Abstract
The relative balance between Th1 and Th2 cytokines appears crucial, since the role of cytokines has been evaluated in several studies by comparison of clinically heterogeneous groups of patients. The aim of this study is to determine the role of proinflammatory Th1 cytokines, interleukin-12 (IL-12) and gamma interferon (IFN-γ), and anti-inflammatory Th2 cytokines, IL-4 and IL-10, in a homogeneous group of patients with uncomplicated Plasmodium falciparum malaria. Levels of IL-12, IFN-γ, Il-4, and IL-10 in serum for 20 adult patients and 15 healthy control subjects were determined by an immunoenzymatic assay. Serum levels of Th1 cytokines, IL-12 (8.6 ± 2.8 pg/ml; controls, 3.2 ± 0.7 pg/ml) and IFN-γ (39.2 ± 67.6 pg/ml; controls, 8.4 ± 6.3 pg/ml), were significantly increased at admission; 3 days later, levels of IL-12 in serum remained significantly high (8.8 ± 2.6 pg/ml), whereas IFN-γ levels returned to control values. The anti-inflammatory response of Th2 cytokines (IL-10 and IL-4) was distinct. Levels of IL-10 in serum were not significantly increased at day 0 and day 3 (306.6 ± 200.4 pg/ml and 56.6 ± 38.4 pg/ml, respectively; controls, 17.4 ± 9.0 pg/ml). In contrast, levels of IL-4 in serum were not increased on admission (3.4 ± 1.2 pg/ml; controls, 2.4 ± 0.8 pg/ml), but at day 3 a moderate and significant increase of IL-4 levels was observed (4.5 ± 1.7 pg/ml). In conclusion, the increase of Th1 cytokine IL-12 and IFN-γ levels during the acute phase of uncomplicated P. falciparum malaria may reflect an early and effective immune response regulated by proinflammatory Th1 cytokines, and in particular IFN-γ may play a role in limiting progression from uncomplicated malaria to severe and life-threatening complications.
Impairment of cell-mediated immunity with specificity for both parasite and nonparasite antigens is a peculiar feature of acute Plasmodium falciparum infection, the causative agent of uncomplicated and severe malaria (1, 5, 15). The CD4 T-cell subset is of major importance for the induction of blood-stage immunity, while the CD8 subset has been shown to be cytolytic against liver stages of the parasite. Stevenson and Tam (7) have shown that the preferential activation of a Th1 response in mice is related to resistance to blood-stage Plasmodium chabaudi AS infection.
High gamma interferon (IFN-γ) production as part of a Th1-driven immune response has been associated with a more favorable outcome in most animal models of malaria (3, 6). This effect has been attributed to the monocyte-macrophage-activating capacity of IFN-γ, with rapid killing of the malarial blood-stage parasites by reactive oxygen and nitrogen intermediates (9). Winkler et al. (14) have shown in patients with uncomplicated P. falciparum malaria the role of IFN-γ as a key molecule in human antimalarial host defense, and they do not support a direct involvement of interleukin-4 (IL-4) in the clearance of P. falciparum parasites.
Several studies have shown that administration of IL-12 before infection of mice with Plasmodium yoelii or of rhesus monkeys with Plasmodium cynomolgi provides full protection in both animal models through an IFN-γ-dependent antiplasmodial mechanism (2, 6). In addition, Stevenson et al. (8) demonstrated that IL-12-induced protection against the blood stage of P. chabaudi AS is mediated by upregulation of IFN-γ and tumor necrosis factor, at least in part by generating high levels of nitric oxide. Thus, these studies suggest that the protective immunity in malaria is mediated by activation of Th1 cytokines, including IL-12, IFN-γ, and tumor necrosis factor.
To confirm experimental and clinical results, particularly in patients with uncomplicated P. falciparum malaria, we attempted to identify the cytokine pattern displayed by these patients during the course of disease, as Th1 cytokines, such as IL-12 and IFN-γ, as well as Th2 cytokines, such as IL-4 and IL-10.
MATERIALS AND METHODS
The study was carried out at the Division of Infectious Diseases, Varese, Italy, and at the Institute of Infectious Diseases, University of Brescia.
Twenty adult patients (17 male, 3 female; mean age ± standard deviation, 35.1 ± 5.0 years) were admitted at the hospitals of Varese and Brescia with a diagnosis of uncomplicated P. falciparum malaria. All patients were African immigrants and had resided in Italy for at least 1 year. Malaria was diagnosed by examination of thick and thin blood films. All patients received adequate antimalarial treatment; most of them received therapy with quinine, given intravenously or orally. Uncomplicated malaria was defined as P. falciparum parasitemia (1,000 to 10,000 parasites/μl) with a hemoglobin level of >8.0 g/dl, glycemia of >60 mg/dl, and no signs of severe malaria. Fifteen Caucasian healthy subjects (10 male, 5 female; mean age, 32.1 ± 5.9 years) served as a negative control group.
Blood samples were taken on admission (before start of the treatment) and on day 3 of the disease. After centrifugation, sera were immediately aliquoted and stored at −80°C until tested.
Samples, taken from all patients on admission, were evaluated for IL-12, IFN-γ, IL-4, and IL-10 by a sandwich enzyme-linked immunosorbent assay.
The assays use a monoclonal antibody specific for human IL-12, IFN-γ, IL-4, and IL-10 (Euroclone, Devon, United Kingdom). The optical density was read at 450 nm.
All cytokine concentrations (in picograms per milliliter) were determined by comparison with the standard curve. The lower detection limits for the IL-12 and the IFN-γ immunoassays were <20 and <5 pg/ml, respectively, whereas those for the IL-4 and the IL-10 immunoassays were <0.5 and <5 pg/ml, respectively.
Data are expressed as means and standard deviations. Statistical analysis was performed using an unpaired t test with Welch's correction. For multiple comparison, the Kruskal-Wallis nonparametric analysis of variance test was used. Coefficients of correlations were calculated by the Spearman rank test. P values are considered significant when P is <0.05.
RESULTS
To evaluate the role of Th1 and Th2 cytokines in the immune response of uncomplicated P. falciparum malaria, levels of Th1 cytokines, IL-12 and IFN-γ, and Th2 cytokines, IL-4 and IL-10, in serum were tested in 20 adult patients and were compared with those detected in 15 seronegative healthy donors. Their demographic, clinical, and laboratory features are shown in Table 1. Our patients quickly improved after antimalarial treatment, including quinine or mefloquine, was started at admission, and all patients completely recovered from the infection within 7 to 10 days.
TABLE 1.
Clinical, parasitological, and laboratory features of study participants
| Patient group (n) | Mean age ± SD (yr) | No. male | No. female | Mean parasitemia ± SD (parasites/μl) | Mean hemoglobin level ± SD (g/dl) | Mean platelet count ± SD (106/liter) | Mean glucose level ± SD (mg/dl) | Mean temp ± SD (°C) |
|---|---|---|---|---|---|---|---|---|
| Healthy control (15) | 32.1 ± 5.9 | 10 | 5 | 0 | NDa | ND | ND | 36.8 ± 0.8 |
| Malaria (20) | 35.1 ± 5.0 | 17 | 3 | 8,080.6 ± 9,912.0 | 13.2 ± 1.9 | 178.9 ± 32.3 | 104.7 ± 46.7 | 38.2 ± 0.5 |
ND, not determined.
Figure 1 shows levels of Th1 cytokines, IL-12 and IFN-γ, in serum. Levels of IL-12 in the sera of our patients on admission were significantly increased (8.6 ± 2.8 pg/ml; controls, 3.2 ± 0.7 pg/ml; P < 0.001), with a persistent increase of IL-12 levels after 3 days (8.8 ± 2.6 pg/ml).
FIG. 1.
Levels of Th1 cytokines, IL-12 and IFN-γ, in sera from 20 patients with uncomplicated P. falciparum malaria and 15 healthy control subjects at day 0 and day 3. The horizontal line within the box stands for the median value; the box includes the 25th and 75th percentiles and the extended T-line stands for standard deviation.
Furthermore, a significant increase of IFN-γ levels was only observed on admission (39.2 ± 67.6 pg/ml; controls, 8.4 ±6.3 pg/ml; P = 0.0285), whereas IFN-γ levels returned to normal levels after 3 days (7.0 ± 5.6 pg/ml).
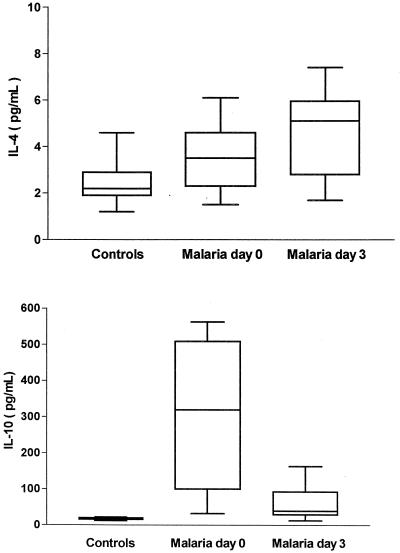
Figure 2 illustrates levels in serum of Th2 cytokines, IL-4 and IL-10. As shown, IL-4 levels were not increased on admission (3.4 ± 1.2 pg/ml; controls, 2.4 ± 0.8 pg/ml), but 3 days later, a moderate and significant increase was observed (4.5 ± 1.7 pg/ml; P = 0.0006). In addition, a nonsignificant increase of IL-10 was observed at days 0 and 3 (306.6 ± 200.4 and 56.6 ± 38.4 pg/ml, respectively; controls, 17.4 ± 3.0 pg/ml).
FIG. 2.
Levels of Th2 cytokines, IL-4 and IL-10, in sera from 20 patients with uncomplicated P. falciparum malaria and in 15 healthy control subjects at day 0 and day 3. The horizontal line within the box stands for the median value; the box includes the 25th and 75th percentiles and the extended T-line stands for the standard deviation.
We also determined a correlation between levels of Th1 cytokines, IL-12 and IFN-γ, in serum; levels of Th2 cytokines, IL-4 and IL-10; and parasitemia. As shown in Fig. 3, parasitemia showed an association only with levels of IL-12 or IFN-γ levels (r = 0.60; P < 0.001).
FIG. 3.
Correlation between Th1 cytokines, IL-12 and IFN-γ, and Th2 cytokines, IL-4 and IL-10, and parasitemia on admission in 20 patients with uncomplicated P. falciparum malaria. Correlation coefficients (r2) and their statistical significance (P) are also indicated.
DISCUSSION
The present study demonstrates a Th1-driven immune response by IL-12 and IFN-γ in adult patients with uncomplicated P. falciparum malaria, whereas the response of Th2 cytokines, IL-4 and IL-10, seems to be not involved during the acute phase of the disease, and these decrease in accordance with improvement and recovery of the disease. More recently, we have demonstrated elevated levels of IL-18 in adult patients with uncomplicated P. falciparum malaria, and these levels decrease in accordance with improvement of and recovery from disease (12). Walker et al. (13) have shown that IL-18 in combination with IL-12 enhanced the rate of transcription of the IFN-γ gene. Increased levels of IL-18, a proinflammatory Th1 cytokine, along with IL-12 in uncomplicated malaria may be crucial to stimulate effective production of IFN-γ.
Thus, IFN-γ represents a key molecule in human antimalarial host defense, as recently demonstrated by Winkler et al. (14) in patients with uncomplicated P. falciparum malaria. These investigators have shown a more pronounced Th2-driven immune response during acute untreated and uncomplicated malaria caused by P. falciparum, with a shift towards Th1 responsiveness induced by parasite clearance.
It is interesting that patients with uncomplicated P. falciparum malaria and higher production of IL-18 at days 0 and 3 had increased levels of IFN-γ (12). It is conceivable to think that an early production of Th1 cytokines such as IL-12, IFN-γ, and IL-18 is crucial in a better response and resolution of malaria infection.
In the murine model of infection with P. chabaudi AS, a Th2-dominated immune response has been demonstrated to be crucial in preventing recrudescent malaria during the course of disease (10). Successively, Taylor-Robinson and Phillips (11) have shown that in the same murine model of infection, the dose of antigen may affect the balance between Th1- and Th2-mediated immune functions; increasing the infective dose in a susceptible mouse strain led to up-regulation of Th2 cytokine (IL-4) production, whereas that in a resistant mouse strain enhanced the Th1 cytokine (IFN-γ). Our study demonstrates up-regulation of the Th1 response through increase of IL-12 and more importantly through increase of IFN-γ.
A more favorable outcome in animal models of malaria has been associated with increased production of IFN-γ (3, 6), and treatment of lethal murine P. vinckei infection with IFN-γ enhanced the effect of antimalarial treatment (4). It is intriguing to postulate that inability to mount a Th1 response, and to maintain an adequately balanced immune response may underlie the progression from uncomplicated P. falciparum malaria to severe, life-threatening complications.
In conclusion, the results of this study suggest a role of Th1 cytokines, IL-12 and IFN-γ, in uncomplicated P. falciparum malaria as a proinflammatory Th1 cytokine, since the elimination of intracellular pathogens, including P. falciparum, requires activation of Th1 cells with consequent cytokines, mainly IFN-γ, to initiate an early and prompt cell-mediated immune response.
REFERENCES
- 1.Brasseur, P., M. Agrapart, J. J. Ballet, P. Druilhe, M. J. Warrell, and S. Tharavanij. 1983. Impaired cell-mediated immunity in Plasmodium falciparum-infected patients with high parasitemia and cerebral malaria. Clin. Immunol. Immunopathol. 27:38-50. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman, S., J. Crutcher, S. Puri, et al. 1997. Sterile protection of monkeys against malaria after administration of interleukin-12. Nat. Med. 3:80-83. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs, P., D. Radzioch, and M. M. Stevenson. 1996. In vivo regulation of nitric oxide production by tumor necrosis factor alpha in the spleen and gamma interferon, but not interleukin-4, during blood stage malaria in mice. Infect. Immun. 64:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kremsner, P. G., S. Neifer, S. Schermick, M. Ferreira Chaves, K. Sliwa, and U. Bienzle. 1991. Interferon-gamma enhances the effect of antimalarial chemotherapy in murine Plasmodium vinckei malaria. J. Infect. Dis. 163:1161-1163. [DOI] [PubMed] [Google Scholar]
- 5.Riley, E. M., G. Andersson, L. N. Otoo, S. Jepsen, and B. M. Greenwood. 1988. Cellular immune responses to Plasmodium falciparum antigens in Gambian children during and after acute attack of falciparum malaria. Clin. Exp. Immunol. 73:17-22. [PMC free article] [PubMed] [Google Scholar]
- 6.Sedegah, M., F. Finkelman, and S. L. Hoffman. 1994. Interleukin 12 induction of interferon gamma-dependent protection against malaria. Proc. Natl. Acad. Sci. USA 91:10700-10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson, M., and M. Tam. 1993. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin. Exp. Immunol. 92:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson, M. M., M. F. Tam, S. F. Wolf, and A. Sher. 1995. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J. Immunol. 155:2545-2556. [PubMed] [Google Scholar]
- 9.Taylor-Robinson, A. W., and M. Looker. 1998. Sensitivity of malaria parasites to nitric oxide at low oxygen tensions. Lancet 351:1630.. [DOI] [PubMed] [Google Scholar]
- 10.Taylor-Robinson, A. W., R. S. Phillips, A. Severn, S. Moncada, and F. Y. Liew. 1993. The role of Th1 and Th2 cells in a rodent malaria infection. Science 260:1931-1934. [DOI] [PubMed] [Google Scholar]
- 11.Taylor-Robinson, A. W., and R. S. Phillips. 1998. Infective dose modulates the balance between Th1- and Th2-regulated immune responses during blood-stage malaria infection. Scand. J. Immunol. 48:527-534. [DOI] [PubMed] [Google Scholar]
- 12.Torre, D., M. Giola, F. Speranza, A. Matteelli, C. Basilico, and G. Biondi. 2001. Serum levels of interleukin-18 in patients with uncomplicated Plasmodium falciparum malaria. Eur. Cytokine Netw. 12:361-364. [PubMed] [Google Scholar]
- 13.Walker, W., M. Aste-Amezaga, R. A. Kastelein, G. Trinchieri, and C. A. Hunter. 1999. IL-18 and CD 28 use distinct molecular mechanisms to enhance NK cell production of IL-12-induced IFN-gamma. J. Immunol. 162:5894-5901. [PubMed] [Google Scholar]
- 14.Winkler, S., M. Willheim, K. Baier, et al. 1998. Reciprocal regulation of Th1- and Th2-cytokine-producing T cells during clearance of parasitemia in Plasmodium falciparum malaria. Infect. Immun. 66:6040-6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. 1997. World malaria in 1994. Wkly. Epidemiol. Rec. 72:269-276.9293226 [Google Scholar]