Abstract
Despite a plethora of studies exploring the transcriptional regulation of the Nanog gene, the role of the enhancer RNAs (eRNAs) derived from Nanog-interacting super-enhancers (SEs) remains under-investigated. In the present study, we examined the functional role of the eRNAs transcribed from the −5 kb Nanog SE in mouse embryonic stem cells (mESCs) and found that an eRNA, here defined as −5KNAR, was essential to maintain the Nanog locus in an epigenetically active configuration, thereby ensuring pluripotency. We found that the here identified −5KNAR functionally interacts with the RAD21 protein, suggesting a role in stabilizing a cohesin complex at the Nanog locus, ensuring the generation and maintenance of an enhancer–promoter loop. Silencing of −5KNAR caused a cascade of events, including the generation of a DNA methylation wave (likely spreading from a single methylated CpG site), substantial chromatin remodeling, and loss of the enhancer–promoter loop, inducing Nanog silencing and mESC differentiation. Under these conditions, exogenous re-expression of Nanog was unable to restore either the endogenous Nanog expression or the enhancer–promoter interaction, suggesting that, at hierarchical level, the expression of the −5KNAR plays a prominent role in maintaining the pluripotency in mESCs.
Graphical Abstract
Graphical Abstract.
Introduction
The maintenance of pluripotency and self-renewal in mouse embryonic stem cells (mESCs) is ensured by an intricate network of molecular events, above all the expression of a specific group of transcription factors (TFs), such as NANOG, OCT4, and SOX2, which together form the core NOS complex [1–3]. Moreover, precise epigenetic footprints, such as DNA hypomethylation and open chromatin state, globally guarantee the identity of mESCs and are the first marks to change when the cells undergo differentiation [4–6]. Finally, stemness is also controlled by chromatin looping events that regulate enhancer–promoter interactions [7–9]. To maintain the pluripotency in mESC, the NOS complex localizes at enhancers [7–9]. Enhancers present features that permit their identification and prediction: they are characterized by p300 occupancy and by specific histone marks, such as H3K27ac and H3K4me1 [7, 10–12]. High-throughput sequencing techniques have revealed the capability of enhancers to be actively transcribed, thus generating long noncoding RNAs (lncRNAs) termed enhancer RNAs (eRNAs) [13–17]. Some eRNAs have been demonstrated to play important roles in governing pluripotency in mESCs [18, 19]. A model of super enhancer (SE) involvement in maintaining the pluripotency is the large 160-kb Nanog locus, with three actively transcribed SEs termed −5, −45, and +60 SEs, based on their distance in kilobases from the Nanog transcriptional start site. Three other genes (Apobec1, Gdf3, and Dppa3) playing critical roles in the maintenance of pluripotency are located at the large 160-kb Nanog locus [20, 21]. All the three SEs structurally interact with Nanog promoter, but their effects on Nanog transcription are different. It has been reported that deletion of the −45 kb SE only partially affects Nanog expression but strongly reduces Dppa3 levels, and that the eRNAs transcribed from the −45 kb SE act by stabilizing the chromatin loop between the Dppa3 promoter and the −45 kb SE [22]. Conversely, the complete deletion of −5 kb SE is not tolerated by mESCs so that only monoallelically deleted mESC clones survive with an ∼50% decrease in Nanog expression [22]. Furthermore, the presence of −5 kb SE is essential for the recruitment and/or phosphorylation of RNA polymerase II to the Nanog promoter, as the −5 kb SE deletion leads to a displacement of RNA polymerase II from Nanog promoter [23]. However, although the enhancer function of the −5 kb SE has been partially elucidated by deletion experiments, the role of its two derivative eRNAs, transcribed from opposite strands of the DNA, is still unknown. Here, we describe for the first time a programmed and selective DNA methylation wave during mESC differentiation in the large region of Nanog −5 kb SE and Nanog gene, identifying an epigenetic crosstalk between DNA methylation and chromatin remodeling. In parallel, we have functionally characterized the nascent transcripts from the −5 kb SE and evaluated their effects on the epigenetic landscape of the Nanog locus. We found that a −5 kb SE eRNA, transcribed from the opposite strand of the genome compared to the Nanog gene, plays a prominent role in the regulation of Nanog transcription by acting extensively on chromatin remodeling and DNA methylation. Moreover, we demonstrate that this eRNA physically interacts with RAD21 protein and its knockdown leads to the displacement of the cohesin complex with the consequent disruption of the chromatin loop between the −5 kb Nanog SE and the Nanog promoter.
Materials and methods
Cell culture
ES-E14TG2a cells (ATCC) were cultured in a 0.2% gelatin-coated plate and in serum/LIF conditions. Briefly, the cells were cultured in Dublecco’s modified Eagle’s medium (Gibco™, #11965092) with the following supplement: 15% embryonic stem cell fetal bovin serum (Gibco™, #6141079), 5% penicillin/streptomycin (10 000 U/ml) (Gibco™, #15140122), 5% MEM nonessential amino acids (100X) (Gibco™, #11140050), 5% 2-mercaptoethanol (50 mM) (Gibco™, #31350010), 5% GlutaMAX™ supplement (Gibco™, #35050061), and ESGRO®recombinant mouse LIF protein (Sigma–Aldrich, #ESG1107). mESCs were incubated at 37°C in 5% CO2; medium was changed daily, and cells were split every 2–3 days. mESC differentiation was achieved as described in [24]. Briefly, ∼2 × 104 cells/ml were resuspended in mESC medium without LIF. Using a multichannel pipette, 20 μl droplets of the cell suspension were plated on the inside of a plastic Petri dish lid (not tissue culture treated and not gelatin coated), allowing the formation of embryo bodies (EBs). After 48 h, EBs were collected and settled on the bottom of the tube for gravity. The supernatant was removed, and the cells were plated in tissue-cultured dishes in DMEM medium without LIF and with 1 μM of retinoic acid (RA). RA was resuspended in Dimethyl Sulfoxide (DMSO) to a concentration of 1 mM. Medium with RA was freshly changed every day for 8 days.
eRNA sequence identification
One thousand nanograms of RNA from mESCs was retrotranscribed using strand-specific primers (Table 1) or with oligo-dT (Euroclone, #EMR433200) using QuantiTect Reverse Transcription Kit (QIAGEN, #205311), following manufacturer’s protocol. The polymerase chain reactions (PCRs) were performed using the HotStarTaq DNA Polymerase Kit (QIAGEN, #203203) with the following protocol: 95°C for 15 min; 95°C for 30 s; 60°C for 40 s; 72°C for 1 min for 35 cycles; 72°C for 10 min. Amplicons were excised from agarose gel (Sigma–Aldrich, #A9539), purified on magnetic beads (AMPure XP Beads, Beckman Coulter, #A63880), and subjected to Sanger sequencing analysis. Sanger sequencing was performed at the Sequencing Facilty of CEINGE Advanced Biotechnologies, Naples.
Table 1.
List of primers used for −5KNSR and −5KNAR sequence characterization
| Primers | ID | Sequence (5′–3′) |
|---|---|---|
| FW1 −5KNSR | SFW1 | GGACTCCAAGGCTAGCGATTCACAC |
| FW2 −5KNSR | SFW2 | GGAGTCTGTAATTCTCTCCAATTG |
| FW3 −5KNSR | SFW3 | TGAGTTCCAGGACAGCCAGGG |
| FW4 −5KNSR | SFW4 | GTCAGGTAAAGCAGCACAAAGCCTTTAA |
| FW5 −5KNSR | SFW5 | GCTTTGTGGGCCTGAGCTCTCAGTGG |
| FW6 −5KNSR | SFW6 | GCCTGGCTGTGTGTGGGTGCACACAG |
| RV1 −5KNSR | SREV1 | TTTGAGAGTCTCTGGTGAAAGATCCGA |
| RV2 −5KNSR | SREV2 | TGCTTAGGAGACGGCGATGGATCC |
| RV3 −5KNSR | SREV3 | TTAAGTTTACCCCAAGTTCTACAAA |
| RV4 −5KNSR | SREV4 | TTCTAGGAACCGTCCGGGAGAGGAATTG |
| RV5 −5KNSR | SREV5 | TTCCTCTCCCTCAGCTACCAGCTCT |
| RV6 −5KNSR | SREV6 | AGTTACATACTTTTGCCACACTCAAGTTT |
| FW1 −5KNAR | AFW1 | ACAATAGGCATTCTGTGATGGCTCTTG |
| FW2 −5KNAR | AFW2 | ACTCACACCTCAGGAGAGGTAGGGGC |
| FW3 −5KNAR | AFW3 | TCTTGCACTTATACAAGTGTGTTTTACC |
| FW4 −5KNAR | AFW4 | TACTTCTGTCTGCCTTAAGGGGATCAAAG |
| FW5 −5KNAR | AFW5 | TGGTTAGCCTGGAACTCACTGTGTAGAC |
| RV1 −5KNAR | AREV1 | AAACTTGAGTGTGGCAAAAGTATGTAACT |
| RV2 −5KNAR | AREV2 | AGAGCTGGTAGCTGAGGGAGAGGAA |
| RV3 −5KNAR | AREV3 | CAATTCCTCTCCCGGACGGTTCCTAGAA |
| RV4 −5KNAR | AREV4 | TTTGTAGAACTTGGGGTAAACTTAA |
| RV5 −5KNAR | AREV5 | GGATCCATCGCCGTCTCCTAAGCA |
| RV6 −5KNAR | AREV6 | TCGGATCTTTCACCAGAGACTCTCAAA |
RNA extraction and real-time PCR
Total RNA was extracted with the RNeasy Plus Mini Kit (QIAGEN, #74104), following the manufacturer’s instructions. Equal amount of RNA (1000 ng) was converted to complementary DNA (cDNA) using the QuantiTect Reverse Transcription Kit (QIAGEN, #205311). Real-time PCR was performed using LightCycler 480 SYBR Green I Master (Roche Diagnostics) in a LightCycler 480 real-time thermocycler. The primers used for the real-time PCR are listed in Supplementary Table S1. All the measurements were normalized to the Actin housekeeping gene. All the RNA quantification experiments were performed by using n = 3 biological replicates and n= 3 technical replicates per biological replicate. The ΔΔCt method was used to analyze all the data. For the quantification of endogenous −5KNAR, serial dilutions of the samples were used to calculate a standard curve with slope (a) and intercept (b). Potential differences in primer efficiencies were corrected. The quantification of the exogenous −5KNAR was calculated using the following formula: 10(Ct−b/a).
DNA extraction, bisulfite conversion, and amplicon library preparation
Total DNA was extracted with DNeasy Blood and Tissue Kit (QIAGEN, #69506), following the manufacturer’s instructions. Equal amount of DNA (1000 ng) was used for bisulfite conversion by using EZ DNA Methylation Kit (Zymo Research, D5002), following the manufacturer’s instructions. The amplicon bisulfite sequencing was performed as previously described [25]. Briefly, the bisulfite-converted DNA was double-step amplified and purified to generate the amplicon library. All the primers were designed with specific overhang to allow indexing. The primers used for the sequencing, with the used thermal protocol for the amplification, are reported in Supplementary Table S1. Amplicons were then quantified using a Qubit 2.0 Fluorometer with the dsDNA broad range assay kit (Invitrogen, Q32850) and diluted to equimolar concentration (4 nM). The final concentration of the amplicon library was 8 pM. The paired-end sequencing was performed by using V2 reagent kits on an Illumina MiSeq system (Illumina) in 251 × 2 cycles. An average of 30 000 reads/sample was obtained. The output of sequencing was bioinformatically analyzed as described in [25].
Chromatin immunoprecipitation and ChIP-qPCR
Approximately 20 × 106 mESCs were cross-linked by addition of formaldehyde to 1% for 10 min at room temperature (RT) and quenched with 0.125 M glycine for 5 min at RT. All the immunoprecipitation assays were performed by using Pierce™ Magnetic ChIP Kit (catalog number 26157, Thermo Scientific) following the manufacturer’s protocol. The nuclei were sonicated with Bioruptor plus (Diagenode) 30 s on/off for 40 cycles at high power. The antibodies used for the chromatin immunoprecipitation (ChIP) assays are listed in Supplementary Table S1. Five micrograms of each antibody was used. ChIP quantitative PCR (qPCR) was performed with LightCycler 480 SYBR Green I Master (Roche) and the analyses were performed using the input percent method. ActB promoter was used as a region, which was not expected to change during differentiation upon H3K27ac, H3K27me3, TET2, and DNMT3a immunoprecipitation (Supplementary Fig. S1A). One percent of chromatin used for the ChIP was used for the input samples. All the ChIP experiments were performed by using n= 4 biological replicates. The primers used for ChIP qPCR are listed in Supplementary Table S1.
Plasmids and RNA interference transfections
For −5KNAR eRNA overexpression, full-length −5 kb SE region codifying for −5KNAR (618 bp) here identified was cloned into pcDNA3.1/Hygro(+) vector purchased from Invitrogen (43-0039). Transcription was driven by CMV promoter. The −5 kb SE region codifying for the −5KNAR eRNA was amplified with a forward primer containing the restriction site for Kpn1 and a reverse primer containing the restriction site for Xho1. Both amplicon and pcDNA3.1/Hygro(+) vector were cut with KpnI (New England Biolabs, R3142S) and XhoI (New England Biolabs, R0146S) restriction enzymes in CutSmart™ Buffer (New England Biolabs, B7204S) and then ligated with the Rapid DNA Ligation Kit (Merck, 11635379001), following the manufacturer’s instructions. The cloned vector was then transformed following manufacturer’s protocol of MAX Efficiency™ DH5α Competent Cells (Invitrogen, 18258012). The overexpression of Nanog was achieved by using Nanog (tGFP-tagged), Mouse Nanog homeobox (MG226678, Origene). Transient transfections of the constructs were performed using Lipofectamine 2000 Transfection Reagent (Invitrogen, 11668019) in Opti-MEM (Gibco™, 31985-047), according to the manufacturer’s protocol. The cells were harvested 48 h after transfection. For silencing the −5KNSR eRNA and −5KNAR eRNA, we used customized siRNAs designed using Horizon Discovery siDESIGN Center (https://horizondiscovery.com/en/ordering-and-calculation-tools/sidesign-center). The sequences of the customized siRNAs are reported in Supplementary Table S1. The efficiency of individual siRNAs and their combination in knocking down the eRNAs was evaluated (Supplementary Fig. S1B). ON-TARGET plus SMARTpool Mouse Nanog (Dharmacon, L-057004-00-0020) was used for Nanog silencing. All the silencing experiments were conducted by using ON-TARGETplus™ Control Pool Non-Targeting Pool as control (Dharmacon, Cat. D-001810-10-20). All siRNAs were transfected for 48 h to a final concentration of 4 nM with DharmaFECT transfection reagents (Dharmacon, T-2001-03), following the manufacturer’s protocol.
Chromosome conformation capture
Chromosome conformation capture (3C) experiments were performed following and modifying the protocol described in [26]. Lysed nuclei were digested with 400 U HaeIII restriction enzyme (New England Biolabs, R0108S) and ligated with 100 U of T4 DNA ligase (New England Biolabs, M0202S) for 4 h at 16°C followed by 30 min at RT. The interaction between Nanog gene and distal −5 kb SE fragments was calculated with 3C-qPCR performed with LightCycler 480 SYBR Green I Master (Roche). All the primers are listed in Supplementary Table S1. Each sample was diluted from two- to eight-fold to calculate a standard curve. The slope (a) and intercept (b) were derived from the standard curves. The interaction ratio was calculated using the following formula: 10(Ct−b/a). To avoid the potential differences in DNA amounts among samples, a region outside of the looping region (internal control) was amplified and absolutely quantified. The obtained values were used to normalize the interaction ratio of each 3C experiment.
RNA immunoprecipitation
6 × 106 mESCs were used to perform RNA immunoprecipitation (RIP) using Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Sigma–Aldrich, 17-700), following the manufacturer’s protocol. Nuclei were incubated with 5 μg Rad21 and 5 μg SMC3 antibodies and IgG antibodies as a negative control. The reactions were incubated on a rotor at 4°C overnight. Isolated RNA was reverse transcribed using QuantiTect Reverse Transcription Kit (QIAGEN, #205311). Real-time qPCR was performed using LightCycler 480 SYBR Green I Master (Roche Diagnostics). The RIP samples were analyzed by applying the percent input method. Real-time PCR was performed with 10% input as well as the immunoprecipitated RNA by using primers amplifying the antisense and sense eRNAs and Nanog messenger RNA (mRNA) listed in Supplementary Table S1. The 10% input Ct values were adjusted to 100% and then the adjusted input was subtracted from the sample Ct values. Finally, the formula “100*2^(adjusted input − Ct(IP)” was used.
Statistical analyses
Statistical analyses were performed with Prism (Prism 7.0, GraphPad). All the experiments were conducted with at least a biological triplicate (n = 3). Student’s t-test or one-way ANOVA followed by multiple comparison analysis was used to determine statistical significance. A P-value < .05 was considered significat (*P < .05, **P < .01, ***P < .001). Error bars represent standard errors.
Results
Definition of the full sequences of the eRNAs transcribed from Nanog −5 kb SE
Due to their high instability, the identification of eRNA sequences often relies on both noncanonical RNA sequencing and other genome-wide techniques [13, 27–29]. Thus, most of the eRNA sequences are only partially characterized, including the eRNAs transcribed from the Nanog −5 kb SE [30]. Therefore, we first aimed to identify the complete RNA sequence of both the eRNAs, which are transcribed from opposite strands of the DNA, by using a walking PCR strategy on cDNA starting from the known eRNA sequences [22]. We used mRNA extracted from ES-E14TG2a cells, which express high levels of the eRNAs [22]. We searched for the full-length sequences for both eRNAs. As depicted in Fig. 1A, we performed first-strand cDNA synthesis using several strand-specific primers (Table 1) and followed by PCR reactions with different complementary strand-specific oligos, respectively (see flowchart shown in Fig. 1A and Supplementary Table S2 for details). In this way, we mapped the putative full-length eRNA sequences, at least detectable by the strategy used. Subsequently, the PCR products obtained with SFW5 and SREV5 and with AFW2 and AREV3, respectively, were sequenced by the Sanger method to confirm the identity and the collinearity with the genomic sequence (data not shown).
Figure 1.
Characterization of the sense (−5KNSR) and antisense (−5KNAR) transcripts derived from Nanog −5 kb SE. (A) Flowchart of the strategy used for the identification of −5KNSR (left) and −5KNAR (right). For −5KNSR, six different reverse transcription reactions were performed using six different reverse, strand-specific primers (represented as arrows in the top left panel). To determine the putative 3′-end of −5KNSR, the obtained six cDNAs were subjected to six PCR reactions using SFW1 as forward primer (known to amplify the −5KNSR transcript) and all the reverse primers used in the reverse transcription reactions (middle left panel). To identify the putative 5′-end of −5KNSR, the cDNA obtained with SREV5 (the primer closest to the 3′-end of the RNA as resulted from previous reactions) was subjected to six PCR reactions using each of the forward primers (SFW1-2-3-4-5-6) and SREV5 (bottom left panel). Green arrows indicate the oligos that generated amplification products (shown in panel B). Red arrows indicate the primers that did not amplify the −5KNSR. For −5KNAR, a similar strategy was used. Six different reverse transcription reactions were performed using six different reverse, strand-specific primers (represented as arrows in top right panel). To determine the putative 3′-end of −5KNAR, the obtained six cDNAs were subjected to six PCR reactions using AFW1 as forward primer (known to be included in the −5KNAR transcript) and all the reverse primers used in the reverse transcription reactions (middle right panel). To identify the putative 5′-end of −5KNAR, the cDNA obtained with AREV3 (the primer closest to the 3′-end of the RNA as resulted from above reactions) was subjected to six PCR reactions using each of the forward primers (AFW1-2-3-4-5) and AREV3 (bottom right panel). Green arrows indicate the primers that generated the amplification products (shown in panel C). Red arrows indicate the primers that did not amplify the −5KNAR. All the primers used for cDNA and PCR reactions are listed in Table 1. All the reactions performed are specified in Supplementary Table S2. (B) Agarose gel of PCR amplifications of cDNA obtained using the strand-specific SREV5 primer and amplified using SFW5 and SREV5, the primers generating the largest amplicon from the −5KNSR transcript. First lane: molecular weight marker (100 bp); second lane: amplicon obtained with reverse transcription (+RT) reaction with SREV5 primer followed by amplification with SFW5 and SREV5; third lane: amplicon obtained with reverse transcription (+RT) reaction with oligo-dT followed by amplification with SFW5 and SREV5; fourth lane: negative control (same PCRs with no reverse transcriptase) (−RT); fifth lane: water. (C) Agarose gel of PCR amplifications of cDNA obtained with the strand-specific AREV3 and amplified using AFW2 and AREV3, the primers generating the largest amplicon from the −5KNAR transcript. First lane: molecular weight marker (100 bp); second lane: amplicon obtained with reverse transcription (+RT) reaction with oligo-dT followed by amplification with AFW2 and AREV3; third lane: amplicon obtained with reverse transcription (+RT) reaction with oligo-dT followed by amplification with AFW2 and AREV3; fourth lane: negative control (same PCRs with no reverse transcriptase) (−RT); fifth lane: water. (D) Identified sequence of −5KNSR and −5KNAR transcripts with genomic coordinates referred to the mouse genome GRCm38/mm10. The overlap region is also indicated with the corresponding genomic coordinates.
To evaluate the possible presence of the poly(A) tail in the natural transcripts, cDNA was then prepared using oligo-dT followed by amplification of internal regions mapped as exclusive for sense or antisense eRNAs (Fig. 1B and C). PCR products were sequenced by the Sanger method in order to verify the identity of the original transcripts (data not shown). Overall, we identified a polyadenylated “sense” eRNA 1075 bp long (putative full-length) with the genomic coordinates chr6:122702066–122703140 (mouse genome: GRCm38/mm10) (Fig. 1B) and a polyadenylated “antisense” eRNA 618 bp long (putative full-length) with the genomic coordinates chr6:122703055–122703672 (mouse genome: GRCm38/mm10) (Fig. 1C). Notably, the two transcripts shared an overlapping region 160 bp long (Fig. 1D). The terms “sense” and “antisense” are arbitrarily used to refer to the direction of Nanog gene transcription. To facilitate the reading, in the rest of the manuscript, we will refer to the above-described transcripts as −5KNSR to indicate the −5 kb Nanog SE sense eRNA and −5KNAR to indicate the −5 kb Nanog antisense eRNA, respectively.
The −5 kb Nanog SE is epigenetically regulated during mESC differentiation
Then, we sought to determine whether, during stem cell differentiation, the regulation of transcription of −5KNSR and −5KNAR may change in parallel with the widely described changes in Nanog mRNA expression [31–33]. We measured the expression levels of both transcripts, along with Nanog mRNA levels (Fig. 2A), at different time points of RA-induced differentiation (Supplementary Fig. S2A). We found that both −5KNSR and −5KNAR levels significantly decreased, starting by the EB generation until the end point of the treatment (day 8) (Fig. 2A). Specifically, we found a five-fold decrease of the −5KNAR at EB time points, likely indicating that the −5KNAR immediately decreased, along with the Nanog mRNA, when the cells started to differentiate, still before the addition of RA (Fig. 2A). Thus, we guessed that these drastic changes in −5KNSR and −5KNAR expression may be related to epigenetic regulation of the −5 kb Nanog SE locus. We first explored the H3K27 acetylation (H3K27ac) and H3K27 trimethylation (H3K27me3) chromatin marker levels in mESCs and at the end point of the RA-induced differentiation (day 8) (Fig. 2B). We found a net and significant switch from the active chromatin marker H3K27ac to the repressive chromatin marker H3K27me3 during differentiation at the −5KNAR genomic region and at the Nanog gene promoter, while no differences were found at the −5KNSR genomic region. We also measured the levels of H3K27ac and H3K27me3 at EB stage in the attempt to detect early events correlating with the strong loss of transcription at Nanog gene promoter and at −5 kb Nanog SE locus occurring at EB stage. At EB stage compared to day 0, we found a significant drop in H3K27ac at −5 kb SE locus and no significant changes in H3K27me3. These data suggest that the loss of transcription of −5KNSR and −5KNAR may initiate with the loss of active H3K27ac chromatin modification (Supplementary Fig. S2B). We then measured DNA methylation in mESCs and at different RA-induced differentiation time points by using an ultra-deep sequencing approach [25, 34] to evaluate DNA methylation levels at each single CpG site (Fig. 2C). To this aim, we divided the −5 kb SE and the Nanog gene into six different amplicons (Fig. 2C). We found in all the regions analyzed on the −5 kb SE locus very low levels of average DNA methylation in mESCs that strikingly increased during the RA-induced differentiation (Fig. 2D). The same was observed in the three analyzed regions of Nanog gene (Fig. 2D), indicating that the DNA methylation wave contextually occurred on the −5 kb SE and on the Nanog gene. Of note, DNA methylation mainly and firstly occurred at specific CpG sites, as in the case of the CpG 122703276, located on the region corresponding to the actively transcribed −5KNAR. While all the CpG sites in the locus were fully unmethylated in mESCs, this CpG was the sole site, with an ∼10% of DNA methylation, strongly increasing early at EB time point, reaching an ∼100% of DNA methylation at the end point of the experiment (Fig. 2E). Likewise, some CpG sites located on Nanog gene, such as CpGs 122707773 and 122707855, were the first to increase, reaching the higher levels of methylation at day 8 of RA treatment (Fig. 2E). To identify the epigenetic actors responsible for the DNA methylation wave, we then performed ChIP assays for the TET2 and DNMT3a enzymes (Fig. 2F). Consistent with our DNA methylation data, we observed a significant decrease in TET2 enzyme levels in favor of a significant increase in the DNMT3a levels during differentiation at the −5 kb SE region and Nanog promoter (Fig. 2F). We did not observe changes in TET1 and TET3 enzyme enrichment at all the analyzed regions (Supplementary Fig. S2C). These data suggest that in mESCs both regions are protected from DNA methylation by the presence of TET2, which gives way to DNMT3a as soon as differentiation starts, permanently triggering the wave of DNA methylation and the silencing of the −5KNSR and −5KNAR and Nanog expression.
Figure 2.
Epigenetic regulation at Nanog and −5 kb SE regions during RA-induced differentiation. (A) Expression levels of −5KNAR, −5KNSR, and Nanog mRNA during RA-induced differentiation. Different time points were selected and analyzed: mESCs = E14TG2a cells before RA treatment; EB = E14TG2a cells after 48 h of LIF deprivation; Day 1-3-6-8 = E14TG2a cells after 24 h, 72 h, 6 days, and 8 days of RA treatment, respectively. The expression data were normalized to mESC expression levels. (B) H3K27ac and H3K27me3 ChIP-qPCR in mESCs and at the end point of RA treatment at the −5 kb SE and at Nanog promoter. The data are expressed as a percentage of the DNA inputs. (C) Schematic representation of the −5 kb SE and the Nanog gene. The rectangles represent the different regions analyzed for DNA methylation. The genomic region transcribing −5KNAR (E1 region) and the regions transcribing −5KNSR (E2 and E3 regions) are shown. For Nanog gene region, the promoter region is shown in red, the exons are indicated in gray, and the introns in black. The arrow indicates the direction of transcription of the Nanog gene. The −5KNSR and −5KNAR transcripts and the overlapping region are shown in yellow, blue, and green, respectively. The reported genomic coordinates of each analyzed CpG site are referred to the mouse genome GRCm38/mm10. (D) Average methylation levels of the −5 kb SE regions and the Nanog regions at all the differentiation stages. (E) Average methylation at all the CpGs analyzed in the −5 kb SE regions and Nanog gene regions. (F) TET2 and DNMT3a ChIP-qPCR in mESCs and at the end point of RA treatment at the −5 kb SE and at Nanog promoter. The data are expressed as a percentage of the DNA inputs. All data are shown as mean ± Standard Error (SE). The statistical analyses of mRNA expression and DNA methylation have been performed by using one-way ANOVA test. The statistical analyses of ChIP-qPCR have been performed by using Student’s t-test. *P-value <.05; ***P-value <.001; ****P-value <.0001; n.s. = nonsignificant comparison.
The −5KNAR regulates Nanog gene expression through epigenetic mechanisms
A recent study by Agrawal et al. demonstrated that the monoallelic deletion of the −5 kb SE led to a strong decrease in Nanog expression, suggesting a structural role of the −5 kb SE in maintaining pluripotency [23]. Thus, to investigate the role of the −5KNSR and −5KNAR in sustaining the expression of Nanog gene, we measured the Nanog mRNA levels in cells transfected with two siRNAs directed against the −5KNSR transcript or with two siRNAs directed against the −5KNAR transcript. We found that the silencing of the −5KNAR (si-5KNAR), but not of the −5KNSR (si-5KNSR), significantly decreased the expression levels of Nanog mRNA compared to the nontargeting siRNA (siCTRL) (Fig. 3A and B). Interestingly, a significant reduction of Nanog expression upon silencing of −5KNAR was observed as well in mESCs cultured in 2i/LIF conditions (Supplementary Fig. S3A). We also found a decrease of NANOG protein after −5KNAR silencing (Supplementary Fig. S3B). To evaluate the involvement of epigenetic processes in the silencing of Nanog after −5KNAR knockdown, we performed ChIP assays for H3K27ac and H3K27me3 markers. −5KNAR silencing resulted in a significant switch from H3K27ac to H3K27me3 at the −5 kb SE region transcribing for the −5KNAR and Nanog promoter (Fig. 3C). We detected a trend to decrease in p300 and BRD4 at −5KNAR region and at Nanog promoter and a significant decrease in enrichment of p300 and BRD4 at −5KNSR region upon silencing of −5KNAR (Supplementary Fig. S3C). Together with chromatin remodeling, we found a significant increased level of DNA methylation at Nanog promoter region, particularly at the same CpG sites (122707773 and 122707855) that strongly increased during the RA-induced differentiation process (Fig. 3D). We also observed a significant increase of DNA methylation in the −5 kb SE region E1, particularly at the 122703276 CpG site, the same CpG site on which DNA methylation bursts when the differentiation process starts (Fig. 3E). Furthermore, after knockdown of the −5KNAR, we observed a displacement of the TET2 enzyme from the antisense −5 kb SE region and Nanog promoter in favor of the positioning of DNMT3a enzyme, concordantly with the increased DNA methylation levels (Fig. 3F). Thus, our results suggest that the −5KNAR silencing not only reduces Nanog mRNA levels, but also primes an epigenetic silencing of Nanog, a process likely difficult to reverse.
Figure 3.
Effects of the silencing of the −5KNSR and −5KNAR on Nanog locus. (A) Expression levels of −5KNSR, −5KNAR, and Nanog gene after silencing of the −5KNSR (siCTRL = nontargeting siRNA; si-5KNSR = siRNA directed to knock down the −5KNSR). Statistical analyses have been performed by using Student’s t-test. The data are normalized to siCTRL expression values. (B) Expression levels of −5KNAR, −5KNSR, and Nanog gene after silencing of the −5KNAR transcript (siCTRL = nontargeting siRNA; si-5KNAR = siRNA directed to knock down the −5KNAR). The data are normalized to siCTRL expression values. (C) H3K27ac and H3K27me3 ChIP-qPCR in the presence of nontargeting and −5KNAR targeting siRNAs. The data are expressed as a percentage of the DNA inputs. (D) DNA methylation levels at Nanog promoter after silencing of the −5KNAR transcript. DNA methylation average (left) and average methylation at single CpGs are shown. (E) DNA methylation average (left) and average methylation at single CpGs (right) at E3 and E1 −5 kb Nanog SE regions after silencing of the −5KNAR. (F) TET2 and DNMT3a ChIP-qPCR at the −5 kb SE regions and at Nanog promoter in the presence of nontargeting and −5KNAR targeting siRNAs. The data are expressed as a percentage of the DNA inputs. All data are shown as mean ± SE. The statistical analyses of mRNA expression and DNA methylation have been performed by using one-way ANOVA test. The statistical analyses of ChIP-qPCR have been performed by using Student's t-test. *P-value <.05; **P-value <.01; ***P-value <.001; n.s. = nonsignificant comparison.
The −5KNAR silencing disrupts the pluripotency network, inducing endoderm differentiation of mESCs
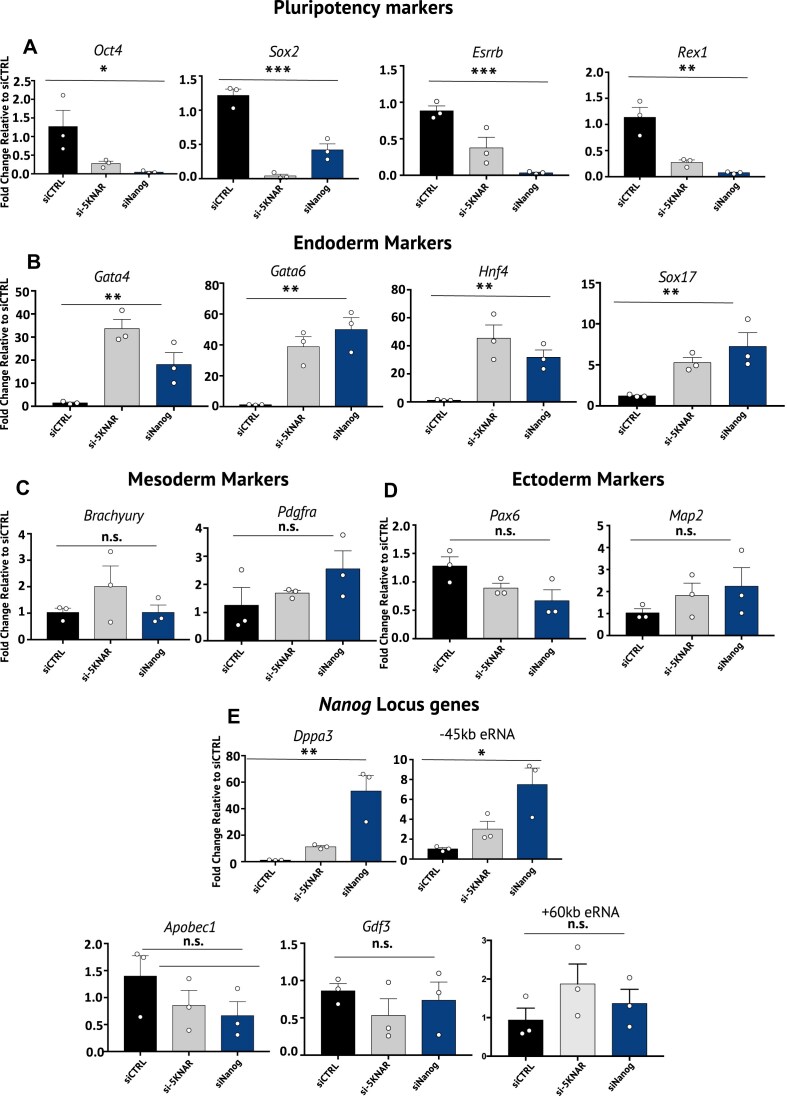
We then checked whether, along with Nanog, the expression of other pluripotency-associated genes may be affected by the −5KNAR knockdown. As Nanog partners in the pluripotency maintenance, we analyzed Oct4 and Sox2 expression, in addition to other pluripotency-associated genes, such as Rex1 and Esrrb. The expression levels of the same genes were also measured upon Nanog silencing to evaluate whether the effects of the −5KNAR silencing were or were not mediated by NANOG levels (Fig. 4). A significant mRNA level reduction of all the analyzed pluripotency-associated genes was found after −5KNAR silencing and the same was observed also with Nanog knockdown (Fig. 4A). We also found a significant decrease of the alkaline phosphatase activity in cells knocked down for the −5KNAR, suggesting that the stemness identity of the cells had been lost (Supplementary Fig. S4). Therefore, to identify the specific layer through which the cells differentiate when treated with −5KNAR siRNAs, we measured the expression levels of Gata4, Gata6, Hnf, and Sox17, markers of endoderm differentiation, of Brachyury and Pdgfra, markers of mesoderm differentiation, and of Pax6 and Map2, markers of ectoderm differentiation (Fig. 4B–D). We did not observe differences in the expression levels of ectoderm and mesoderm markers (Fig. 4C and D), but we did find a significant mRNA increase of all the analyzed endoderm markers (Fig. 4B). The same phenomena were observed, when Nanog gene was silenced, suggesting also in this case that the effect of the −5KNAR silencing was likely mediated by Nanog loss. Finally, to evaluate whether the silencing of −5KNAR impacts the expression of other transcripts on the same genomic locus, we explored the expression levels of Apobec1, Gdf3, and Dppa3 genes together with the expression levels of the eRNA actively transcribed from the −45 kb SE and +60 kb SE (Fig. 4E). We did not observe changes in the expression levels of Gdf3, Apobec1, and +60 kb SE with both −5KNAR and Nanog interference (Fig. 4E). Moreover, we found a significant increase of Dppa3 and −45 kb eRNA after −5KNAR knockdown (Fig. 4E). These observations are in line with a previous study from Blinka et al. demonstrating that the monoallelic deletion of the −5 kb SE led to an increase of Dppa3 gene. Also, the silencing of Nanog mRNA induced an increase of Dppa3 levels together with increased levels of the −45 kb eRNA (as also previously described in [23]), likely due to the capability of NANOG protein to bind Dppa3 promoter and repress Dppa3 expression [35]. Overall, our results show that the −5KNAR knockdown induces a reduction of stemness markers and the beginning of mESC differentiation toward endoderm layer, a likely consequence of the effects on Nanog gene.
Figure 4.
Effects of the silencing of −5KNAR and silencing of Nanog on pluripotency markers, differentiation markers, and genes on the Nanog locus. (A) mRNA expression levels of Oct4, Sox2, Esrrb, and Rex1 genes as markers of stemness, after silencing of −5KNAR (si-5KNAR) and Nanog (siNanog). (B) mRNA expression levels of Gata4, Gata6, Hnf4, and Sox17, as markers of endoderm differentiation, after silencing of −5KNAR (si-5KNAR) and Nanog (siNanog). (C) mRNA expression levels of Brachyury and Pdgfra, as markers of mesoderm differentiation, after silencing of −5KNAR (si-5KNAR) and Nanog (siNanog). (D) mRNA expression levels of Pax6 and Map2, as markers of ectoderm differentiation, after silencing of −5KNAR (si-5KNAR) and Nanog (siNanog). (E) mRNA expression levels of the genes located on the same locus of Nanog (Dppa3, Apobec1, and Gdf3) together with the expression levels of the −45 kb Nanog SE and +60 kb Nanog SE after silencing of −5KNAR (si-5KNAR) and Nanog (siNanog). All the expression data are normalized to siCTRL values. All data are shown as mean ± SE. Statistical analyses have been performed by applying one-way ANOVA tests. *P-value <.05; **P-value <.01; ***P-value <.001; n.s. = nonsignificant comparison.
The overexpression of −5KNAR delays RA-induced differentiation in mESCs
Our data demonstrate that −5KNAR abrogation generates a cascade of mechanisms involving the entire pluripotency network. However, we were not able to discriminate whether the phenomena were due to the −5KNAR depletion or the consequent reduction of Nanog expression. To this end, we artificially overexpressed the −5KNAR in mESCs by cloning the entire −5KNAR sequence into a pcDNA3.1(+) construct (here defined as pcDNA3.1+-5KNAR). First, we found that the exogenous overexpression of the −5KNAR significantly increased the levels of the endogenous −5KNAR, both in the presence and in the absence of RA treatment, suggesting that the −5KNAR positively regulates itself (Supplementary Fig. S5A). Moreover, the overexpression of the −5KNAR induced a significant increase (about five-fold) of Nanog expression in mESCs (Fig. 5A). Conversely, no changes in the expression levels of other pluripotency-associated markers, such as Oct4 and Sox2, were observed in transfected mESCs (Fig. 5A). Subsequently, to evaluate the effects of −5KNAR overexpression on differentiation, we treated pcDNA3.1+-5KNAR transfected mESCs and empty-pcDNA3.1 transfected mESCs with RA. We found that Nanog mRNA expression decreased slightly in mESCs overexpressing −5KNAR compared to mESCs transfected with an empty vector upon RA treatment (Fig. 5A). Similarly, Oct4 and Sox2 gene expression was only partially affected by the RA treatment in −5KNAR overexpressing cells (Fig. 5A). Thus, the overexpression of the −5KNAR delayed the differentiation process induced by the RA treatment (Supplementary Fig. S5B). Moreover, the overexpression of −5KNAR made the mESCs resistant to the DNA hypermethylation induced by the differentiation process at the Nanog promoter and E3 −5 kb enhancer regions (Fig. 5B and C). Altogether, our data indicate that the −5KNAR delays the differentiation process, by preventing the DNA methylation wave on Nanog gene and by ensuring the expression of the NOS complex.
Figure 5.
Effects of the overexpression of the −5KNAR eRNAs upon differentiation stimulus. (A) mRNA expression levels of Nanog, Oct4,and Sox2 after overexpression of the −5KNAR in the absence (−RA) and in the presence (+RA) of retinoic acid. (B) DNA methylation average (left) and average methylation at single CpGs (right) at Nanog promoter region in nontransfected mESCs and in mESCs transfected with pcDNA3.1 overexpressing the −5KNAR in the absence (−RA) and in the presence (+RA) of retinoic acid. (C) DNA methylation average (left) and average methylation at single CpGs (right) at E3 −5 kb Nanog SE in nontransfected mESCs and in mESCs transfected with pcDNA3.1 overexpressing the −5KNAR in the absence (−RA) and in the presence (+RA) of retinoic acid. All data are shown as mean ± SE. All the statistical analyses have been performed by applying one-way ANOVA followed by multiple comparison. *P-value <.05; **P-value <.01; ***P-value <.001; n.s. = nonsignificant comparison.
The −5KNAR acts by stabilizing the loop between −5 kb SE and Nanog promoter, functionally interacting with the cohesin complex
Some eRNAs have been reported to function by stabilizing enhancer–promoter loops [36–41]. Thus, we aimed to determine whether the −5KNAR acted on Nanog expression stabilizing the loop between the −5 kb SE and Nanog promoter. For this purpose, we performed a 3C assay, evaluating the previously described Nanog enhancer–promoter looping locus [22] (Fig. 6A). We found that interaction between the −5 kb SE and Nanog gene was completely lost after −5KNAR silencing (Fig. 6B). We also evaluated the loops between the Nanog promoter and the −45 kb SE and +60 kb SE after −5KNAR depletion (Supplementary Fig. S6A), and we did not observe changes in the interaction ratio. To discriminate whether the disruption of the loop was directly related to the −5KNAR absence and not to the consequent decrease of Nanog expression or to cell differentiation, we artificially overexpressed NANOG protein in mESCs deprived of the −5KNAR (Fig. 6C). Because NANOG has been reported to be part of an autoregulatory complex [42, 43], first we checked whether exogenous Nanog could influence endogenous Nanog expression. We found that the levels of endogenous Nanog gene were not restored (Fig. 6C). Instead, other pluripotency-associated genes, such as Oct4 and Sox2, rescued their levels after supplementation of exogenous NANOG, guaranteeing the stemness features of the mESCs (Supplementary Fig. S6B). Moreover, even maintaining stemness by Nanog exogenous expression, knockdown of the −5KNAR was sufficient to disrupt the loop and, consequently, to block the endogenous Nanog expression (Fig. 6D). Then, we investigated the possible role of −5KNAR in recruiting of cohesin complex at the −5 kb SE region. We performed a ChIP assay for RAD21 and SMC3 proteins after knockdown of −5KNAR, and we found a significant displacement of RAD21 and SMC3 proteins at both −5 kb SE and Nanog gene regions (Fig. 6E). To test the possible direct involvement of the −5KNAR in cohesin recruitment to the Nanog −5 kb SE, we performed RIP assay for RAD21 and SMC3 in mESCs. We found that −5KNAR, but not −5KNSR or Nanog mRNA, was significantly enriched upon RAD21 immunoprecipitation (Fig. 6F), suggesting, but not definitively proving, a direct interaction between RAD21 and −5KNAR. No significant interactions were found between eRNAs and Nanog mRNA with SMC3 (Fig. 6F). However, this could not exclude a role of SMC3 in loop stabilization not involving a direct interaction with -5KNAR. To confirm our model involving RAD21 and possibly, SMC3 in the stabilization of the loop between Nanog promoter and −5 kb SE, we silenced both Rad21 and Smc3 and evaluated the loop between −5 kb SE and Nanog promoter. We found that the absence of RAD21 as well as of SMC3 proteins disrupted the enhancer–promoter loop between Nanog promoter and −5 kb SE (Supplementary Fig. S6C and D), similarly to what happened when the −5KNAR was silenced (Fig. 6F). Overall, our data suggested that the presence of −5KNAR is required to directly stabilize the loop and activate the Nanog gene expression.
Figure 6.
The silencing of −5KNAR prevents the formation of the 3D chromatin structure between −5 kb SE and Nanog promoter. (A) UCSC schematic representation of the primers used for the 3C experiment (Anchor = anchor primer located on Nanog promoter; E1, E2, E3, E4, and E5 = different primers located on the −5 kb SE region previously used by Blinka et al.for the loop identification). The HaeIII restriction sites used for the digestion reactions in the 3C experiments are shown. (B) 3C assay performed on mESCs treated with nontargeting siRNA (siCTRL) and siRNA against −5KNAR (si-5KNAR). The x-axis indicates the specific primer located on the −5 kb SE used for the real-time PCR. The y-axis shows the interaction ratio normalized with a specific internal control. (C) mRNA expression levels of endogenous and exogenous Nanog gene in mESCs treated with nontargeting siRNA (siCTRL), with siRNA against the −5KNAR (si-5KNAR), and with both siRNA against the -5KNAR and Nanog overexpressing plasmid (tGFP Nanog + si-5KNAR). (D) 3C assay performed on mESCs treated with nontargeting siRNA (siCTRL), siRNA against −5KNAR (si-5KNAR), and siRNA against the −5KNAR in combo with Nanog overexpressing plasmid (tGFP Nanog + si-5KNAR). The x-axis indicates the specific primer located on the −5 kb SE used for the real-time PCR. The y-axis shows the interaction ratio normalized with a specific internal control. (E) RAD21 and SMC3 ChIP-qPCR in mESCs at the −5 kb SE regions and at Nanog promoter. The data are expressed as a percentage of the DNA inputs. (F) RIP with RAD21, SMC3, and IgG antibodies. The associated RNAs were reverse transcribed and amplified with specific primers for −5KNSR, −5KNAR, and Nanog transcripts. Relative enrichment was measured by qRT-PCR and normalized to the input. All data are shown as mean ± SE. The statistical analyses of mRNA expression, DNA methylation, and RIP-qPCR have been performed by using one-way ANOVA test. The statistical analyses of 3C-qPCR have been performed by using Student’s t-test. *P-value <.05; **P-value <.01; ***P-value <.001; n.s. = nonsignificant comparison.
Discussion
In the present study, we explored the role of the eRNAs actively transcribed from the −5 kb Nanog SE, investigating in detail how the −5 kb SE region is regulated in mESCs and in differentiated cells, and what are the effects of the eRNA modulation in mESCs.
Regulation of the −5 kb SE region
We found a well-orchestrated epigenetic regulation of the −5 kb SE region during RA-induced stem cell differentiation, involving both DNA methylation and chromatin remodeling. We found that the Nanog locus in mESCs is maintained demethylated thanks to the presence of the TET2 enzyme, which leaves the way to DNMT3a upon differentiation, in line with previous observations identifying TET2 as a pluripotency marker [44] that localizes to chromatin to regulate enhancers associated with naïve pluripotency genes. [45]. This induces a DNA methylation increase specifically and firstly at certain −5 kb SE regions, thereby delineating CpG sites that are more prone to be methylated. Specifically, we found that a single CpG (122703276) located at the −5 kb SE region where the −5KNAR originates remains methylated in 10% of undifferentiated mESCs although all the rest of the CpGs are fully unmethylated. We hypothesize that this may be the result of a dynamic oscillation of the methylation state that favors the firing of the methylation wave observed upon differentiation stimulus. This phenomenon would be consistent with previous observations showing oscillatory dynamics of DNA methylation resulting in cell-to-cell heterogeneity of epiallele profiles at locus-specific and genome-wide levels [46–50]. On the same regions, we also detected a switch from the active H3K27ac mark to the repressive H3K27me3 mark during RA-induced differentiation, revealing a tight collaboration between DNA methylation and chromatin remodeling, as widely described in different regulatory networks [51–53].
Functional roles of the −5KNAR
We found that the −5KNAR physically interacts with the RAD21 protein at the Nanog locus. The ability of eRNAs to interact with the cohesin complex has been previously described for eRNAs that regulate genes whose transcription is sensitive to estrogen [36]. By modulating −5KNAR levels, we observed a complex chain of events. The silencing of the −5KNAR induces a displacement of RAD21 and SMC3 cohesin proteins to the Nanog locus with the consequent loss of the loop between −5 kb Nanog SE and Nanog promoter, inducing Nanog silencing. Several lines of evidence have demonstrated that eRNAs may act to dynamically stabilize the enhancer–promoter loop by interacting with the cohesin complex. For instance, during myogenic differentiation, a specific eRNA interacts with the cohesin complex to regulate the expression of Myogenin [37]. Loss of the enhancer–promoter loop induced by the silencing of the −5KNAR blocks Nanog expression and is accompanied by an epigenetic switch from the active H3K27ac to the repressive H3K27me3 chromatin mark and from TET2 to DNMT3a occupancy along the entire Nanog locus. Along with these processes, we observed a reduction in the expression of the other pluripotency-associated genes Oct4, Sox2, Esrrb, and Rex1 upon −5KNAR knockdown. Under these conditions, the differentiation process initiates, specifically with the activation of the main markers of endoderm differentiation, similarly to what happens when the Nanog gene is silenced [54].
Limitations
In this study, we did not make use also of genome editing at the Nanog −5 kb enhancer, and we cannot exclude that the silencing of −5KNAR may also affect the binding of some, here unidentified, TFs. Our experiments did not address the precise mechanisms by which cohesin acts in Nanog enhancer–promoter interactions. However, the results indicate the presence of cohesin components at −5 kb enhancer and that the silencing of Rad21 and Smc3 disrupted the Nanog enhancer–promoter loop. We cannot conclude whether this effect depends on a direct mechanism or is an indirect consequence of other broader 3D remodeling due to the induced decrease in RAD21 and/or SMC3.
Summary and conclusions
By recapitulating, the here identified −5KNAR transcript is essential to maintain the demethylated and active state of the Nanog locus, thus ensuring pluripotency. Silencing of −5KNAR caused a cascade of events, including the generation of a DNA methylation wave, loss of the pluripotency-associated loop between the −5 kb SE and the Nanog promoter, and induced Nanog silencing and mESC differentiation. To discriminate which of these events were directly related to the absence of −5KNAR and which to the consequential loss of NANOG, we artificially induced either NANOG or −5KNAR overexpression. We found that exogenous Nanog expression was not able to restore either the endogenous Nanog expression or the enhancer–promoter interaction, albeit it guaranteed the pluripotency. Conversely, the overexpression of the −5KNAR counteracted the RA-induced DNA hypermethylation and prevented the exit from the pluripotent state. Thus, on a hierarchical level, the expression of −5KNAR occurs even before the Nanog expression and is therefore essential to directly maintain the pluripotency in mESCs. Although widely investigated, the regulation of the Nanog gene is still an unsolved puzzle involving an intricated network of factors acting in concert. These factors regulate the dynamics of cell differentiation state and other functions that may be disrupted in different pathologies. In this complex scenario, we propose that the −5KNAR may play a prominent role that deserves to be further evaluated in different contexts, such as cancer cells and other pathological conditions involving Nanog dysregulation.
Supplementary Material
Acknowledgements
The authors thank the cell culture, the advanced light microscopy, and the sequencing facilities of CEINGE-Advanced Biotechnologies “Franco Salvatore” for their support.
Author contributions: Mariella Cuomo: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft. Davide Costabile: Methodology, Validation, Investigation, Writing - Original Draft. Rosa Della Monica: Validation. Michela Buonaiuto: Validation. Federica Trio: Writing - Review & Editing. Giulia De Riso: Formal analysis. Roberta Visconti: Writing - Review & Editing. Lorenzo Chiariotti: Conceptualization, Writing - Original Draft, Writing - Review & Editing, Funding acquisition.
Contributor Information
Mariella Cuomo, Department of Molecular Medicine and Medical Biotechnologies, University of Naples “Federico II”, Via S. Pansini 5, Naples 80131, Italy; CEINGE Advanced Biotechnologies “Franco Salvatore”, Naples 80145, Italy.
Davide Costabile, CEINGE Advanced Biotechnologies “Franco Salvatore”, Naples 80145, Italy; SEMM-European School of Molecular Medicine, Department of Molecular Medicine and Medical Biotechnologies, University of Naples “Federico II”, Naples 80131, Italy.
Rosa Della Monica, Department of Molecular Medicine and Medical Biotechnologies, University of Naples “Federico II”, Via S. Pansini 5, Naples 80131, Italy; CEINGE Advanced Biotechnologies “Franco Salvatore”, Naples 80145, Italy.
Michela Buonaiuto, Department of Molecular Medicine and Medical Biotechnologies, University of Naples “Federico II”, Via S. Pansini 5, Naples 80131, Italy; CEINGE Advanced Biotechnologies “Franco Salvatore”, Naples 80145, Italy.
Federica Trio, CEINGE Advanced Biotechnologies “Franco Salvatore”, Naples 80145, Italy.
Giulia De Riso, Department of Molecular Medicine and Medical Biotechnologies, University of Naples “Federico II”, Via S. Pansini 5, Naples 80131, Italy.
Roberta Visconti, CEINGE Advanced Biotechnologies “Franco Salvatore”, Naples 80145, Italy; Institute for the Experimental Endocrinology and Oncology “G. Salvatore”, Italian National Council of Research, Via S. Pansini 5, 80131 Naples, Italy.
Lorenzo Chiariotti, Department of Molecular Medicine and Medical Biotechnologies, University of Naples “Federico II”, Via S. Pansini 5, Naples 80131, Italy; CEINGE Advanced Biotechnologies “Franco Salvatore”, Naples 80145, Italy.
Supplementary data
Supplementary data is available at NAR online.
Conflict of interest
None declared.
Funding
This work was supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministero dell’Università e della Ricerca (MUR) of Italy, National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006)—a multiscale integrated approach to the study of the nervous system in health and disease [DN. 1553 11.10.2022] (to L.C.). This project was also funded by the Ministero dell’Università e della Ricerca (MUR) of Italy, PRIN 2022 PNRR [P20225P45M] (to L.C. and R.V.) and by PRIN 2022 [20227MC8ZS] (to L.C.). Funding to pay the Open Access publication charges for this article was provided by Ministero dell'Università e della Ricerca (PRIN2022-20227MC8ZS).
Data availability
DNA methylation raw data generated in this study are available on European Nucleotide Archive (ENA) database with the accession number PRJEB77658.
References
- 1. Loh YH, Wu Q, Chew JL et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006; 38:431–40. 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 2. Chen CY, Cheng YY, Yen CY et al. Mechanisms of pluripotency maintenance in mouse embryonic stem cells. Cell Mol Life Sci. 2017; 74:1805–17. 10.1007/s00018-016-2438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Z, Oron E, Nelson B et al. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012; 10:440–54. 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 4. Jackson M, Krassowska A, Gilbert N et al. Severe global DNA hypomethylation blocks differentiation and induces histone hyperacetylation in embryonic stem cells. Mol Cell Biol. 2004; 24:8862–71. 10.1128/MCB.24.20.8862-8871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arabac DH, Terzioğlu G, Bayırbaş B et al. Going up the hill: chromatin-based barriers to epigenetic reprogramming. FEBS J. 2021; 288:4798–811. 10.1111/febs.15628. [DOI] [PubMed] [Google Scholar]
- 6. Sun L, Fu X, Ma G et al. Chromatin and epigenetic rearrangements in embryonic stem cell fate transitions. Front Cell Dev Biol. 2021; 9:637309. 10.3389/fcell.2021.637309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agrawal P, Rao S Super-enhancers and CTCF in early embryonic cell fate decisions. Front Cell Dev Biol. 2021; 9:653669. 10.3389/fcell.2021.653669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moorthy SD, Davidson S, Shchuka VM et al. Enhancers and super-enhancers have an equivalent regulatory role in embryonic stem cells through regulation of single or multiple genes. Genome Res. 2017; 27:246–58. 10.1101/gr.210930.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Apostolou E, Ferrari F, Walsh RM et al. Genome-wide chromatin interactions of the Nanog locus in pluripotency, differentiation, and reprogramming. Cell Stem Cell. 2013; 12:699–712. 10.1016/j.stem.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rada-Iglesias A, Bajpai R, Swigut T et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011; 470:279–83. 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Narita T, Ito S, Higashijima Y et al. Enhancers are activated by p300/CBP activity-dependent PIC assembly, RNAPII recruitment, and pause release. Mol Cell. 2021; 81:2166–82. 10.1016/j.molcel.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 12. Heintzman ND, Stuart RK, Hon G et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007; 39:311–8. 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 13. Song C, Zhang G, Mu X et al. eRNAbase: a comprehensive database for decoding the regulatory eRNAs in human and mouse. Nucleic Acids Res. 2024; 52:D81–91. 10.1093/nar/gkad925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sartorelli V, Lauberth SM Enhancer RNAs are an important regulatory layer of the epigenome. Nat Struct Mol Biol. 2020; 27:521–8. 10.1038/s41594-020-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li W, Notani D, Rosenfeld MG Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nat Rev Genet. 2016; 17:207–23. 10.1038/nrg.2016.4. [DOI] [PubMed] [Google Scholar]
- 16. Arnold CD, Gerlach D, Stelzer C et al. Genome-wide quantitative enhancer activity maps identified by STARR-seq. Science. 2013; 339:1074–7. 10.1126/science.1232542. [DOI] [PubMed] [Google Scholar]
- 17. Kim TK, Hemberg M, Gray JM et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010; 465:182–7. 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang C, Jia L, Wang Y et al. Genome-wide interaction target profiling reveals a novel Peblr20-eRNA activation pathway to control stem cell pluripotency. Theranostics. 2020; 10:353–70. 10.7150/thno.39093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan P, Lu JY, Niu J et al. LncRNA Platr22 promotes super-enhancer activity and stem cell pluripotency. J Mol Cell Biol. 2021; 13:295–313. 10.1093/jmcb/mjaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levasseur DN, Wang J, Dorschner MO et al. Oct4 dependence of chromatin structure within the extended Nanog locus in ES cells. Genes Dev. 2008; 22:575–80. 10.1101/gad.1606308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blinka S, Rao S Nanog expression in embryonic stem cells—an ideal model system to dissect enhancer function. Bioessays. 2017; 39:10.1002/bies.201700086. 10.1002/bies.201700086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blinka S, Reimer MH Jr, Pulakanti K et al. Super-enhancers at the Nanog locus differentially regulate neighboring pluripotency-associated genes. Cell Rep. 2016; 17:19–28. 10.1016/j.celrep.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agrawal P, Blinka S, Pulakanti K et al. Genome editing demonstrates that the −5 kb Nanog enhancer regulates Nanog expression by modulating RNAPII initiation and/or recruitment. J Biol Chem. 2021; 296:100189. 10.1074/jbc.RA120.015152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Witteveldt J, Macias S Differentiation of mouse embryonic stem cells to neuronal cells using hanging droplets and retinoic acid. Bio Protoc. 2019; 9:e3417. 10.21769/BioProtoc.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cuomo M, Florio E, Della Monica R et al. Epigenetic remodelling of Fxyd1 promoters in developing heart and brain tissues. Sci Rep. 2022; 12:6471. 10.1038/s41598-022-10365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hagège H, Klous P, Braem C, Splinter E et al. Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nat Protoc. 2007; 2:1722–33. 10.1038/nprot.2007.243. [DOI] [PubMed] [Google Scholar]
- 27. Andersson R, Gebhard C, Miguel-Escalada I et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014; 507:455–61. 10.1038/nature12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Project Consortium E An integrated encyclopedia of DNA elements in the human genome. Nature. 2012; 489:57–74. 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blow MJ, McCulley DJ, Li Z et al. ChIP-seq identification of weakly conserved heart enhancers. Nat Genet. 2010; 42:806–10. 10.1038/ng.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pulakanti K, Pinello L, Stelloh C et al. Enhancer transcribed RNAs arise from hypomethylated, Tet-occupied genomic regions. Epigenetics. 2013; 12:1303–20. 10.4161/epi.26597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang K, Chen Y, Chang EA et al. Dynamic epigenetic regulation of the Oct4 and Nanog regulatory regions during neural differentiation in rhesus nuclear transfer embryonic stem cells. Cloning Stem Cells. 2009; 11:483–96. 10.1089/clo.2009.0019. [DOI] [PubMed] [Google Scholar]
- 32. Hattori N, Imao Y, Nishino K et al. Epigenetic regulation of Nanog gene in embryonic stem and trophoblast stem cells. Genes Cells. 2007; 12:387–96. 10.1111/j.1365-2443.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 33. Carey TS, Choi I, Wilson CA et al. Transcriptional reprogramming and chromatin remodeling accompanies Oct4 and Nanog silencing in mouse trophoblast lineage. Stem Cells Dev. 2014; 23:219–29. 10.1089/scd.2013.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Riso G, Cuomo M, Di Risi T et al. Ultra-deep DNA methylation analysis of X-linked genes: GLA and AR as model genes. Genes. 2020; 11:620. 10.3390/genes11060620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharov AA, Masui S, Sharova LV et al. Identification of Pou5f1, Sox2, and Nanog downstream target genes with statistical confidence by applying a novel algorithm to time course microarray and genome-wide chromatin immunoprecipitation data. BMC Genomics. 2008; 9:269. 10.1186/1471-2164-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li W, Notani D, Ma Q et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013; 498:516–20. 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsai PF, Dell’Orso S, Rodriguez J et al. A muscle-specific enhancer RNA mediates cohesin recruitment and regulates transcription in trans. Mol Cell. 2018; 71:129–41. 10.1016/j.molcel.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lai F, Orom UA, Cesaroni M et al. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013; 494:497–501. 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiao W, Chen Y, Song H et al. HPSE enhancer RNA promotes cancer progression through driving chromatin looping and regulating hnRNPU/p300/EGR1/HPSE axis. Oncogene. 2018; 37:2728–45. 10.1038/s41388-018-0128-0. [DOI] [PubMed] [Google Scholar]
- 40. Xiang JF, Yin QF, Chen T et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014; 24:513–31. 10.1038/cr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hsieh CL, Fei T, Chen Y et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc Natl Acad Sci USA. 2014; 111:7319–24. 10.1073/pnas.1324151111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mitsui K, Tokuzawa Y, Itoh H et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003; 113:631–42. 10.1016/S0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 43. Chambers I, Colby D, Robertson M et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003; 113:643–55. 10.1016/S0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 44. Costa Y, Ding J, Theunissen TW et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature. 2013; 495:370–4. 10.1038/nature11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pantier R, Tatar T, Colby D et al. Endogenous epitope-tagging of Tet1, Tet2 and Tet3 identifies TET2 as a naïve pluripotency marker. Life Sci Alliance. 2019; 2:e201900516. 10.26508/lsa.201900516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rulands S, Lee HJ, Clark SJ et al. Genome-scale oscillations in DNA methylation during exit from pluripotency. Cell Syst. 2018; 7:63–76. 10.1016/j.cels.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Affinito O, Palumbo D, Fierro A et al. Nucleotide distance influences co-methylation between nearby CpG sites. Genomics. 2020; 112:144–50. 10.1016/j.ygeno.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 48. Florio E, Keller S, Coretti L et al. Tracking the evolution of epialleles during neural differentiation and brain development: D-aspartate oxidase as a model gene. Epigenetics. 2017; 12:41–54. 10.1080/15592294.2016.1260211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cuomo M, Keller S, Punzo D et al. Selective demethylation of two CpG sites causes postnatal activation of the Dao gene and consequent removal of D-serine within the mouse cerebellum. Clin Epigenetics. 2019; 11:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Improda T, Morgera V, Vitale M et al. Specific methyl-CpG configurations define cell identity through gene expression regulation. Int J Mol Sci. 2023; 24:9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mohn F, Weber M, Rebhan M et al. Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol Cell. 2008; 30:755–66. [DOI] [PubMed] [Google Scholar]
- 52. Schlesinger Y, Straussman R, Keshet I et al. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet. 2007; 39:232–6. [DOI] [PubMed] [Google Scholar]
- 53. Brinkman AB, Gu H, Bartels SJ et al. Sequential ChIP-bisulfite sequencing enables direct genome-scale investigation of chromatin and DNA methylation cross-talk. Genome Res. 2012; 22:1128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hyslop L, Stojkovic M, Armstrong L et al. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005; 23:1035–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA methylation raw data generated in this study are available on European Nucleotide Archive (ENA) database with the accession number PRJEB77658.