Abstract
Antibiotic resistance and the persistence of sessile cells within biofilms complicate the eradication of biofilm-related infections using conventional antibiotics. This highlights the necessity for alternate therapy methods. The objective of this study was to investigate the biofilm destruction activity of α-tocopherol against Staphylococcus aureus, Proteus mirabilis, and Pseudomonas aeruginosa on polystyrene. α-Tocopherol showed significant biofilm destruction activity on the pre-formed biofilms of S. aureus (45%–46%), Pr. mirabilis (42%–54%), and Ps. aeruginosa (28%). Resazurin assay showed that α-tocopherol disrupted all bacterial biofilms without interfering with their cell viability. Scanning electron microscope images showed lower bacterial cell count and less compacted cell aggregates on polystyrene surfaces after treatment with α-tocopherol. This study demonstrated the biofilm destruction activity of α-tocopherol against S. aureus, Pr. mirabilis, and Ps. aeruginosa. α-Tocopherol could potentially be used to decrease biofilm-associated infections of these bacteria.
Keywords: anti-biofilm, antimicrobial, biofilm inhibition, Gram-negative bacteria, Gram-positive bacteria, vitamin E
α-tocopherol, a member of the vitamin E family, exhibits biofilm destruction activity against pathogenic bacteria such as Staphylococcus aureus, Proteus mirabilis, and Pseudomonas aeruginosa.
Introduction
There are two major states of bacteria: the planktonic and biofilm states, where bacteria exist predominantly in biofilm (Kang et al. 2012, Seth et al. 2012). The adherent bacteria in biofilms differ profoundly from their planktonic counterparts in their intrinsic defense and survival mechanisms (Cos et al. 2010, Seth et al. 2012). It is estimated that ~68% of human microbial infections are associated with bacteria biofilms, as they can colonize living surfaces such as host tissue or non-living surfaces such as surfaces of medical implants (Crouzet et al. 2014, Hong et al. 2014, Jamal et al. 2018, Ghosh et al. 2020). Biofilm has been implicated as an independent contributor that impedes wound healing in patients with chronic wound infections, where the bacteria can colonize the wound surface through biofilm formation (Seth et al. 2012). The sessile bacteria in biofilm often cause chronic infections as they are tolerant to conventional antimicrobials. Even in healthy individuals, biofilm infections can rarely be resolved until the biofilm is eradicated (Cos et al. 2010). As a result of the chronicity, resistance to antimicrobial therapies, and immunological evasion ability of biofilm, clinical biofilm-related infections in humans often lead to significant morbidity (Hernández-Jiménez et al. 2013, Suresh et al. 2019). This further complicates and limits the therapeutic options in the management of biofilm infections in humans (Seth et al. 2012, Hernández-Jiménez et al. 2013). As several resistance mechanisms of biofilms are different from the resistance mechanisms of planktonic bacteria to antimicrobial agents, an increase in resistance of biofilms can occur if these resistance mechanisms contribute together (Yong et al. 2019a).
Staphylococcus aureus is one of the most frequently found opportunistic pathogens in biofilm-associated nosocomial infections, including medical device-related infections (Lister and Horswill 2014, Mawang et al. 2017, Kong et al. 2018, Suresh et al. 2019). The staphylococcal species is accountable for ~70% of implantable device-associated infections, with the majority of these infections being associated with S. aureus (Moormeier and Bayles 2017, Khatoon et al. 2018). As Proteus mirabilis has the ability to form crystalline biofilms on the surface of the catheter upon chemical reaction with urine, this makes it frequently associated with catheter-associated urinary tract infections in humans. The bacteria embedded in the crystalline biofilms are highly recalcitrant to the human immune system and many conventional antibiotics (Wasfi et al. 2020). Pseudomonas aeruginosa usually establishes itself in immunocompromised patients, such as patients with cystic fibrosis or patients in intensive care units in hospitals (de bentzmann and Plésiat 2011). Pseudomonas aeruginosa can also cause several chronic wound infections similar to S. aureus, including chronic leg ulcers, skin, and diabetic ulcers. About 52% of patients with chronic leg ulcers are colonized by Ps. aeruginosa, where the virulence factor expression of the bacteria is increased, leading to severe infections and antibiotic resistance (Crouzet et al. 2014, Serra et al. 2015). These three pathogens share the same characteristic where they are the common opportunistic pathogens for biofilm-related infections in the human body.
The occurrence of antibiotic resistance of bacteria in biofilm and the persistence of sessile cells have necessitated the development of effective alternative treatments for biofilm-associated infections. Antimicrobials derived from plants have more structural and biochemical diversity than synthetic drugs. It would be more difficult for the bacteria to interact with the high complexity of natural compounds, and this could contribute to the development of alternative therapies to treat biofilm-related infections (Roy et al. 2018). Tocopherols are the major vitamin E components in most edible oils, such as sunflower and olive oils (Shahidi and de Camargo 2016). Tocopherol has been shown to reduce bacterial adherence of some Staphylococcus epidermidis and S. aureus strains on ultra-high molecular weight polyethylene (UHMWPE; Gómez-Barrena et al. 2011), reduce bacterial adhesive ability of S. aureus and Escherichia coli on various types of UHMWPE (Banche et al. 2014), reduce bacterial adhesion and biofilm accumulation of S. epidermidis and S. aureus on polylactic acid (Campoccia et al. 2015), and prevent bacterial adherence and biofilm formation of S. aureus on a cross-linked polyethylene surface with a poly(2-methacryloyloxyethyl phosphorylcholine) layer (Kyomoto et al. 2015). Biofilm formation of S. aureus, S. epidermidis, E. coli, Klebsiella pneumoniae, Pr. mirabilis, Acinetobacter baumannii, Ps. aeruginosa, and Ps. putida has been shown to reduce with α-tocopheryl acetate application on polystyrene (Vergalito et al. 2015). Vitamin E reduced 62% and 67% of the biofilm formation of Ps. aeruginosa PAO1 in a flat-bottomed 96-well plate (Soltani et al. 2021) and a molecular imprinting polymer (Tajani et al. 2022), respectively. The objective of this study was to investigate the biofilm destruction activity of α-tocopherol against S. aureus, Pr. mirabilis, and Ps. aeruginosa on polystyrene.
Materials and methods
Bacterial strains
Cultures of S. aureus ATCC 6538P, Pr. mirabilis ATCC 12453, and Ps. aeruginosa ATCC 27853 were obtained from American Type Culture Collection (ATCC) Manassas, VA, USA.
Preparation of α-tocopherol solution
α-Tocopherol was prepared by dissolving 4 mg of α-tocopherol ( 96%, Sigma–Aldrich, Steinheim, Germany) in 10 µl of dimethyl sulfoxide (DMSO; Calbiochem, CA, USA), followed by 990 µl of brain–heart infusion (BHI) broth to achieve a final concentration of 4 mg ml−1 and subjected to 45 s of sonication.
96%, Sigma–Aldrich, Steinheim, Germany) in 10 µl of dimethyl sulfoxide (DMSO; Calbiochem, CA, USA), followed by 990 µl of brain–heart infusion (BHI) broth to achieve a final concentration of 4 mg ml−1 and subjected to 45 s of sonication.
Antimicrobial test
An antimicrobial test using the broth microdilution method was conducted to determine the minimum inhibitory concentration (MIC) of a sample (Clinical and Laboratory Standards Institute 2012). MIC is the lowest concentration of a compound that will cause inhibition of the visible growth of a microorganism. The bacteria were incubated in 10 ml BHI broth (Oxoid, Hampshire, UK) at 37°C for 18 h. Then, the bacteria suspension was adjusted to 0.5 McFarland standard using BHI broth (absorbance of 0.08–0.11 at 625 nm), equivalent to ~1 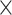 108 colony-forming unit (CFU) per ml. The adjusted bacterial suspension was diluted 1:1000 in sterile BHI broth (equivalent to ~1
108 colony-forming unit (CFU) per ml. The adjusted bacterial suspension was diluted 1:1000 in sterile BHI broth (equivalent to ~1 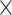 105 CFU ml−1). α-Tocopherol solution (100 µl) was serially diluted into 96-well microtiter plates with BHI broth before inoculation of 100 µl of diluted bacterial suspension (the highest concentration of α-tocopherol will be 2 mg ml−1 after inoculating with bacteria suspension). The concentration of DMSO in the wells was 0.5% DMSO. After 24 h incubation at 37°C, the absorbance was measured at 625 nm using a microplate reader (Tecan, Männedorf, Switzerland). The negative control (NC) was a bacterial suspension in BHI broth with 0.5% DMSO, and the positive control (PC) was a bacterial suspension in BHI broth with chloramphenicol (Nacalai Tesque, Kyoto, Japan). Chloramphenicol (100 mg ml−1) was first prepared by dissolving 20 mg of chloramphenicol in 200 µl of absolute ethanol, followed by taking 10 µl of this solution and adding 990 µl of BHI broth to achieve the starting concentration of 1 mg ml−1 to be used for serial dilution.
105 CFU ml−1). α-Tocopherol solution (100 µl) was serially diluted into 96-well microtiter plates with BHI broth before inoculation of 100 µl of diluted bacterial suspension (the highest concentration of α-tocopherol will be 2 mg ml−1 after inoculating with bacteria suspension). The concentration of DMSO in the wells was 0.5% DMSO. After 24 h incubation at 37°C, the absorbance was measured at 625 nm using a microplate reader (Tecan, Männedorf, Switzerland). The negative control (NC) was a bacterial suspension in BHI broth with 0.5% DMSO, and the positive control (PC) was a bacterial suspension in BHI broth with chloramphenicol (Nacalai Tesque, Kyoto, Japan). Chloramphenicol (100 mg ml−1) was first prepared by dissolving 20 mg of chloramphenicol in 200 µl of absolute ethanol, followed by taking 10 µl of this solution and adding 990 µl of BHI broth to achieve the starting concentration of 1 mg ml−1 to be used for serial dilution.
Crystal violet assay
Crystal violet assay was carried out to determine the biofilm destruction activity of a sample (Jeyaraj et al. 2022). This assay allowed the determination of the minimum biofilm destruction concentration (MBDC) of a sample. MBDC is the lowest sample concentration that can destroy a pre-formed biofilm. The bacteria were incubated in BHI broth at 37°C for 18 h. Then, the bacteria suspension was adjusted to 0.5 McFarland standard. The bacterial suspension was then diluted 1:100 (equivalent to ~1 × 106 CFU ml−1) in BHI broth supplemented in 1% glucose (Oxoid, Hampshire, UK) to stress the biofilm formation by bacteria. To determine biofilm destruction activity, diluted bacterial suspension (200 µl) was inoculated into 96-well microtiter plates. This was followed by incubation at 37°C for 24 h to allow biofilm formation. Then, the broth in the wells was removed, and the plate was rinsed gently with sterile phosphate-buffered saline (PBS; Oxoid, Hampshire, UK) three times. Next, a two-fold serial dilution of the α-tocopherol solution (200 µl) was added to the wells accordingly. The concentration of DMSO in the wells was 0.5% DMSO. The plate was incubated for 24 h at 37°C. Then, the contents of the wells were removed, and the wells were rinsed with water gently three times. The wells were heat-fixed at 60°C for 1 h, followed by staining with 0.2 ml of 0.1% crystal violet for 10 min. The wells were then rinsed with water and air-dried. Lastly, 0.2 ml of 95% ethanol was added, and absorbance was measured at 570 nm. The NC was bacteria suspension in BHI broth with 0.5% DMSO, while the PC was bacteria suspension in BHI broth with 1% sodium hypochlorite. Results were expressed as % destruction = [(absorbance of NC at 570 nm − absorbance of treated sample at 570 nm)/absorbance of NC at 570 nm] 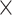 100.
100.
Resazurin assay
This assay allowed the evaluation of the effect of a substrate on the cellular viability of bacterial cells within a biofilm matrix (Toté et al. 2008). The bacteria were incubated in BHI broth at 37°C for 18 h. Next, the bacteria suspension was adjusted to 0.5 McFarland standard. Then, the bacterial suspension was diluted 1:100 in BHI with 1% glucose. The diluted bacterial suspension (200 µl) was inoculated into wells and incubated for 24 h at 37°C to allow biofilm formation. The broth was discarded after 24 h of incubation, and the adherent cells in the wells were washed with sterile PBS two times before adding a two-fold serial dilution of the α-tocopherol solution (200 µl) followed by incubation for 24 h at 37°C. The concentration of DMSO in the wells was 0.5% DMSO. The NC was bacteria suspension in BHI broth with 0.5% DMSO, while the PC was bacteria suspension in BHI broth with 1% sodium hypochlorite. The plates were rinsed with sterile distilled water before adding 5 µg ml−1 of resazurin (Sigma–Aldrich, Steinheim, Germany) in sterile distilled water. The plate was incubated for 2 h at 37°C. After the incubation, the fluorescence intensity (λex: 560 nm and λem: 590 nm) was measured. Results were expressed as % cell viability = fluorescence units of treated sample/fluorescence units of NC 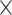 100.
100.
Scanning electron microscopy
Scanning electron microscopy (SEM) was used to observe bacterial cell density and distribution of biofilm after α-tocopherol treatment at MBDC (Yong et al. 2021). Polystyrene discs with dimensions of 1 cm  1 cm were cleaned by soaking them in 70% ethanol for 5 min. This was followed by rinsing them with sterile distilled water and placing them in a clean Petri dish. The bacteria were incubated in BHI broth at 37°C for 18 h. Next, the bacteria suspension was adjusted to 0.5 McFarland standard (absorbance of 0.08–0.11 at 625 nm), equivalent to ~1 × 108 CFU ml−1 in BHI broth. Then, the bacterial suspension was diluted 1:100 in BHI with 1% glucose. The adjusted bacterial suspension was added to a 24-well microtiter plate and incubated for 24 h at 37°C. Then, the discs were washed gently with sterile PBS and placed into a new 24-well microtiter plate inoculated with a sample at MBDC. Incubation for 24 h at 37°C was carried out. Then, the discs were washed gently with sterile PBS. The discs were prepared for SEM according to the following procedures: fixing with 2.5% (v/v) glutaraldehyde in sterile PBS for 4 h at room temperature and soaking in sterile PBS for 10 min afterward. Ethanol dehydration was carried out using ethanol with concentrations of 20, 40, 60, 70, 80, 90, 95, and 100% (v/v) in sterile PBS sequentially with 10 min for each concentration. The discs were kept in a desiccator overnight. The surfaces of the discs were gold-sputtered using a sputter coater (Quorum, Laughton, UK) before examination using a scanning electron microscope (Hitachi S-3400N-II, Chiyoda, Japan). Images were taken under 2500× magnification at 10 kV. Bacterial cell numbers were estimated using Fiji (distribution of the ImageJ software, US National Institutes of Health, Bethesda, USA).
1 cm were cleaned by soaking them in 70% ethanol for 5 min. This was followed by rinsing them with sterile distilled water and placing them in a clean Petri dish. The bacteria were incubated in BHI broth at 37°C for 18 h. Next, the bacteria suspension was adjusted to 0.5 McFarland standard (absorbance of 0.08–0.11 at 625 nm), equivalent to ~1 × 108 CFU ml−1 in BHI broth. Then, the bacterial suspension was diluted 1:100 in BHI with 1% glucose. The adjusted bacterial suspension was added to a 24-well microtiter plate and incubated for 24 h at 37°C. Then, the discs were washed gently with sterile PBS and placed into a new 24-well microtiter plate inoculated with a sample at MBDC. Incubation for 24 h at 37°C was carried out. Then, the discs were washed gently with sterile PBS. The discs were prepared for SEM according to the following procedures: fixing with 2.5% (v/v) glutaraldehyde in sterile PBS for 4 h at room temperature and soaking in sterile PBS for 10 min afterward. Ethanol dehydration was carried out using ethanol with concentrations of 20, 40, 60, 70, 80, 90, 95, and 100% (v/v) in sterile PBS sequentially with 10 min for each concentration. The discs were kept in a desiccator overnight. The surfaces of the discs were gold-sputtered using a sputter coater (Quorum, Laughton, UK) before examination using a scanning electron microscope (Hitachi S-3400N-II, Chiyoda, Japan). Images were taken under 2500× magnification at 10 kV. Bacterial cell numbers were estimated using Fiji (distribution of the ImageJ software, US National Institutes of Health, Bethesda, USA).
Statistical analysis
Antimicrobial test, crystal violet assay, and resazurin assay were carried out in independent triplicates, whereas SEM was carried out in duplicates. Then, all data were analysed using one-way ANOVA. Post hoc Tukey’s test was carried out at P < .05.
Results
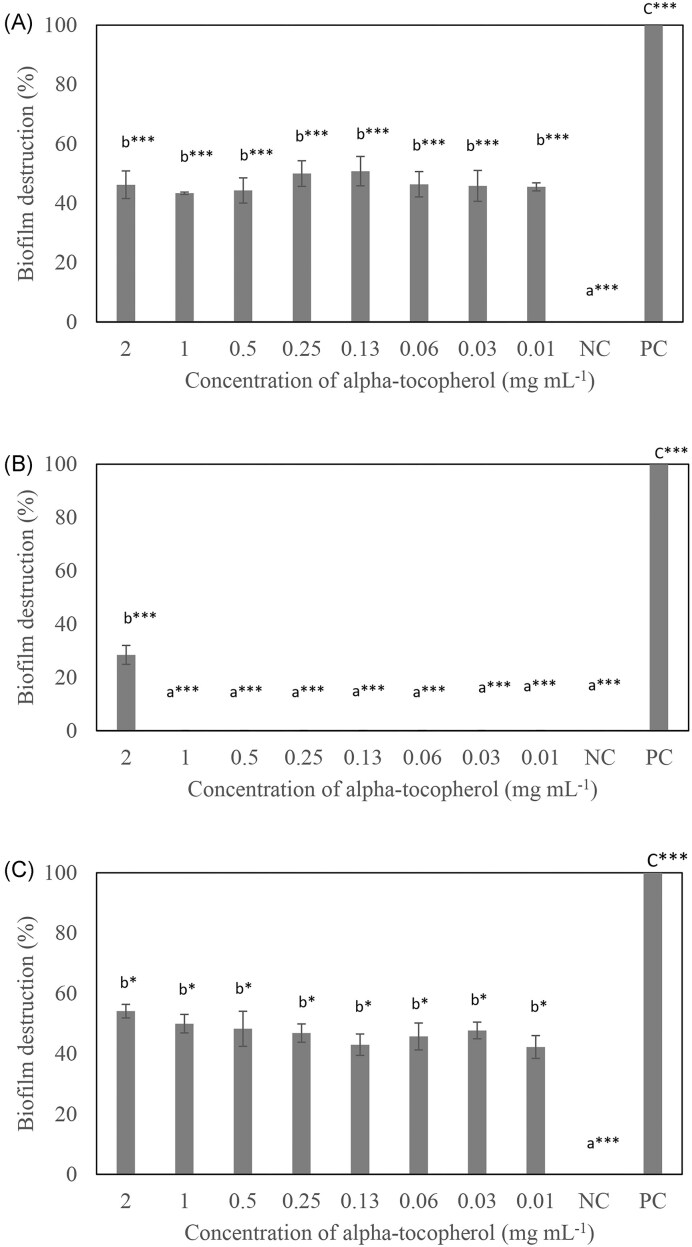
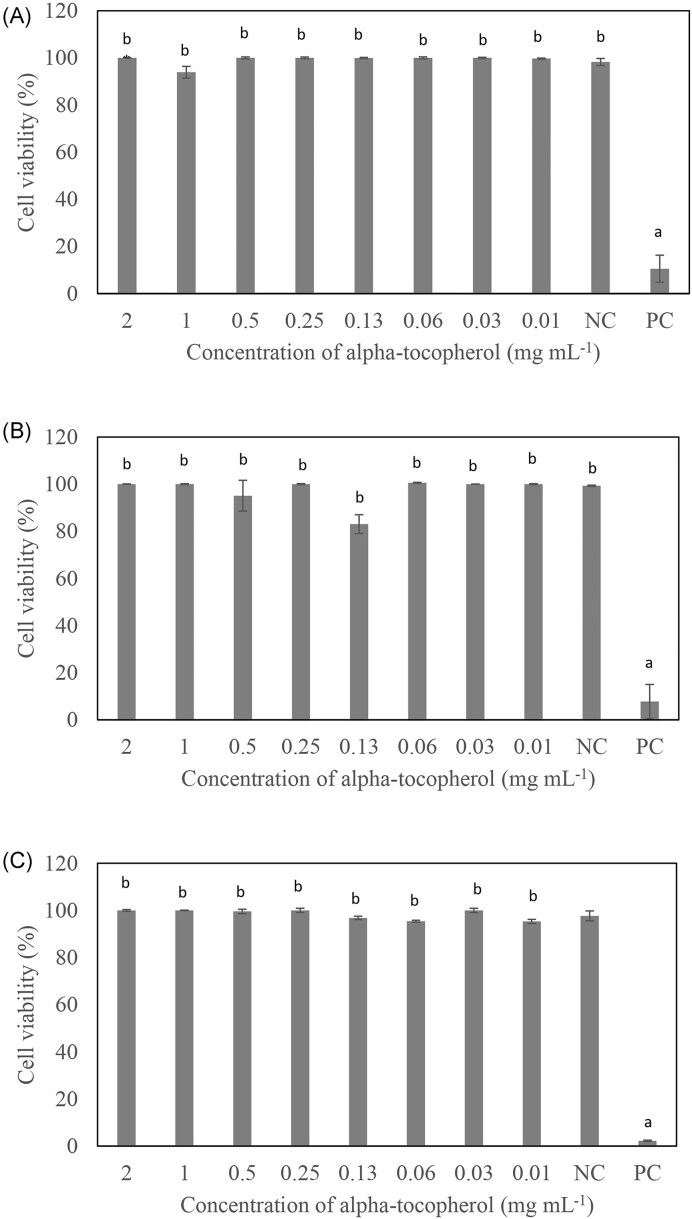
The MIC values of α-tocopherol were more than 2 mg ml−1 for S. aureus ATCC 6538P, Pr. mirabilis ATCC 12453, and Ps. aeruginosa ATCC 27853 (Table 1). α-Tocopherol was able to disrupt the biofilms formed by S. aureus ATCC 6538P (45%–46%) and Pr. mirabilis ATCC 12453 (42%–54%) at all the concentrations of α-tocopherol tested, whereas it was only effective in disrupting Ps. aeruginosa ATCC 27853 biofilm at 2 mg ml−1 (28%) to a lower extent compared to the other two bacteria tested (Fig. 1). The MBDC of α-tocopherol was determined to be 0.01 mg ml−1 for S. aureus ATCC 6538P and Pr. mirabilis ATCC 12453, and 2 mg ml−1 for Ps. aeruginosa ATCC 27853. α-Tocopherol was shown to have no significant effect on the cell viability of S. aureus ATCC 6538P, Pr. mirabilis ATCC 12453, and Ps. aeruginosa ATCC 27853 from 0.01 to 2 mg ml−1 (Fig. 2).
Table 1.
MIC of α-tocopherol against S. aureus ATCC 6538, Pr. mirabilis ATCC 12453, and Ps. aeruginosa ATCC 27853.
| Bacteria strains | α-Tocopherol MIC (mg ml−1) | Chloramphenicol MIC (mg ml−1) |
|---|---|---|
| Staphylococcus aureus ATCC 6538P (MSSA) | >2 | 0.008 |
| Proteus mirabilis ATCC 12 453 | >2 | 0.004 |
| Pseudomonas aeruginosa ATCC 27 853 | >2 | 0.250 |
All experiments were carried out in independent triplicates.
Figure 1.
Biofilm destruction after treatment with α-tocopherol at various concentrations against (A) S. aureus ATCC 6538P, (B) Ps. aeruginosa ATCC 27853, and (C) Pr. mirabilis ATCC 12453. The NC was bacteria suspension in BHI broth with 0.5% DMSO water, while the PC was bacteria suspension in BHI broth with 1% sodium hypochlorite. All experiments were carried out in triplicates, and results were expressed as mean percentage biofilm destruction ± standard deviation. abcDifferent letters indicate significant differences in biofilm destruction at ***P < .001 or *P < .05.
Figure 2.
Cell viability within bacterial biofilms after treatment with α-tocopherol at various concentrations against (A) S. aureus ATCC 6538P, (B) Ps. aeruginosa ATCC 27853, and (C) Pr. mirabilis ATCC 12453. The NC was bacteria suspension in BHI broth with 0.5% DMSO, while the PC was bacteria suspension in BHI broth with 1% sodium hypochlorite. All experiments were carried out in triplicates, and results were expressed as mean percentage cell viability ± standard deviation. abDifferent letters indicate significant differences in cell viability at P < .001.
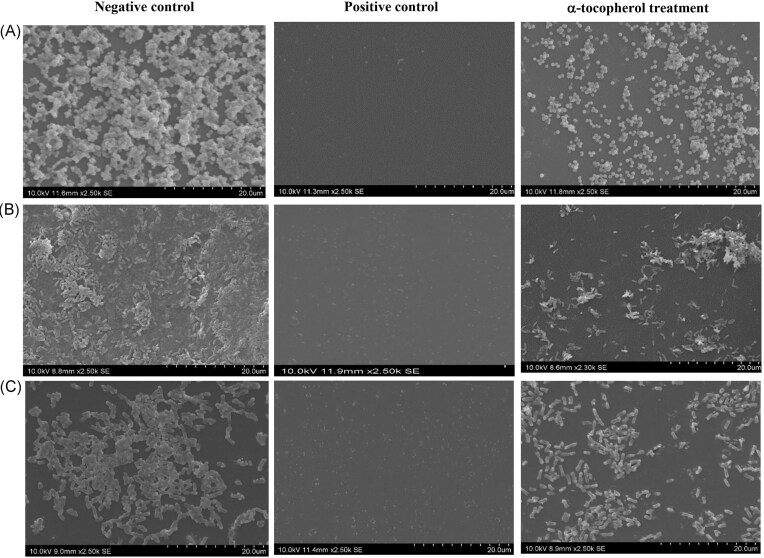
The effect of α-tocopherol treatment at MBDC on the biofilm destruction of treated groups compared to the NC is also shown in Fig. 3, whereby the SEM images of treated groups show a reduction in bacterial cell number (Table 2) and less compact aggregation of bacterial cells on the polystyrene surfaces.
Figure 3.
Effect of α-tocopherol on the bacterial cell density and distribution of biofilm after treatment with α-tocopherol at MBDC for (A) S. aureus ATCC 6538P, (B) Ps. aeruginosa ATCC 27853, and (C) Pr. mirabilis ATCC 12453. Images were taken under 2500× magnification at 10 kV. The NC was bacteria suspension in BHI broth with 0.5% DMSO. The PC was bacteria suspension in BHI broth with 1% sodium hypochlorite. The MDBC was 0.01 mg ml−1 for S. aureus ATCC 6538P and Pr. mirabilis ATCC 12453, and 2 mg ml−1 for Ps. aeruginosa ATCC 27853.
Table 2.
Effect of α-tocopherol on the number of bacterial cells on SEM images of S. aureus ATCC 6538P, Ps. aeruginosa ATCC 27853, and Pr. mirabilis ATCC 12453.
| Bacteria | Bacterial cell number (log cells cm−2) | |
|---|---|---|
| NC | Treatment | |
| Staphylococcus aureus ATCC 6538P | 9.65 ± 0.01a | 9.59 ± 0.00bA |
| Pseudomonas aeruginosa ATCC 27 853 | 10.15 ± 0.00a | 9.35 ± 0.01bB |
| Proteus mirabilis ATCC 12 453 | 9.56 ± 0.00a | 9.44 ± 0.00bC |
All experiments were carried out in duplicates, and the number of bacterial cells was expressed as mean log cells cm−2 ± standard deviation. Different lowercase letters indicate significant differences in bacterial cell number within the same bacterial strain at P < .05 as analysed by paired t-test. Different uppercase letters indicate significant differences in bacterial cell number between bacterial strains at P < .05, as analysed by two-way ANOVA.
Discussion
The MIC results are consistent with previous studies (Andrade et al. 2014, Tintino et al. 2016), where the MIC values for S. aureus and Ps. aeruginosa were more than 1 mg ml−1 of α-tocopherol. α-Tocopherol did not exhibit antimicrobial activity from 0.01 to 2 mg ml−1 against S. aureus ATCC 6538P, Pr. mirabilis ATCC 12453, and Ps . aeruginosa ATCC 27853. To the authors’ best knowledge, the antimicrobial effect of α-tocopherol on P. mirabilis is not reported. Chloramphenicol was used as a PC in this broth microdilution assay. The MIC value of chloramphenicol was similar to those reported in the literature, indicating the validity of the tests (Andrews 2001, Yong et al. 2017).
α-Tocopherol was tested for its biofilm destruction activity against S. aureus ATCC 6538P, Pr. mirabilis ATCC 12453, and Ps. aeruginosa ATCC 27853 (Fig. 1). The concentration of α-tocopherol tested ranges from 2 to 0.01 mg ml−1. α-Tocopherol was able to disrupt the biofilms formed by S. aureus ATCC 6538P and Pr. mirabilis ATCC 12453 at all the concentrations of α-tocopherol tested, whereas it only disrupted Ps. aeruginosa ATCC 27853 biofilm at 2 mg ml−1 (Fig. 1). To the authors’ best knowledge, this is the first study to report on the biofilm destruction activity of α-tocopherol on Ps. aeruginosa ATCC 27853 and Pr. mirabilis ATCC 12453, which are Gram-negative bacteria.
The result obtained for S. aureus ATCC 6538P is in accordance with another study where α-tocopherol was reported to destroy the biofilm of S. aureus ATCC 6538P at 0.01 mg ml−1 (Mawang et al. 2017). Nonetheless, there is no significant difference in the percentage of biofilm destruction between 2 and 0.01 mg ml−1 α-tocopherol for S. aureus ATCC 6538P and Pr. mirabilis ATCC 12453.
Ps. aeruginosa biofilm is mainly composed of extracellular DNA (eDNA), with the other three exopolysaccharides (EPS), which are alginate, Psl, and Pel (Ciofu and Tolker-Nielsen 2019). The synergistic effect between the three EPS affects the biofilm architecture and formation. Pel, eDNA, and live bacterial cells establish a connected meshwork that shows increased cell-to-cell interaction. This adds up to the increased compactness of the biofilm of Ps. aeruginosa when compared to the biofilms of S. aureus and Pr. mirabilis (Ghafoor et al. 2011, Ciofu and Tolker-Nielsen 2019). This may explain the lower percentage of biofilm destruction of α-tocopherol towards Ps. aeruginosa biofilm compared to the other two bacteria tested. Nevertheless, further studies are needed to substantiate this.
α-Tocopherol was assessed for its effect on cell viability of S. aureus ATCC 6538P, Pr. mirabilis ATCC 12453, and Ps. aeruginosa ATCC 27853 (Fig. 2). Resazurin assay was used to determine the viability of cells within the biofilm after treatment with α-tocopherol. The non-fluorescent, blue resazurin can be reduced to fluorescent pink resorufin by cells that are metabolically active. The fluorescent intensity of resazurin assay is proportional to the number of active cells that are present (Welch et al. 2012). This enables the study of the effect of α-tocopherol on the survival of cells within the biofilm matrix, which is not reflected in the crystal violet assay.
It was observed that α-tocopherol at various concentrations tested did not show a significant reduction in the viability of S. aureus ATCC 6538P, Pr. mirabilis ATCC 12453, and Ps. aeruginosa ATCC 27853 (Fig. 2). This signifies that α-tocopherol does not have a killing effect on the bacteria. Instead, it disrupts the biofilm matrix to achieve the biofilm destruction effect. Without any bacteriostatic and bactericidal effect by α-tocopherol, there will be weaker selective pressure towards the bacterial cells. Therefore, this will be advantageous for α-tocopherol as an anti-biofilm agent because the bacteria are less likely to develop resistance against it (Mawang et al. 2017).
The SEM images in Fig. 3 show a reduction in bacterial cell number of S. aureus ATCC 6538P, Pr. mirabilis ATCC 12453, and Ps. aeruginosa ATCC 27853 (Table 2), and less compacted aggregates of cells on the polystyrene surfaces after α-tocopherol treatment when compared with the NC. These observations support the biofilm destruction activity of the crystal violet assay (Fig. 1).
Chemical cleaners such as hydrogen peroxide can have a negative impact on the users and the environment. Natural anti-biofilm compounds could be used with chemicals to reduce these adverse effects (Yong et al. 2019b). The reduction of bacterial cell number (Table 2) using α-tocopherol in mature biofilm is lower when compared to treatment with betacyanin, a natural biofilm-inhibiting agent. Betacyanin possesses biofilm inhibition activity against S. aureus and Ps. aeruginosa. It was effective in reducing 1.1–1.2 log orders of Ps. aeruginosa and S. aureus (Yong et al. 2021). The bacterial cells in a premature biofilm are more metabolically active than the dormant cells in a mature biofilm, making them more susceptible to antibiotic treatment (Shahidi and de Camargo 2016, Ghosh et al. 2020). This may be the same for biofilm inhibiting agents, as they can interfere with biofilms that are not fully developed through multiple mechanisms, such as inhibition of bacteria adhesion to a surface by interfering with the quorum-sensing system that takes part in biofilm formation, whereas biofilm destruction agents mainly rely on the disassembly of mature biofilms by interfering with EPS matrix (Shahidi and de Camargo 2016). The effect of α-tocopherol on the biofilms of the three bacteria may be due to the variation in the EPS components of these bacteria but this requires further investigation.
The α-tocopherol was shown to be able to disrupt the biofilms of S. aureus, Pr. mirabilis, and Ps. aeruginosa. As of now, there are limited studies that investigate the mechanism of the biofilm-disrupting of α-tocopherol. One commonly known mechanism in relation to biofilm disruption is the solubilization of the matrix components of biofilms, such as polysaccharides (Mawang et al. 2017, Yong et al. 2019b). As the biofilms of S. aureus, Pr. mirabilis, and Ps. aeruginosa contain polysaccharides, this could be the potential mechanism of biofilm disruption by α-tocopherol. However, further study is required to investigate the mechanism of biofilm destruction.
Conclusions
α-Tocopherol was effective in disrupting biofilms formed by S. aureus ATCC 6538P (46%) and Pr. mirabilis ATCC 12453 (42%) at 0.01 mg ml−1, as well as Ps. aeruginosa ATCC 27853 (28%) at 2 mg ml−1. Further study of the dose-dependence of the biofilm destruction capability of α-tocopherol towards S. aureus ATCC 6538P and Pr. mirabilis ATCC 12453 should be conducted using lower α-tocopherol concentrations. As Ps. aeruginosa ATCC 27853 biofilms were only reduced at the highest concentration of 2 mg ml−1 α-tocopherol used, further studies should use even higher concentrations to establish the dose–response curve. As this study investigated 24 h biofilm formation, this could lead to premature biofilm conditions. Further hours of biofilm formation, such as 48 and 72 h, could be investigated in the future to provide a more comprehensive understanding of the effect of α-tocopherol on mature biofilms. As the effect of α-tocopherol on biofilms from reference strains investigated in this study is promising, clinical isolates should be investigated as they produce different biofilms. Besides, as biofilm-associated infections usually involve polymicrobial biofilms, polymicrobial biofilms can be investigated. Resazurin assay revealed that α-tocopherol does not affect the viability of cells within biofilms. Hence, it was deduced that α-tocopherol destroys biofilm by affecting the biofilm matrix only, thus degrading the structural integrity of the biofilms.
Images of scanning electron microscopy show that α-tocopherol significantly reduced bacterial cell number as well as compacted cell aggregates of S. aureus ATCC 6538P, Ps. aeruginosa ATCC 27853, and Pr. mirabilis ATCC 12453 on polystyrene surface. Materials for making catheters will be good choices of surfaces to investigate in the future. It is also important to investigate the mechanism for biofilm destruction of these bacteria by α-tocopherol. α-Tocopherol can potentially be a natural biofilm destruction agent against S. aureus ATCC 6538P, Ps. aeruginosa ATCC 27853, and Pr. mirabilis ATCC 12453.
Contributor Information
Pui Yee Leong, School of Science, Monash University Malaysia, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor, Malaysia.
Wei Qi Tan, School of Science, Monash University Malaysia, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor, Malaysia.
Wee Sim Choo, School of Science, Monash University Malaysia, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor, Malaysia.
Conflict of interest
None declared.
Funding
Funding was received from the School of Science of Monash University Malaysia.
References
- Andrade JC, Morais-Braga MFB, Guedes GMM et al. Enhancement of the antibiotic activity of aminoglycosides by alpha-tocopherol and other cholesterol derivates. Biomed Pharmacother. 2014;68:1065–9. [DOI] [PubMed] [Google Scholar]
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48:5–16. [DOI] [PubMed] [Google Scholar]
- Banche G, Allizond V, Bracco P et al. Interplay between surface properties of standard, vitamin E blended and oxidised ultra high molecular weight polyethylene used in total joint replacement and adhesion of Staphylococcus aureus and Escherichia coli. Bone Joint J. 2014;96-B:497–501. [DOI] [PubMed] [Google Scholar]
- Campoccia D, Visai L, Renò F et al. Bacterial adhesion to poly-(d,l)lactic acid blended with vitamin E: toward gentle anti-infective biomaterials. J Biomed Mater Res. 2015;103:1447–58. [DOI] [PubMed] [Google Scholar]
- Ciofu O, Tolker-Nielsen T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—how P. aeruginosa can escape antibiotics. Front Microbiol. 2019;10:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard CLSI Document M07-A9 (9th edn). Wayne: Clinical and Laboratory Standards Institute, 2012. [Google Scholar]
- Cos P, Tote K, Horemans T et al. Biofilms: an extra hurdle for effective antimicrobial therapy. Curr Pharm Des. 2010;16:2279–95. [DOI] [PubMed] [Google Scholar]
- Crouzet M, Le Senechal C, Brözel VS et al. Exploring early steps in biofilm formation: set-up of an experimental system for molecular studies. BMC Microbiol. 2014;14:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bentzmann S, Plésiat P. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ Microbiol. 2011;13:1655–65. [DOI] [PubMed] [Google Scholar]
- Ghafoor A, Hay ID, Rehm BHA. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl Environ Microbiol. 2011;77:5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Jayaraman N, Chatterji D. Small-molecule inhibition of bacterial biofilm. ACS Omega. 2020;5:3108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Barrena E, Esteban J, Molina-Manso D et al. Bacterial adherence on UHMWPE with vitamin E: an in vitro study. J Mater Sci: Mater Med. 2011;22:1701–6. [DOI] [PubMed] [Google Scholar]
- Hernández-Jiménez E, Del Campo R, Toledano V et al. Biofilm vs. planktonic bacterial mode of growth: which do human macrophages prefer?. Biochem Biophys Res Commun. 2013;441:947–52. [DOI] [PubMed] [Google Scholar]
- Hong W, Claus M, Heng-Zhuang W et al. Strategies for combating bacterial biofilm infections. Int J Oral Sci. 2014;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M, Ahmad W, Andleeb S et al. Bacterial biofilm and associated infections. J Chin Med Assoc. 2018;81:7–11. [DOI] [PubMed] [Google Scholar]
- Jeyaraj EJ, Nathan S, Lim YY et al. Antibiofilm properties of Clitoria ternatea flower anthocyanin-rich fraction towards Pseudomonas aeruginosa. Access Microbiol. 2022;4:000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Kim T, Tak Y et al. Cyclic voltammetry for monitoring bacterial attachment and biofilm formation. J Ind Eng Chem. 2012;18:800–7. [Google Scholar]
- Khatoon Z, McTiernan CD, Suuronen EJ et al. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4:e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong C, Chee C-F, Richter K et al. Suppression of Staphylococcus aureus biofilm formation and virulence by a benzimidazole derivative, UM-C162. Sci Rep. 2018;8:2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyomoto M, Shobuike T, Moro T et al. Prevention of bacterial adhesion and biofilm formation on a vitamin E-blended, cross-linked polyethylene surface with a poly (2-methacryloyloxyethyl phosphorylcholine) layer. Acta Biomater. 2015;24:24–34. [DOI] [PubMed] [Google Scholar]
- Lister JL, Horswill AR. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol. 2014;4:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawang CI, Lim YY, Ong KS et al. Identification of α-tocopherol as a bioactive component of Dicranopteris linearis with disrupting property against preformed biofilm of Staphylococcus aureus. J Appl Microbiol. 2017;123:1148–59. [DOI] [PubMed] [Google Scholar]
- Moormeier DE, Bayles KW. Staphylococcus aureus biofilm: a complex developmental organism. Mol Microbiol. 2017;104:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Tiwari M, Donelli G et al. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9:522–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Grande R, Butrico L et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther. 2015;13:605–13. [DOI] [PubMed] [Google Scholar]
- Seth KA, Geringer RM, Gurjala NA et al. Treatment of Pseudomonas aeruginosa biofilm–infected wounds with clinical wound care strategies: a quantitative study using an in vivo rabbit ear model. Plast Reconstr Surg. 2012;129:262e–74e. [DOI] [PubMed] [Google Scholar]
- Shahidi F, de Camargo AC. Tocopherols and tocotrienols in common and emerging dietary sources: occurrence, applications, and health benefits. Int J Mol Sci. 2016;17:1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani S, Bazzaz BSF, Hadizadeh F et al. New insight into vitamins E and K1 as anti-quorum-sensing agents against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2021;65:10–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh MK, Biswas R, Biswas L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int J Med Microbiol. 2019;309:1–12. [DOI] [PubMed] [Google Scholar]
- Tajani AS, Soheili V, Moosavi F et al. Ultra selective and high-capacity dummy template molecular imprinted polymer to control quorum sensing and biofilm formation of Pseudomonas aeruginosa. Anal Chim Acta. 2022;1199:339574. [DOI] [PubMed] [Google Scholar]
- Tintino SR, Morais-Tintino CD, Campina FF et al. Action of cholecalciferol and alpha-tocopherol on Staphylococcus aureus efflux pumps. EXCLI J. 2016;15:315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toté K, Bergh DV, Maes L et al. A new colorimetric microtitre model for the detection of Staphylococcus aureus biofilms. Lett Appl Microbiol. 2008;46:249–54. [DOI] [PubMed] [Google Scholar]
- Vergalito F, Pietrangelo L, Petronio Petronio G et al. Vitamin E for prevention of biofilm-caused healthcare-associated infections. Open Med. 2015;20:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasfi R, Hamed SM, Amer MA et al. Proteus mirabilis biofilm: development and therapeutic strategies. Front Cell Infect Microbiol. 2020;10:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K, Cai Y, Strømme M. A method for quantitative determination of biofilm viability. J Funct Biomater. 2012;3:418–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong YY, Dykes G, Choo WS. Biofilm inhibiting activity of betacyanins from red pitahaya (Hylocereus polyrhizus) and red spinach (Amaranthus dubius) against Staphylococcus aureus and Pseudomonas aeruginosa biofilms. J Appl Microbiol. 2019a;126:68–78. [DOI] [PubMed] [Google Scholar]
- Yong YY, Dykes G, Choo WS. Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit Rev Microbiol. 2019b;45:201–22. [DOI] [PubMed] [Google Scholar]
- Yong YY, Dykes G, Choo WS. Comparative study of betacyanin profile and antimicrobial activity of red pitahaya (Hylocereus polyrhizus) and red spinach (Amaranthus dubius). Plant Foods Hum Nutr. 2017;72:41–47. [DOI] [PubMed] [Google Scholar]
- Yong YY, Ong MWK, Dykes G et al. Betacyanin-inhibited biofilm formation of co-culture of Staphylococcus aureus and Pseudomonas aeruginosa on different polymer surfaces. FEMS Microbiol Lett. 2021;368:fnaa214. [DOI] [PubMed] [Google Scholar]