anti-CD4 MAb resulted in significant depletion of CD3+ CD4+ T lymphocytes (P < 0.05) and CD4+ CD45R+ (naive) T lymphocytes (P < 0.01) from blood, spleen, and lymph nodes in both experiments between days 7 and 56, compared to results for thymectomized control calves treated with a subclass-matched MAb (Fig. 1 and 2). In experiment 1, differences in depletion of CD4+ CD45RO+ (memory) T lymphocytes between treatment groups were significant (P < 0.05) only in lymph nodes and not spleen or blood between days 7 and 56 (Fig. 3). In experiment 2, memory T lymphocytes were significantly (P < 0.05) depleted from blood, spleen, and lymph nodes of thymectomized anti-CD4 MAb-treated calves between days 7 and 56, compared to the tissues of thymectomized control calves treated with a subclass-matched MAb (Fig. 3). With the exception of concomitant decreases in percentages of CD2+ and CD3+ T lymphocytes, as well as absolute reductions in total numbers of lymphocytes (identified on complete blood counts), no other subpopulations of lymphocytes (CD8+, γδ, or B lymphocytes) were depleted by anti-CD4 MAb administration in thymectomized, anti-CD4 MAb-treated calves (data not shown). In all calves, regardless of treatment group, the percentages of B lymphocytes in the peripheral blood and the percentages of CD2+ γδ T lymphocytes in the spleen progressively increased following infection with A. marginale for the duration of the study (data not shown).
FIG. 1.
Depletion of CD3+ CD4+ T lymphocytes. Mean percentages (± standard errors of the mean) of CD3+ CD4+ T lymphocytes in blood, spleen, or lymph nodes of thymectomized calves treated with a subclass-matched control MAb (•) or thymectomized calves treated with anti-CD4 MAb (▾). The arrows indicate the day of infection for each experiment (experiment 1, day 5 following the commencement of MAb treatment; and experiment 2, day 12 following the commencement of MAb treatment).
FIG. 2.
Depletion of naive CD4+ T lymphocytes (CD4+ CD45R+ T lymphocytes). Mean percentages (± standard errors of the means) of CD4+ CD45R+ T lymphocytes in blood, spleen, or lymph nodes of thymectomized calves treated with a subclass-matched control MAb (•) or thymectomized calves treated with anti-CD4 MAb (▾). The arrows indicate the day of infection for each experiment (experiment 1, day 5 following the commencement of MAb treatment; and experiment 2, day 12 following the commencement of MAb treatment).
FIG. 3.
Depletion of memory CD4+ T lymphocytes (CD4+ CD45RO+ T lymphocytes). Mean percentages (± standard errors of the means) of CD4+ CD45RO+ T lymphocytes in blood, spleen, or lymph nodes of thymectomized calves treated with a subclass-matched control MAb (•) or thymectomized calves treated with anti-CD4 MAb (▾). The arrows indicate the day of infection for each experiment (experiment 1, day 5 following the commencement of MAb treatment; and experiment 2, day 12 following the commencement of MAb treatment).
Changes in PCV and PPE in CD4+-T-lymphocyte-depleted and nondepleted A. marginale-infected calves.
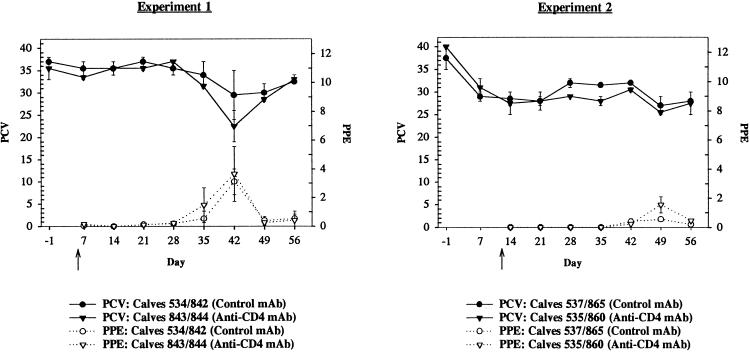
The kinetics of changes in PCV and PPE were essentially identical between treatment groups in both experiments (Fig. 4). Thymectomized calves treated with anti-CD4+ MAb had greater decreases in PCV and a higher PPE in both experiments than did thymectomized control calves treated with a subclass-matched MAb (Fig. 4). Neither of these differences, however, was statistically significant. The maximum changes in pre- versus postinfection PCV within treatment groups and the maximum changes in PCV at peak parasitemia between treatment groups were greater for thymectomized anti-CD4 MAb-treated calves than for thymectomized control calves treated with a subclass-matched MAb, although these differences were not statistically significant (Fig. 4). Doubling the infectious dose from 2 × 104 parasitized erythrocytes to 4 × 104 parasitized erythrocytes and delaying the day of infection by 1 week in experiment 2 did not significantly alter the prepatent period, PCV, or PPE between treatment groups (Fig. 4). Transient pyrexia was observed just prior to and during peak parasitemia in all calves (data not shown).
FIG. 4.
Relationship of PCV to PPE. Mean PCV (± standard error of the mean) of thymectomized calves treated with an isotype-matched MAb (•, control MAb) or anti-CD4 MAb (▾, anti-CD4 MAb) or mean PPE (± standard error of the mean) of thymectomized calves treated with an isotype-matched MAb (○, control MAb) or anti-CD4 MAb (▿, anti-CD4 MAb). The arrows indicate the day of infection for each experiment (experiment 1, day 5 following the commencement of MAb treatment; and experiment 2, day 12 following the commencement of MAb treatment).
Effect of MAb treatment on IgG1 and IgG2 antibody titers to A. marginale.
A. marginale antibody responses to MSP-2, an immunodominant 36-kDa protein (36, 37), were assessed at the conclusion of each study, 2 months postinfection immediately following resolution of acute infection (Table 1). In experiment 1 there were no differences in anti-A. marginale MSP-2 IgG1 or IgG2 antibody titers between treatment groups. In experiment 2, however, treatment of thymectomized calves with anti-CD4 MAb resulted in four- to sixfold reductions in anti-A. marginale MSP-2 IgG1 titers and no detectable IgG2 antibody titers in contrast to anti-A. marginale MSP-2 IgG2 antibody titers of thymectomized control calves treated with a subclass-matched MAb (Table 1).
TABLE 1.
Anti-A. marginale MSP-2 IgG1 and IgG2 antibody titers
| Animala | Titerb
|
|
|---|---|---|
| IgG1 | IgG2 | |
| Expt 1 | ||
| Control MAb | ||
| 534 | 32,000 | <10 |
| 842 | 32,000 | <10 |
| Mean ± SEM | 32,000 ± 0 | <10 ± 0 |
| Anti-CD4 MAb | ||
| 843 | 32,000 | <10 |
| 844 | 32,000 | <10 |
| Mean ± SEM | 32,000 ± 0 | <10 ± 0 |
| Expt 2 | ||
| Control MAb | ||
| 537 | 128,000 | 4,000 |
| 865 | 64,000 | 1,000 |
| Mean ± SEM | 96,000 ± 32,000 | 2,500 ± 1,500 |
| Anti-CD4 MAb | ||
| 535 | 16,000 | <10 |
| 860 | 16,000 | <10 |
| Mean ± SEM | 16,000 ± 0 | <10 ± 0 |
Calves 534 and 842 (experiment 1) and 537 and 865 (experiment 2) were thymectomized and treated with a subclass-matched control MAb. Calves 843 and 844 (experiment 1) and 535 and 860 (experiment 2) were thymectomized and treated with an anti-CD4 MAb. All calves were infected with A. marginale, and isotype-specific antibody responses were assessed 8 weeks postinfection following resolution of acute infection.
Sera were diluted 1:10 to 1:128,000 and were tested for reactivity against A. marginale Florida homogenate on Western blots. The titer is defined as the reciprocal of the highest dilution giving a positive signal on the blots. <10, no reaction at a dilution of 1:10 or higher.
DISCUSSION
Following experimental infection, all calves experienced expected changes in parameters of clinical anaplasmosis, including transient pyrexia just prior to and during peak parasitemia and observed reductions in PCV that coincided with increases in PPE. Despite significant depletion of CD4+ T lymphocytes between treatment groups and a reduction in IgG1 and IgG2 anti-A. marginale MSP-2 antibody titers in experiment 2, no significant differences in PCV or PPE were observed between anti-CD4 MAb-treated thymectomized calves and thymectomized control calves treated with a subclass-matched MAb. In addition, in an attempt to assess differences in T-lymphocyte function, lymphoproliferative responses to A. marginale antigen were evaluated, as described previously (6). There were no detectable significant lymphoproliferative responses of PBMC to A. marginale antigen in anti-CD4 MAb-treated thymectomized calves or in thymectomized control calves in either experiment 1 or 2 (data not shown). At the level of CD4+-T-lymphocyte depletion achieved and acute anaplasmosis induced in this study, thymectomized anti-CD4 MAb-treated calves were able to control acute infection with A. marginale, contrary to the prediction that significant depletion of CD4+ T lymphocytes between treatment groups of animals would abrogate resistance to acute infection.
The lack of observable significant differences in parameters of clinical disease monitored between depleted and nondepleted calves in the present study was unexpected and differed from a previous study in which similar methods were utilized to achieve long-term in vivo depletion of functional CD4+ T lymphocytes to ovalbumin (42). In the previous study, treatment of thymectomized calves with anti-CD4 MAb resulted in depletion of ovalbumin-specific CD4+ T lymphocytes of sufficient magnitude to observe significant differences in immunological responses between treatment groups (42). Thymectomized calves depleted of CD4+ T lymphocytes and immunized with ovalbumin had complete abrogation of lymphoproliferative responses to ovalbumin with significant reduction of IgG1 and no detectable IgG2 ovalbumin-specific antibody responses, compared to thymus-intact anti-CD4 MAb-treated calves (42). One possible reason for the lack of complete abrogation of functional immune responses to A. marginale in the present study might have been lack of absolute depletion of CD4+ T lymphocytes specific for A. marginale antigen. Complete and absolute depletion of CD4+ T lymphocytes from blood, spleen, and lymph nodes of calves infected with A. marginale would certainly provide optimum conditions for testing of the hypothesis; however, these conditions may be very difficult to achieve, if possible at all. The rapid generation of a bovine anti-mouse antibody response directed against the injected mouse anti-bovine CD4 MAb may have protected reappearing CD4+ T lymphocytes from depletion by subsequent anti-CD4 MAb treatment (7, 21, 42). In the present study, the production of bovine anti-mouse antibody was not assessed. Since administration of MAb remained unchanged from the previous study in which the dose of MAb required for selective, long-term, in vivo depletion of functional CD4+ T lymphocytes from thymectomized calves and the frequency of injection were established (42), presumptively the kinetics of the bovine anti-mouse antibody response in the present study was essentially identical to that of the previous study. In the previous study, all calves treated with MAb developed a bovine anti-mouse antibody response (42). Polyclonal expansion of mouse protein-specific activated T lymphocytes may also have partially contributed to the increase in the numbers of activated or memory CD4+ T lymphocytes in blood and peripheral lymphoid organs. Any or a combination of these factors may have contributed to the reappearance of antigen-specific CD4+ T lymphocytes and subsequent resistance to depletion despite continuous anti-CD4 MAb treatment, which may have precluded the observation of significant differences in parameters of clinical disease monitored.
Despite significant differences in depletion of CD4+ T lymphocytes observed between days 7 and 56 between treatment groups, differences in the outcome of results of this study and other previously reported in vivo depletion studies in cattle (22, 33) are likely due to differences in the type of pathogen and incubation period of the various pathogens investigated. Many viral and bacterial pathogens have short incubation and prepatent periods and are suitable for in vivo depletion studies in which only transient depletion of T-lymphocyte subpopulations is necessary to identify a significant treatment effect between depleted and nondepleted animals. In the present study, because of the long prepatent period of A. marginale, the period of optimum CD4+-T-lymphocyte depletion in blood and lymphoid organs may not necessarily have correlated with the period of maximum challenge with replicating microorganisms; therefore, significant differences in clinical disease may not have been recognized. It is possible that use of a higher dose for experimental infection or a different strain of A. marginale may decrease the prepatent period sufficiently to result in observable differences between depleted and nondepleted calves.
Although the present model of protective immunity in cattle during acute anaplasmosis hypothesizes that complete clearance of the hemoparasite requires induction of high titers of opsonizing IgG2 antibody (6), parasite-specific IgG1 antibody may also play a role in control of acute anaplasmosis. All animals in both treatment groups in the present study had strong and predominant IgG1 antibody responses, and all calves controlled acute experimental infection with A. marginale. Control calves in experiment 2 infected with a higher dose of A. marginale parasitized erythrocytes had higher anti-A. marginale MSP-2 IgG1 and IgG2 antibody responses than seen in experiment 1, possibly a reflection of the initial higher infective dose of A. marginale parasitized erythrocytes used to infect calves in experiment 2. Despite four- to sixfold lower IgG1 and no detectable IgG2 anti-A. marginale MSP-2 antibody titers observed in experiment 2, thymectomized anti-CD4 MAb-treated calves were still able to adequately control acute anaplasmosis. That IgG1 plays a predominant role, however, is neither clearly established nor strongly supported by these data. Although these results also suggest that high IgG1 and/or IgG2 anti-A. marginale MSP-2 antibody titers might not be necessary for control of acute anaplasmosis in calves, the minimum amount of specific antibody necessary and sufficient for control of acute anaplasmosis in calves is unknown. Factors other than antibody alone may be responsible for control of acute anaplasmosis. Passive transfer studies have been previously completed to assess the role of A. marginale-specific antibody and have determined that antibody alone may not be sufficient for protection during acute anaplasmosis (17). The passive transfer of antibodies from A. marginale-immune cattle, using the same basic procedure shown to transfer immunity against Babesia bovis (27), failed to protect recipient calves from experimental challenge (17).
The results of the present study differ from those reported previously, in which calves immunized with purified outer membranes of the Florida strain of A. marginale and completely protected against homologous challenge developed only transient and weak IgG1 and strong IgG2 antibody titers prior to challenge (6). Since different antigens and conditions of immunization lead to responses that vary quantitatively and qualitatively, differences in anti-A. marginale MSP-2 antibody responses between the previously reported and the present study may be due to differences in the type of antigen (purified outer membrane proteins versus whole infectious initial bodies), route of entry (subcutaneous immunization versus intravenous inoculation), adjuvant (saponin versus no adjuvant), dose (microgram quantities of purified protein versus whole initial bodies), numbers and types of accessory cells that initially interact with the antigen, and the nature of the responding lymphocytes. The predominant IgG1 antibody response observed in all calves in the present study is similar to that reported previously in which immunization of calves with a plasmid (pVCL/MSP1a) encoding and expressing the complete msp1a gene of A. marginale resulted in restricted IgG1 anti-MSP1a responses (3). In cattle, both IgG1 and IgG2 have been shown to fix bovine complement and mediate phagocytosis by cultured monocytes (28), suggesting that after macrophage activation both IgG antibody isotypes could play a role in control of acute anaplasmosis.
Finally, the results of the present study confirm earlier reports of resistance of calves to anaplasmosis (23, 39). In contrast to adult cattle, although calves are susceptible to infection with A. marginale, they are relatively resistant to clinical disease. The specific mechanisms of resistance of calves to anaplasmosis are unknown, although innate immunity may play an important role. In young calves, a relatively high number (up to 75%) of circulating T cells are CD2− WC1+ γδ T lymphocytes (20). The specific role of γδ T lymphocytes in the peripheral blood and spleen of ruminants is largely unknown, although γδ T lymphocytes in cattle have been shown to present antigen to CD4+ T lymphocytes (10) and are activated by and have been shown to lyse Theileria parva-infected cells by recognizing conserved parasite-induced or parasite-derived antigens in a major histocompatibility complex-unrestricted fashion (12). In the present study, progressive expansion of γδ T lymphocytes was observed in the peripheral blood and spleens of all infected calves (data not shown); all infected calves, regardless of treatment group, were able to control acute anaplasmosis, suggesting a possible role for γδ T lymphocytes in control of acute anaplasmosis.
In conclusion, despite significant differences in depletion of CD4+ T lymphocytes between treatment groups and four- and sixfold reductions in IgG1 and IgG2 anti-A. marginale MSP-2 antibody titers in experiment 2, thymectomized calves treated with anti-CD4 MAb were able to control acute experimental anaplasmosis induced in this study, in contrast to the prediction that significant depletion of CD4+ T lymphocytes between treatment groups would abrogate resistance to acute infection.
Acknowledgments
We thank Mary Jo Hamilton, Will Harwood, and Beverly Hunter for excellent technical laboratory assistance and Duane Chandler, Robert Finch, Ralph Horn, and Pete Steiner for assistance with livestock husbandry. We also thank Teri Olson and Robert Keegan for assistance with surgical preparation and anesthesia of calves.
This work was supported by grants from the American Veterinary Medical Foundation (97-06), National Institutes of Health, National Institute of Allergy and Infectious Diseases (K08AI01447), USDA-ARS-CWU (5348-32000-008-00D), and USDA-CSREES-NRI (2000-02087).
REFERENCES
- 1.Ajayi, S. A., J. A. Wilson, and R. S. Campbell. 1978. Experimental bovine anaplasmosis: clinico-pathological and nutritional studies. Res. Vet. Sci. 25:76-81. [PubMed] [Google Scholar]
- 2.Allen, P. C., K. L. Kuttler, and T. E. Amerault. 1981. Clinical chemistry of anaplasmosis: blood chemical changes in infected mature cows. Am. J. Vet. Res. 42:322-325. [PubMed] [Google Scholar]
- 3.Arulkanthan, A., W. C. Brown, T. C. McGuire, and D. P. Knowles. 1999. Biased immunoglobulin G1 isotype responses induced in cattle with DNA expressing msp1a of Anaplasma marginale. Infect. Immun. 67:3481-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aydintug, M. K., T. J. Inzana, T. Letonja, W. C. Davis, and L. B. Corbeil. 1989. Cross-reactivity of monoclonal antibodies to Escherichia coli J5 with heterologous gram-negative bacteria and extracted lipopolysaccharides. J. Infect. Dis. 160:846-857. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin, C. L., A. J. Teale, J. G. Naessens, B. M. Goddeeris, N. D. MacHugh, and W. I. Morrison. 1986. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J. Immunol. 136:4385-4391. [PubMed] [Google Scholar]
- 6.Brown, W. C., V. Shkap, D. Zhu, T. C. McGuire, W. Tuo, T. F. McElwain, and G. H. Palmer. 1998. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect. Immun. 66:5406-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce, C. J., C. J. Howard, L. H. Thomas, P. R. Tempest, and G. Taylor. 1999. Depletion of CD8+ T cells with chCC63, a chimaeric mouse-bovine antibody. Vet. Immunol. Immunopathol. 71:215-231. [DOI] [PubMed] [Google Scholar]
- 8.Chace, J. H., J. S. Cowdery, and E. H. Field. 1994. Effect of anti-CD4 on CD4 subsets. I. Anti-CD4 preferentially deletes resting, naive CD4 T cells and spares activated CD4 cells. J. Immunol. 152:405-412. [PubMed] [Google Scholar]
- 9.Coleman, G. L., M. L. Crandall, and A. J. Guidry. 1966. Surgical ablation of the thymus gland in neonatal dairy calves. Am. J. Vet. Res. 27:1123-1126. [PubMed] [Google Scholar]
- 10.Collins, R. A., D. Werling, S. E. Duggan, A. P. Bland, K. R. Parsons, and C. J. Howard. 1998. γδ T cells present antigen to CD4+ αβ T cells. J. Leukoc. Biol. 63:707-714. [DOI] [PubMed] [Google Scholar]
- 11.Dame, J. B., S. M. Mahan, and C. A. Yowell. 1992. Phylogenetic relationship of Cowdria ruminantium, agent of heartwater, to Anaplasma marginale and other members of the order Rickettsiales determined on the basis of 16S rRNA sequence. Int. J. Syst. Bacteriol. 42:270-274. [DOI] [PubMed] [Google Scholar]
- 12.Daubenberger, C. A., E. N. Taracha, L. Gaidulis, W. C. Davis, and D. J. McKeever. 1999. Bovine γδ T-cell responses to the intracellular protozoan parasite Theileria parva. Infect. Immun. 67:2241-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis, W. C., and G. S. Splitter. 1991. Individual antigens of cattle. Bovine CD2 (BoCD2). Vet. Immunol. Immunopathol. 27:43-50. [DOI] [PubMed] [Google Scholar]
- 14.Davis, W. C., J. E. Davis, and M. J. Hamilton. 1995. Use of monoclonal antibodies and flow cytometry to cluster and analyze leukocyte differentiation molecules, p. 149-167. In W. C. Davis (ed.), Monoclonal antibody protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 15.Davis, W. C., N. D. MacHugh, Y. H. Park, M. J. Hamilton, and C. R. Wyatt. 1993. Identification of a monoclonal antibody reactive with the bovine orthologue of CD3 (BoCD3). Vet. Immunol. Immunopathol. 39:85-91. [DOI] [PubMed] [Google Scholar]
- 16.Davis, W. C., W. C. Brown, M. J. Hamilton, C. J. Wyatt, J. A. Orden, A. M. Khalid, and J. Naessens. 1996. Analysis of monoclonal antibodies specific for the gamma delta TcR. Vet. Immunol. Immunopathol. 52:275-283. [DOI] [PubMed] [Google Scholar]
- 17.Gale, K. R., G. Leatch, M. Gartside, and C. M. Dimmock. 1992. Anaplasma marginale: failure of sera from immune cattle to confer protection in passive-transfer experiments. Parasitol. Res. 78:410-415. [DOI] [PubMed] [Google Scholar]
- 18.Gale, K. R., M. G. Gartside, C. M. Dimmock, H. Zakrewski, and G. Leatch. 1996. Peripheral blood lymphocyte proliferative responses in cattle infected with or vaccinated against Anaplasma marginale. Parasitol. Res. 82:551-562. [DOI] [PubMed] [Google Scholar]
- 19.Goff, W. L., W. C. Johnson, C. R. Wyatt, and C. W. Cluff. 1996. Assessment of bovine mononuclear phagocytes and neutrophils for induced l-arginine-dependent nitric oxide production. Vet. Immunol. Immunopathol. 55:45-62. [DOI] [PubMed] [Google Scholar]
- 20.Hein, W. R., and C. R. Mackay. 1991. Prominence of γδ T lymphocytes in the ruminant immune system. Immunol. Today 12:30-34. [DOI] [PubMed] [Google Scholar]
- 21.Howard, C. J., P. Sopp, K. R. Parsons, and J. Finch. 1989. In vivo depletion of BoT4 (CD4) and of non-T4/T8 lymphocyte subsets in cattle with monoclonal antibodies. Eur. J. Immunol. 19:757-764. [DOI] [PubMed] [Google Scholar]
- 22.Howard, C. J., M. C. Clarke, P. Sopp, and J. Brownlie. 1992. Immunity to bovine virus diarrhoea virus in calves: the role of different T-cell subpopulations analysed by specific depletion in vivo with monoclonal antibodies. Vet. Immunol. Immunopathol. 32:303-314. [DOI] [PubMed] [Google Scholar]
- 23.Jones, E. W., I. O. Kliewer, B. B. Norman, and W. E. Brock. 1968. Anaplasma marginale infection in young and aged cattle. Am. J. Vet. Res. 29:535-544. [PubMed] [Google Scholar]
- 24.Kocan, K. M., E. F. Blouin, and A. F. Barbet. 2000. Anaplasmosis control. Past, present and future. Ann N. Y. Acad. Sci. 916:501-509. [DOI] [PubMed] [Google Scholar]
- 25.Kuttler, K. L., J. L. Zaugg, and L. W. Johnson. 1984. Serologic and clinical responses of premunized, vaccinated, and previously infected cattle to challenge exposure by two different Anaplasma marginale isolates. Am. J. Vet. Res. 45:2223-2226. [PubMed] [Google Scholar]
- 26.Larsen, R. A., M. L. Monaghan, Y. H. Park, M. J. Hamilton, J. A. Ellis, and W. C. Davis. 1990. Identification and characterization of monoclonal antibodies reactive with bovine, caprine and ovine T-lymphocyte determinants by microfluorimetry. Vet. Immunol. Immunopathol. 25:195-208. [DOI] [PubMed] [Google Scholar]
- 27.Mahoney, D. F., J. D. Kerr, B. V. Goodger, and I. G. Wright. 1979. The immune response of cattle to Babesia bovis (syn. B. argentina). Studies on the nature and specificity of protection. Int. J. Parasitol. 9:297-306. [DOI] [PubMed] [Google Scholar]
- 28.McGuire, T. C., A. J. Musoke, and T. Kurtti. 1979. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils and skin. Immunology 38:249-256. [PMC free article] [PubMed] [Google Scholar]
- 29.McGuire, T. C., G. H. Palmer, W. L. Goff, M. I. Johnson, and W. C. Davis. 1984. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect. Immun. 45:697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naessens, J., and C. J. Howard. 1991. Individual antigens of cattle. Monoclonal antibodies reacting with bovine B cells (BoWC3, BoWC4 and BoWC5). Vet. Immunol. Immunopathol. 27:77-85. [DOI] [PubMed] [Google Scholar]
- 31.Naessens, J., C. J. Howard, and J. Hopkins. 1997. Nomenclature and characterization of leukocyte differentiation antigens in ruminants. Immunol. Today 18:365-368. [DOI] [PubMed] [Google Scholar]
- 32.Naessens, J., and J. Hopkins. 1996. Third workshop on ruminant leukocyte antigens. Introduction and summary of workshop findings. Vet. Immunol. Immunopathol. 52:213-235. [PubMed] [Google Scholar]
- 33.Oldham, G., J. C. Bridger, C. J. Howard, and K. R. Parsons. 1993. In vivo role of lymphocyte subpopulations in the control of virus excretion and mucosal antibody responses of cattle infected with rotavirus. J. Virol. 67:5012-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer, G. H., and T. C. McGuire. 1984. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J. Immunol. 133:1010-1015. [PubMed] [Google Scholar]
- 35.Palmer, G. H., F. R. Rurangirwa, K. M. Kocan, and W. C. Brown. 1999. Molecular basis for vaccine development against the ehrlichial pathogen A. marginale. Parasitol. Today 15:281-286. [DOI] [PubMed] [Google Scholar]
- 36.Palmer, G. H., G. Eid, A. F. Barbet, T. C. McGuire, and T. F. McElwain. 1994. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect. Immun. 62:3808-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer, G. H., S. M. Oberle, A. F. Barbet, W. L. Goff, W. C. Davis, and T. C. McGuire. 1988. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous challenge. Infect. Immun. 56:1526-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richey, E. J., and G. H. Palmer. 1990. Bovine anaplasmosis. Comp. Contin. Educ. Pract. Vet. 12:1661-1666. [Google Scholar]
- 39.Roby, T. O., D. W. Gates, and L. O. Mott. 1961. The comparative susceptibility of calves and adult cattle to bovine anaplasmosis. Am. J. Vet. Res. 22:982-985. [PubMed]
- 40.Stich, R. W., L. K. Shoda, M. Dreewes, B. Alder, T. W. Jungi, and W. C. Brown. 1998. Stimulation of nitric oxide production in macrophages by Babesia bovis. Infect. Immun. 66:4130-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuo, W., G. H. Palmer, T. C. McGuire, D. Zhu, and W. C. Brown. 2000. Interleukin-12 as an adjuvant promotes immunoglobulin G and type 1 cytokine recall responses to major surface protein 2 of the ehrlichial pathogen Anaplasma marginale. Infect. Immun. 68:270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valdez, R. A., T. C. McGuire, W. C. Brown, W. C. Davis, and D. P. Knowles. 2001. Long-term in vivo depletion of functional CD4+ T lymphocytes from calves requires both thymectomy and anti-CD4 monoclonal antibody treatment. Immunology 102:426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varma, S., and A. M. Shatry. 1980. A technique for partial marsupialisation of the spleen in calves. Vet. Rec. 106:127-128. [DOI] [PubMed] [Google Scholar]
- 44.Walker, D. H., and J. S. Dumler. 1996. Emergence of the ehrlichioses as human health problems. Emerg. Infect. Dis. 2:18-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zar, J. H. 1984. Biostatistical analysis, 2nd ed. Prentice-Hall, Englewood Cliffs, N.J.