Abstract
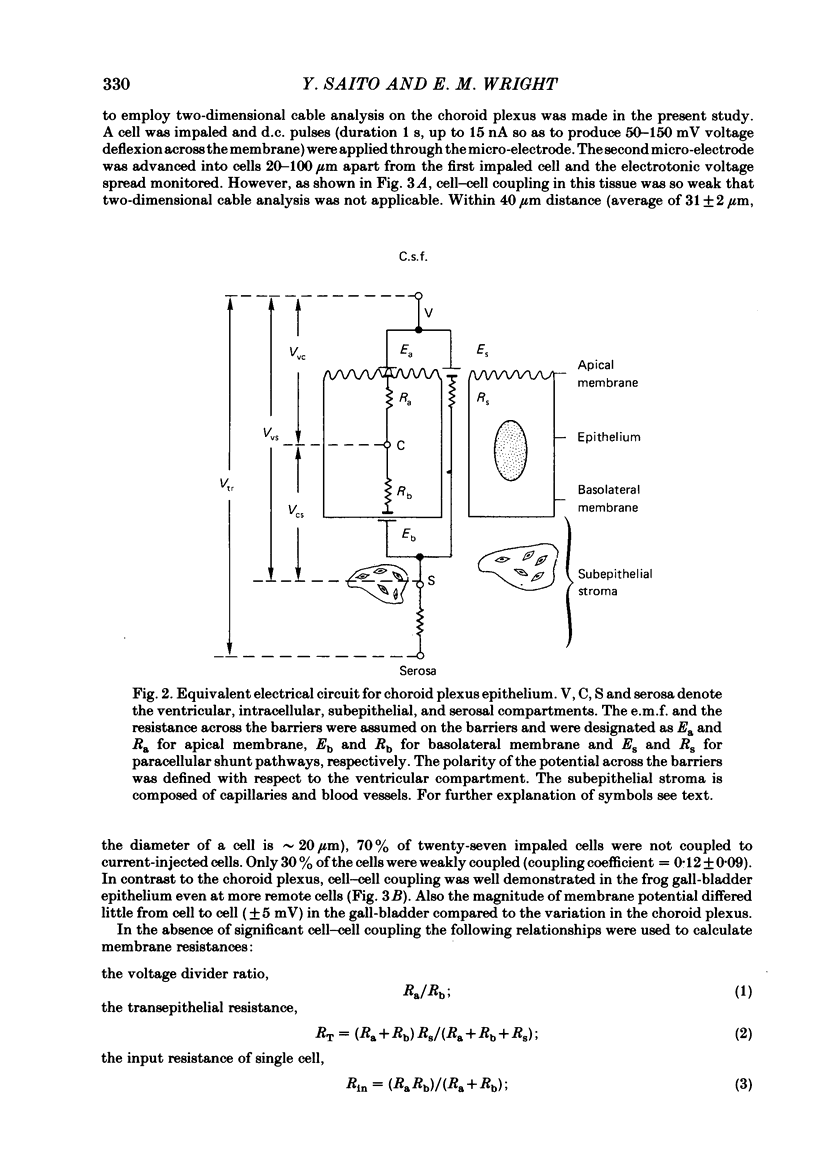
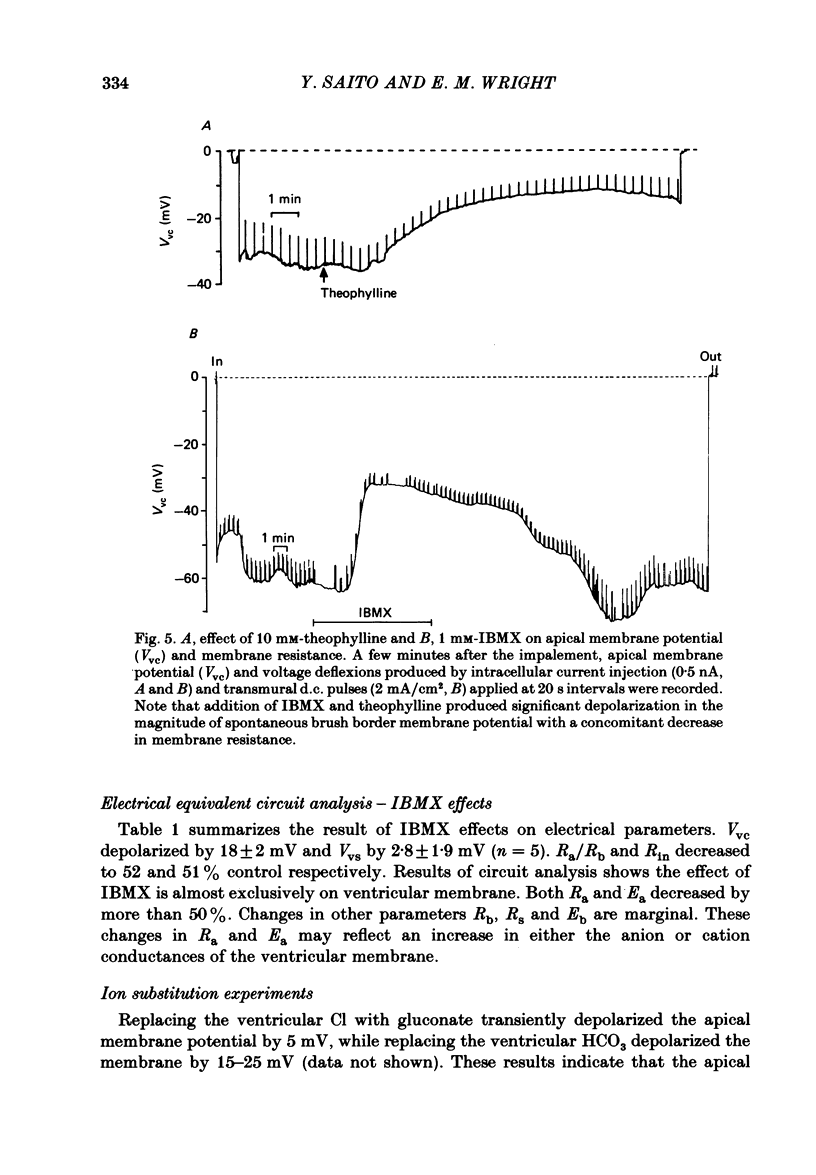
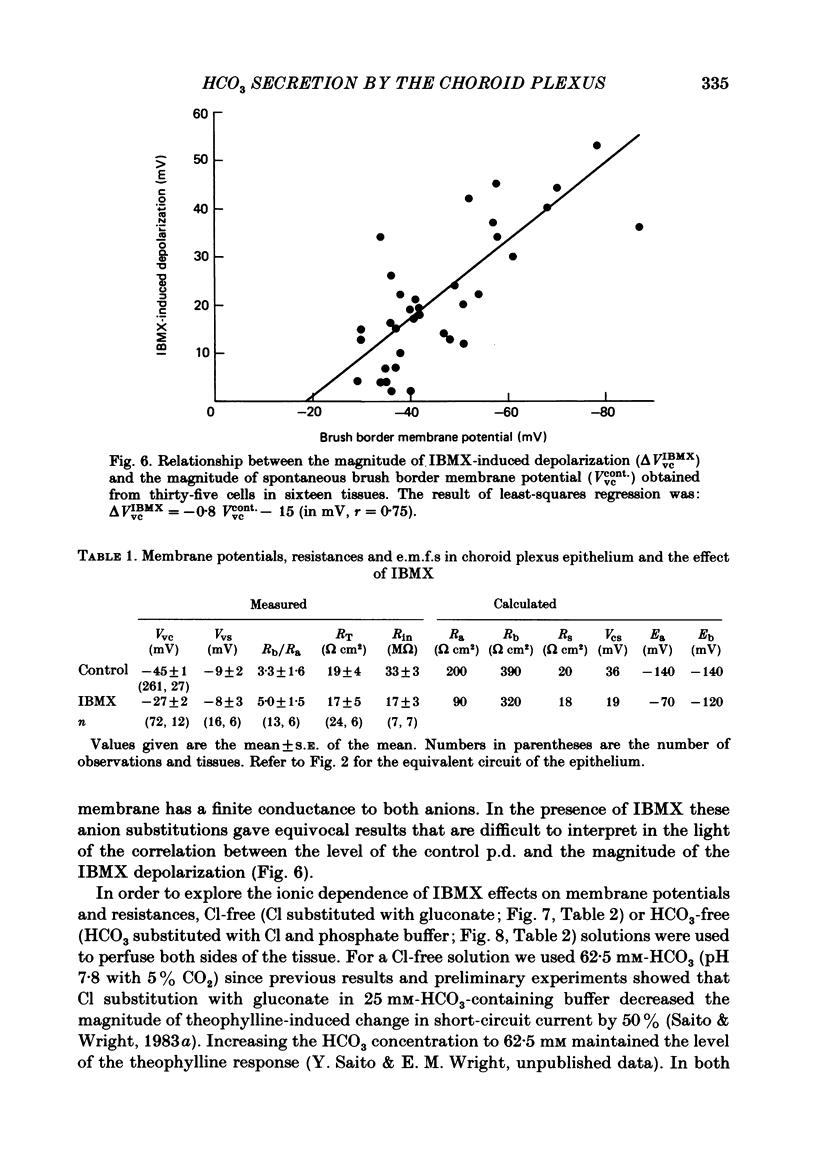
Our previous study of the choroid plexus showed that elevation of cellular cyclic AMP stimulated HCO3 secretion into the cerebrospinal fluid (c.s.f.). To explore the mechanism of this stimulation, we have used micro-electrodes to observe the effect of phosphodiesterase inhibitors on membrane potentials, resistances and electromotive forces (e.m.f.) in the bull-frog plexus. The potential profile under control conditions was -45 mV across the apical membrane (Vvc) and -9 mV across the epithelium (Vvs) with respect to the c.s.f. Two-dimensional cable analysis indicated that 70% of adjacent epithelial cells were electrically uncoupled, and that the coupling coefficient for the remaining cells was only 0.12. The results of circuit analysis gave values for membrane resistance and e.m.f. across the apical membrane of 200 omega cm2 and -135 mV, across the basolateral membrane of 386 omega cm2 and -138 mV, and across the paracellular shunt of 20 omega cm2 (the shunt e.m.f. was assumed to be negligible). Addition of phosphodiesterase inhibitors (10 mM-theophylline or 1 mM-3-isobutyl-1-methylxanthine (IBMX) ) depolarized Vvc by 18 mV and Vvs by 2 mV. Circuit analysis showed that the inhibitor reduced the apical membrane resistance by more than 60% and depolarized the apical e.m.f. by 70 mV without affecting the basolateral membrane or the shunt parameters. In Cl-free solutions IBMX depolarized Vvc and decreased the apical membrane resistance similar to that observed in regular buffer solution. However, in HCO3-free buffer solutions, the effects of IBMX on Vvc and membrane resistances were insignificant. In thirty-five cells in sixteen tissues a linear relationship was observed between the magnitude of the spontaneous membrane potential (Vvc) and the magnitude of IBMX-induced depolarization (delta VIBMXvc): delta VIBMXvc = -0.8 Vvc -15 (in mV). This suggests that HCO3 is accumulated within the epithelium above electrochemical equilibrium. We conclude that cyclic AMP increases HCO3 secretion across the choroid plexus by increasing the apical membrane HCO3 conductance.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Lindley B. D., Prince W. T. Membrane permeability changes during stimulation of isolated salivary glands of Calliphora by 5-hydroxytryptamine. J Physiol. 1975 Jan;244(3):549–567. doi: 10.1113/jphysiol.1975.sp010812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curci S., Frömter F. Micropuncture of lateral intercellular spaces of Necturus gallbladder to determine space fluid K+ concentration. Nature. 1979 Mar 22;278(5702):355–357. doi: 10.1038/278355a0. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Gupta B. L., Hall T. A., Naftalin R. J. Microprobe measurement of Na, K and Cl concentration profiles in epithelial cells and intercellular spaces of rabbit ileum. Nature. 1978 Mar 2;272(5648):70–73. doi: 10.1038/272070a0. [DOI] [PubMed] [Google Scholar]
- Kanno Y. [Electrophysiology of epithelia]. Nihon Seirigaku Zasshi. 1968;30(7):419–430. [PubMed] [Google Scholar]
- Khuri R. N., Agulian S. K., Bogharian K., Nassar R., Wise W. Intracellular bicarbonate in single cells of Necturus kidney proximal tubule. Pflugers Arch. 1974;349(4):295–299. doi: 10.1007/BF00588415. [DOI] [PubMed] [Google Scholar]
- Klyce S. D., Wong R. K. Site and mode of adrenaline action on chloride transport across the rabbit corneal epithelium. J Physiol. 1977 Apr;266(3):777–799. doi: 10.1113/jphysiol.1977.sp011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel W., Reinach P. Mechanism of stimulation by epinephrine of active transepithelial Cl transport in isolated frog cornea. J Membr Biol. 1980 Aug 21;56(1):73–79. doi: 10.1007/BF01869354. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Wright E. M. The distribution, activity, and function of the cilia in the frog brain. J Physiol. 1974 Nov;243(1):63–78. doi: 10.1113/jphysiol.1974.sp010742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Irimajiri A., Inouye A. Electrical properties and active solute transport in rat small intestine. II. Conductive properties of transepithelial routes. J Membr Biol. 1977 Mar 8;31(3):221–232. doi: 10.1007/BF01869406. [DOI] [PubMed] [Google Scholar]
- Petersen K. U., Reuss L. Cyclic AMP-induced chloride permeability in the apical membrane of Necturus gallbladder epithelium. J Gen Physiol. 1983 May;81(5):705–729. doi: 10.1085/jgp.81.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. I. Circuit analysis and steady-state effects of mucosal solution ionic substitutions. J Membr Biol. 1975 Dec 4;25(1-2):115–139. doi: 10.1007/BF01868571. [DOI] [PubMed] [Google Scholar]
- Rose R. C., Schultz S. G. Studies on the electrical potential profile across rabbit ileum. Effects of sugars and amino acids on transmural and transmucosal electrical potential differences. J Gen Physiol. 1971 Jun;57(6):639–663. doi: 10.1085/jgp.57.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Wright E. M. Bicarbonate transport across the frog choroid plexus and its control by cyclic nucleotides. J Physiol. 1983 Mar;336:635–648. doi: 10.1113/jphysiol.1983.sp014602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Electrical potential differences and electromotive forces in epithelial tissues. J Gen Physiol. 1972 Jun;59(6):794–798. doi: 10.1085/jgp.59.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorofsky S. R., Field M., Fozzard H. A. Electrophysiology of Cl secretion in canine trachea. J Membr Biol. 1983;72(1-2):105–115. doi: 10.1007/BF01870318. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Frömter E. The potential and resistance profile of Necturus gallbladder cells. Pflugers Arch. 1977 Oct 19;371(1-2):109–117. doi: 10.1007/BF00580778. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Miller C., Webb W. W. Isolated-patch recording from liposomes containing functionally reconstituted chloride channels from Torpedo electroplax. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7749–7753. doi: 10.1073/pnas.79.24.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: III. Membrane resistances and electromotive forces. J Membr Biol. 1983;71(3):209–218. doi: 10.1007/BF01875462. [DOI] [PubMed] [Google Scholar]
- White J. F. Chloride transport and intracellular chloride activity in the presence of theophylline in Amphiuma small intestine. J Physiol. 1981 Dec;321:331–341. doi: 10.1113/jphysiol.1981.sp013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M. Mechanisms of ion transport across the choroid plexus. J Physiol. 1972 Oct;226(2):545–571. doi: 10.1113/jphysiol.1972.sp009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B. Structural aspects of brain barriers, with special reference to the permeability of the cerebral endothelium and choroidal epithelium. Int Rev Cytol. 1980;65:117–191. doi: 10.1016/s0074-7696(08)61960-9. [DOI] [PubMed] [Google Scholar]


