Abstract
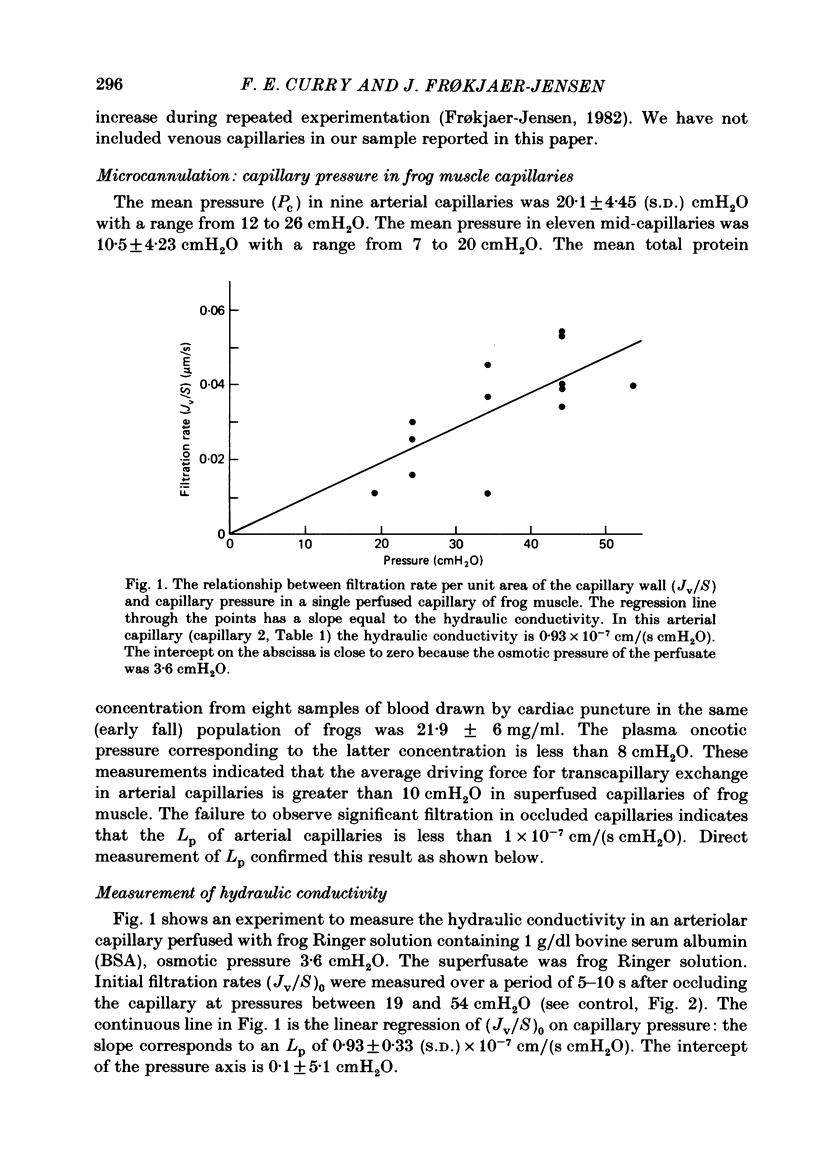
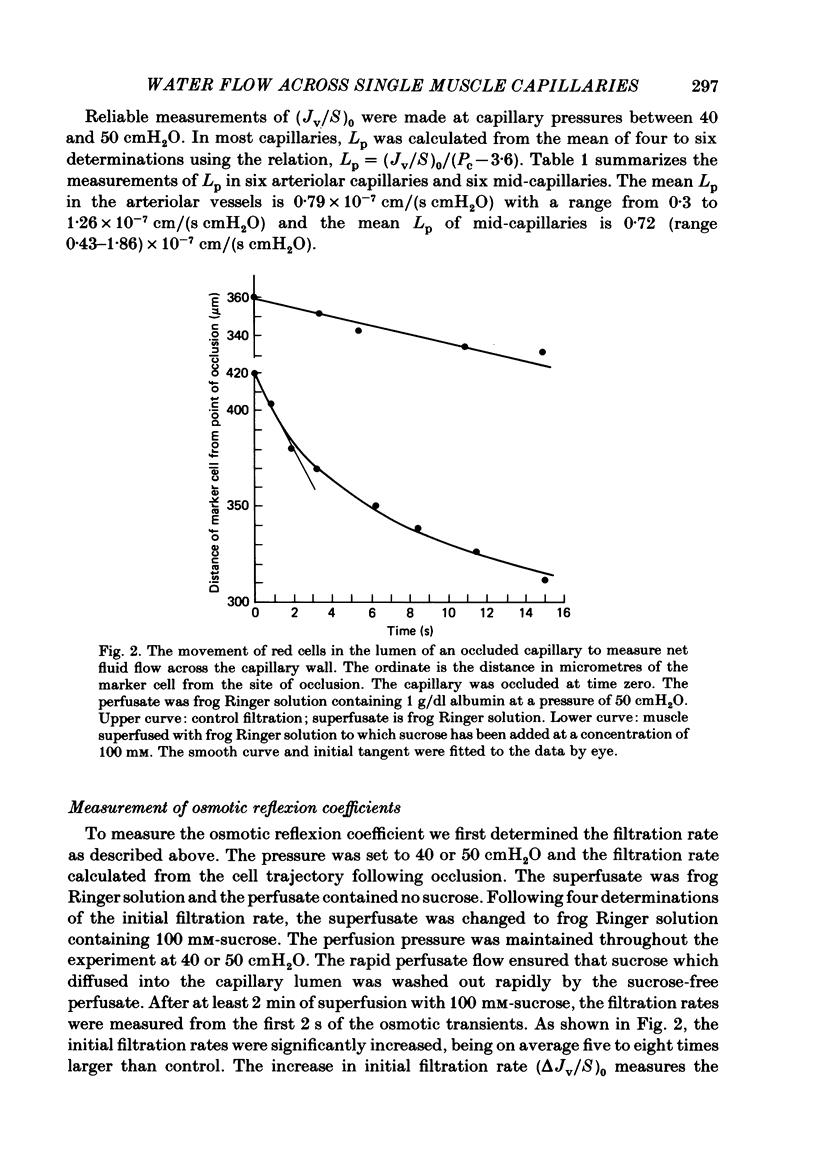
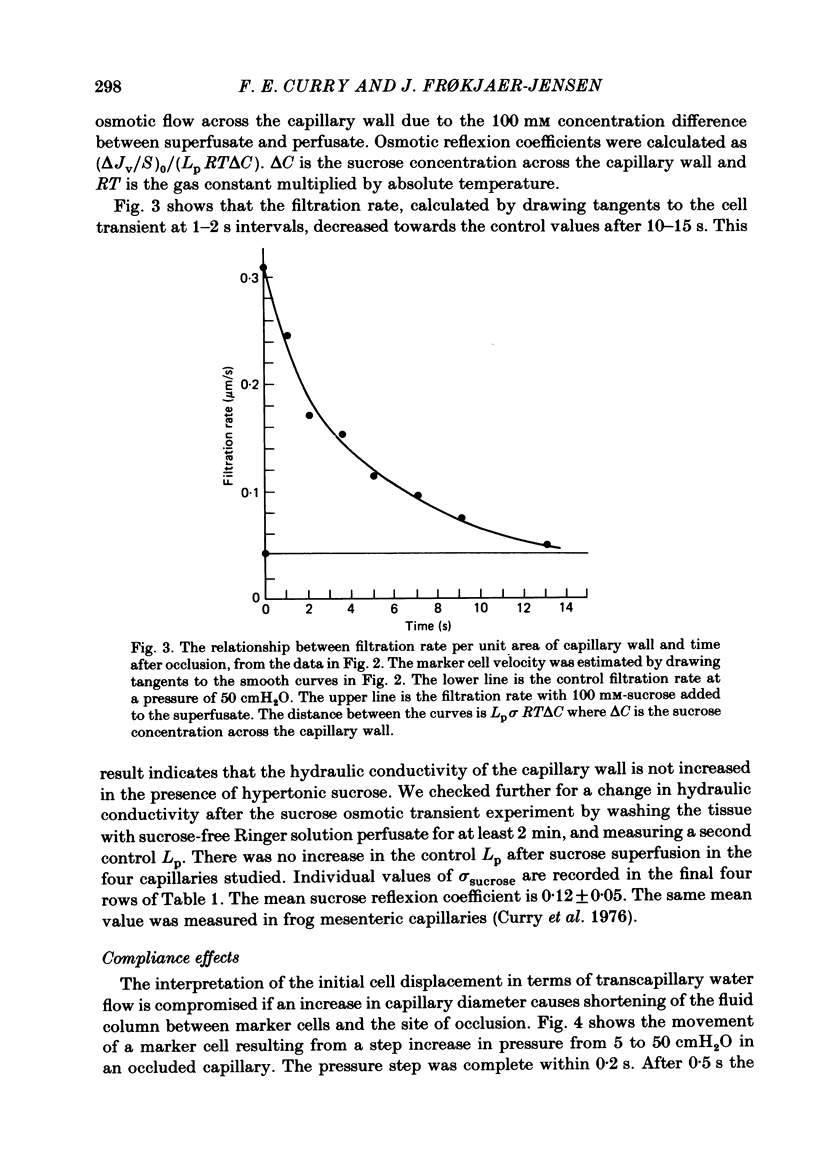
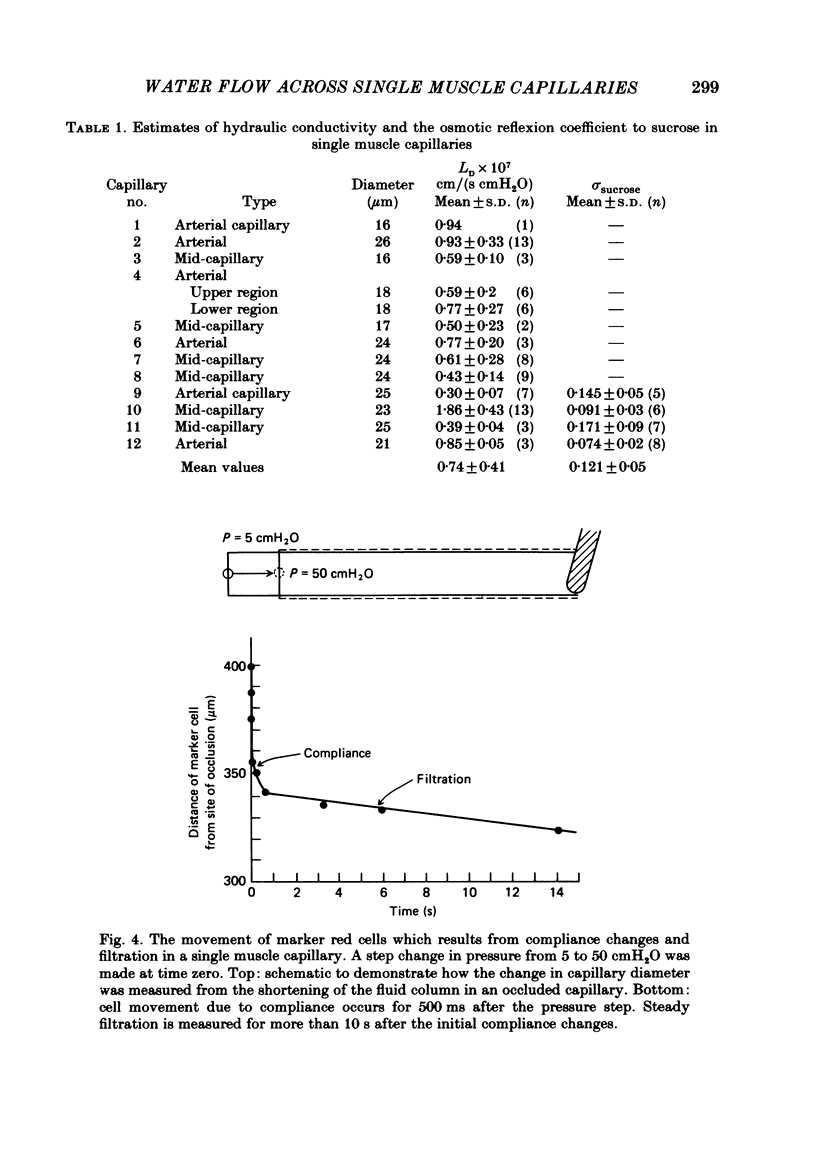
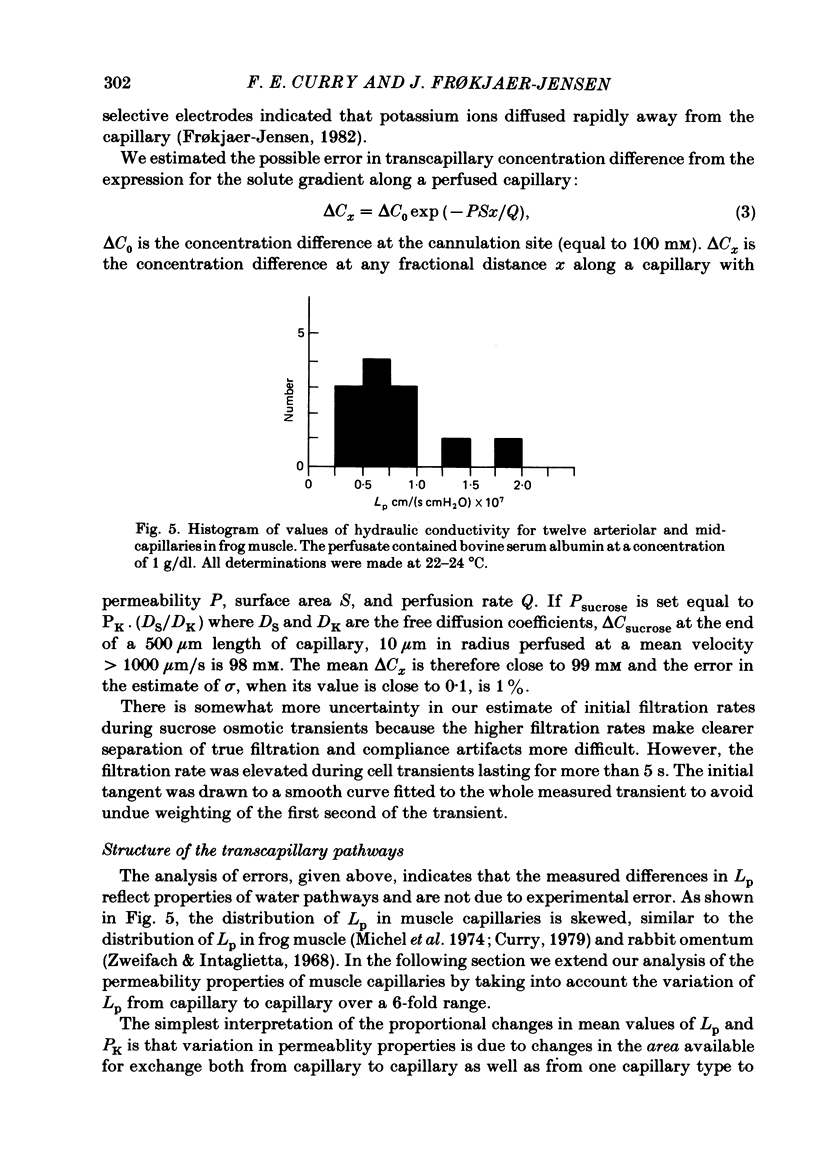
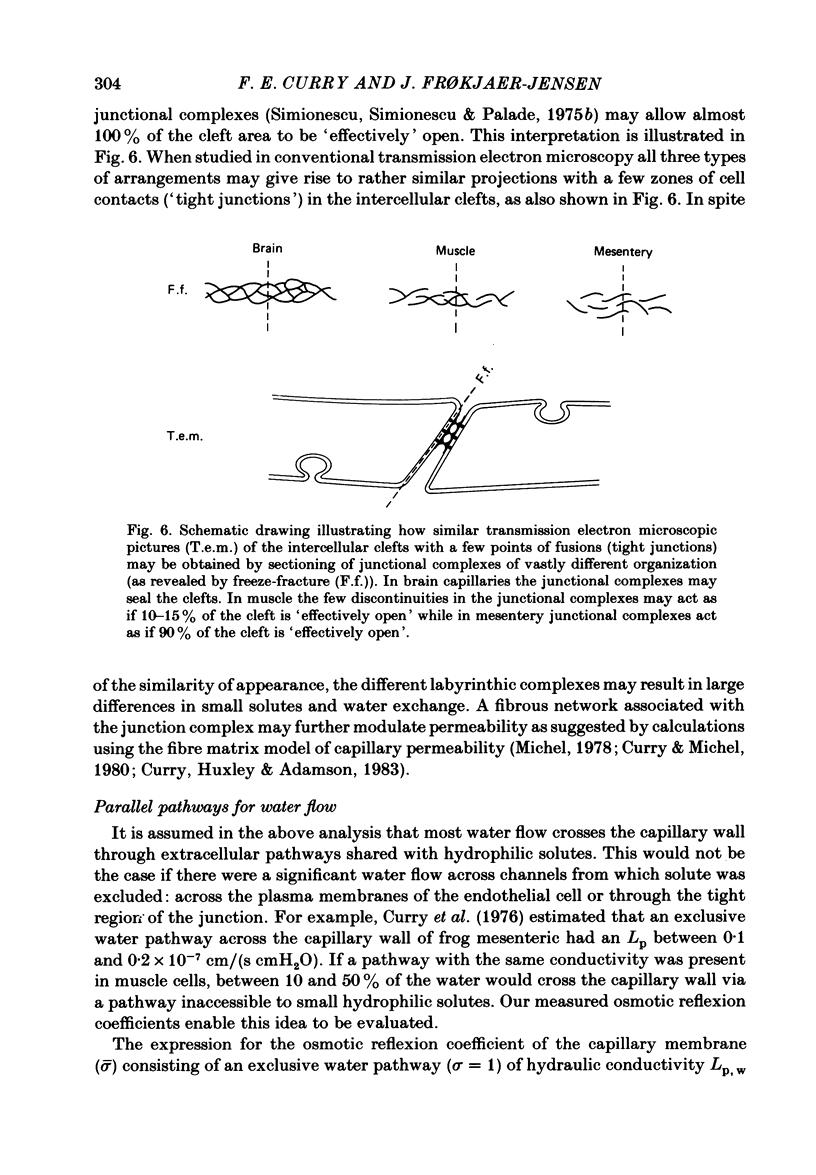
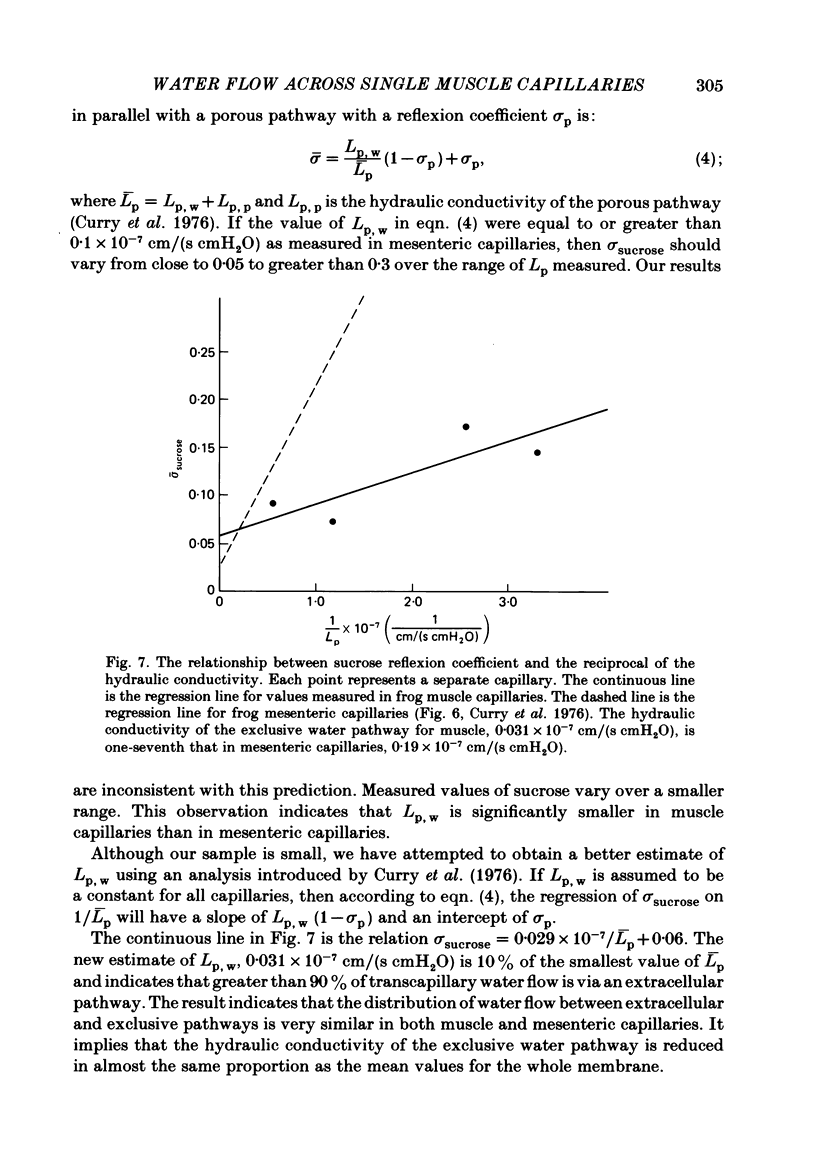
Individual capillaries of the transilluminated frog muscle cutaneous pectoris were perfused with suspensions of human red cells in frog Ringer solution containing 1 g/dl bovine serum albumin. The modified Landis technique (Michel, Mason, Curry & Tooke, 1974) was used to measure hydraulic conductivities of the capillary wall. Sucrose osmotic reflexion coefficients of the capillary wall were measured in four capillaries when the superfusate contained 100 mM-sucrose. All experiments were made at 22-24 degrees C. The hydraulic conductivity of arterial capillaries varied from 0.3 to 1.26 X 10(-7) cm/(s cmH2O) with a mean of 0.79 X 10(-7) cm/(s cmH2O) (six capillaries). The hydraulic conductivities of mid-capillaries varied from 0.43 to 1.86 X 10(-7) cm/(s cmH2O) with a mean value of 0.72 X 10(-7) cm/(s cmH2O) (six capillaries). The mean reflexion coefficient to sucrose was 0.12 +/- 0.05 (S.D.). The measured reflexion coefficients to sucrose conform to the hypothesis that 90% of the transcapillary water flow crosses the capillary wall via the principal hydrophilic pathway. The remaining 10% crosses via an exclusive water pathway. The distribution of water flow is similar to that previously described in frog mesenteric capillaries. The mean value of the hydraulic conductivity of frog muscle capillaries is about one-seventh the mean value of the hydraulic conductivity of frog mesenteric capillaries measured at the same temperature. The result conforms to the hypothesis that only a small fraction (mean 10%) of the area of junctional contact between adjacent endothelial cells is available for water and solute exchange in frog muscle capillaries. The hydraulic and diffusional conductances per unit length of open junction appear to be very similar when muscle capillaries are compared to mesenteric capillaries in the frog. Our results lead us to speculate that structures within the intercellular junctions determine the extent of open junction and may modulate the hydraulic conductivity of both the principal water pathway and the exclusive water pathway.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bundgaard M., Frøkjaer-Jensen J. Functional aspects of the ultrastructure of terminal blood vessels: a qualitative study on consecutive segments of the frog mesenteric microvasculature. Microvasc Res. 1982 Jan;23(1):1–30. doi: 10.1016/0026-2862(82)90028-0. [DOI] [PubMed] [Google Scholar]
- Bundgaard M. Ultrastructure of frog cerebral and pial microvessels and their impermeability to lanthanum ions. Brain Res. 1982 Jun 3;241(1):57–65. doi: 10.1016/0006-8993(82)91228-8. [DOI] [PubMed] [Google Scholar]
- Crone C., Frøkjaer-Jensen J., Friedman J. J., Christensen O. The permeability of single capillaries to potassium ions. J Gen Physiol. 1978 Feb;71(2):195–220. doi: 10.1085/jgp.71.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E., Huxley V. H., Adamson R. H. Permeability of single capillaries to intermediate-sized colored solutes. Am J Physiol. 1983 Sep;245(3):H495–H505. doi: 10.1152/ajpheart.1983.245.3.H495. [DOI] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C. A fiber matrix model of capillary permeability. Microvasc Res. 1980 Jul;20(1):96–99. doi: 10.1016/0026-2862(80)90024-2. [DOI] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C., Mason J. C. Osmotic reflextion coefficients of capillary walls to low molecular weight hydrophilic solutes measured in single perfused capillaries of the frog mesentery. J Physiol. 1976 Oct;261(2):319–336. doi: 10.1113/jphysiol.1976.sp011561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E. Permeability coefficients of the capillary wall to low molecular weight hydrophilic solutes measured in single perfused capillaries of frog mesentery. Microvasc Res. 1979 May;17(3 Pt 1):290–308. doi: 10.1016/s0026-2862(79)80005-9. [DOI] [PubMed] [Google Scholar]
- Frøkjaer-Jensen J. Permeability of single muscle capillaries to potassium ions. Microvasc Res. 1982 Sep;24(2):168–183. doi: 10.1016/0026-2862(82)90054-1. [DOI] [PubMed] [Google Scholar]
- Frøkjaer-Jensen J. Three-dimensional organization of plasmalemmal vesicles in endothelial cells. An analysis by serial sectioning of frog mesenteric capillaries. J Ultrastruct Res. 1980 Oct;73(1):9–20. doi: 10.1016/0022-5320(80)90111-2. [DOI] [PubMed] [Google Scholar]
- Gore R. W. Fluid exchange across single capillaries in rat intestinal muscle. Am J Physiol. 1982 Feb;242(2):H268–H287. doi: 10.1152/ajpheart.1982.242.2.H268. [DOI] [PubMed] [Google Scholar]
- Gore R. W., McDonagh P. F. Fluid exchange across single capillaries. Annu Rev Physiol. 1980;42:337–357. doi: 10.1146/annurev.ph.42.030180.002005. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Smaje L. H., Zweifach B. W. Fluid movement in occluded single capillaries of rabbit omentum. Circ Res. 1971 Mar;28(3):358–370. doi: 10.1161/01.res.28.3.358. [DOI] [PubMed] [Google Scholar]
- Michel C. C. Filtration coefficients and osmotic reflexion coefficients of the walls of single frog mesenteric capillaries. J Physiol. 1980 Dec;309:341–355. doi: 10.1113/jphysiol.1980.sp013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C., Mason J. C., Curry F. E., Tooke J. E., Hunter P. J. A development of the Landis technique for measuring the filtration coefficient of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1974 Oct;59(4):283–309. doi: 10.1113/expphysiol.1974.sp002275. [DOI] [PubMed] [Google Scholar]
- Michel C. C. The measurement of permeability in single capillaries. Arch Int Physiol Biochim. 1978 Aug;86(3):657–667. doi: 10.3109/13813457809055934. [DOI] [PubMed] [Google Scholar]
- Mollgøard K., Saunders N. R. Complex tight junctions of epithelial and of endothelial cells in early foetal brain. J Neurocytol. 1975 Aug;4(4):453–468. doi: 10.1007/BF01261375. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Crone C. Electrical resistance of muscle capillary endothelium. Biophys J. 1983 Apr;42(1):31–41. doi: 10.1016/S0006-3495(83)84366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975 Dec;67(3):863–885. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Siminoescu M., Palade G. E. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975 Mar;64(3):586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaje L. H., Fraser P. A., Clough G. The distensibility of single capillaries and venules in the cat mesentery. Microvasc Res. 1980 Nov;20(3):358–370. doi: 10.1016/0026-2862(80)90064-3. [DOI] [PubMed] [Google Scholar]
- Smaje L., Zweifach B. W., Intaglietta M. Micropressures and capillary filtration coefficients in single vessels of the cremaster muscle of the rat. Microvasc Res. 1970 Jan;2(1):96–110. doi: 10.1016/0026-2862(70)90055-5. [DOI] [PubMed] [Google Scholar]
- Wissig S. L. Identification of the small pore in muscle capillaries. Acta Physiol Scand Suppl. 1979;463:33–44. [PubMed] [Google Scholar]


