Abstract
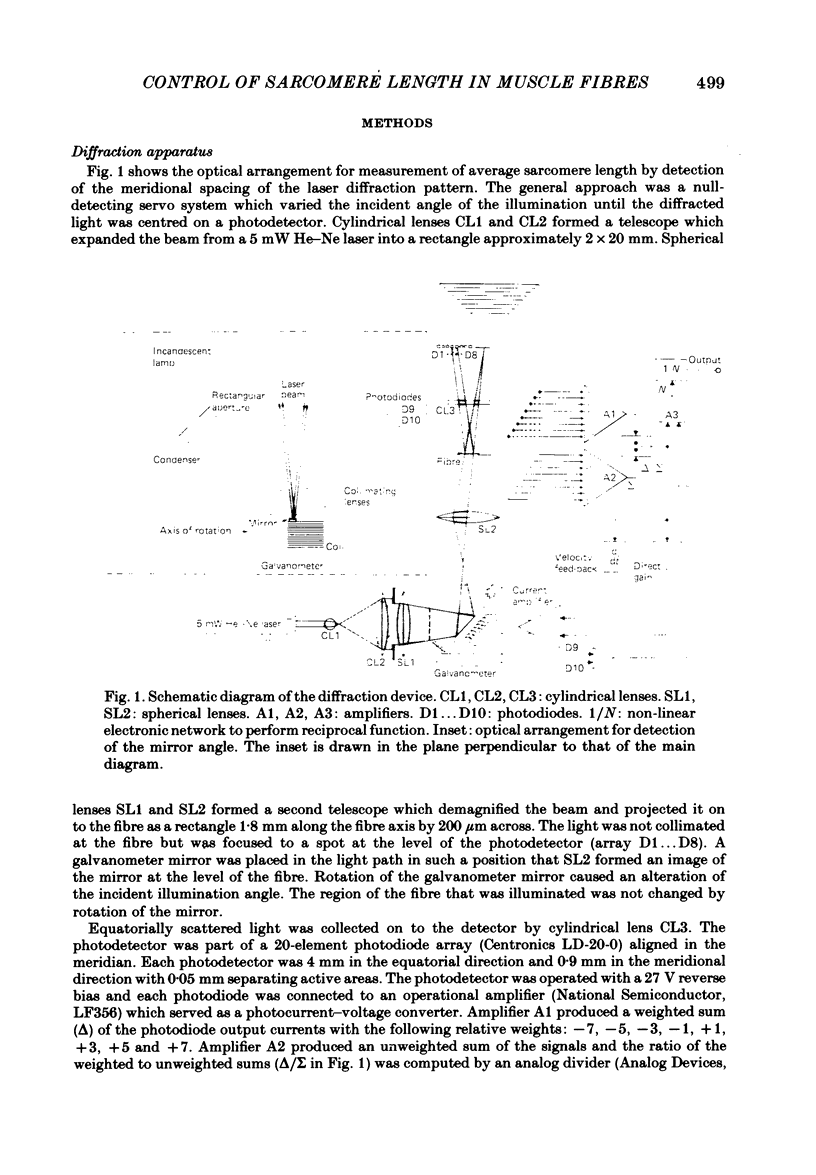
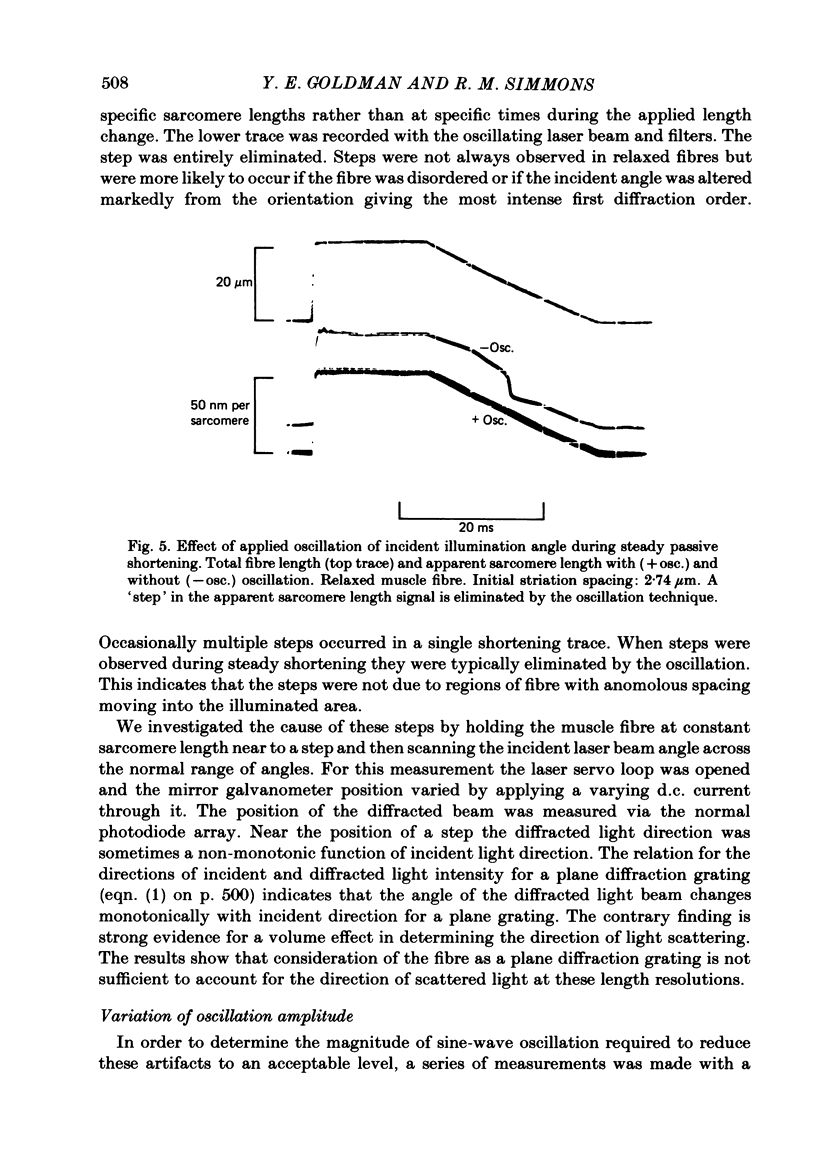
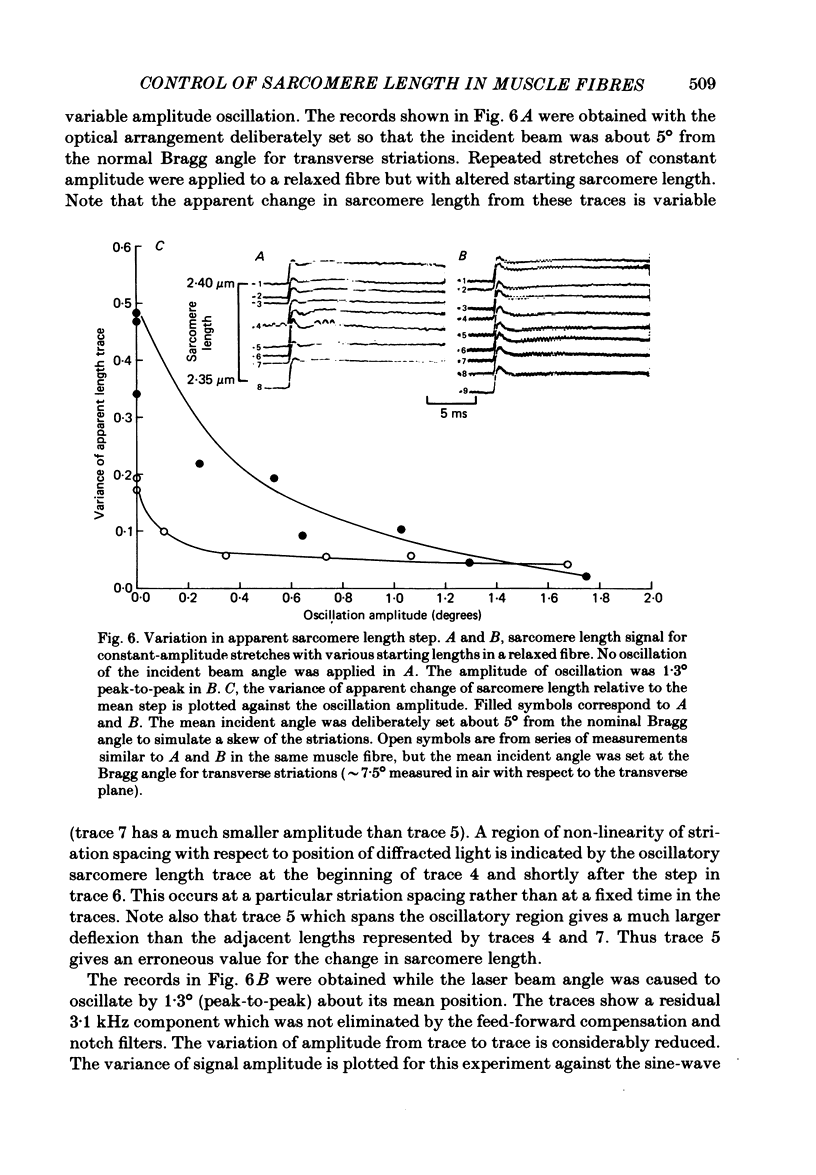
A new technique is described for high-speed measurement of striation spacing of single skinned muscle fibres. A galvanometer mirror directs helium-neon laser light on to the muscle fibre at a variable angle. Light diffracted by the cross-striations is collected by a position-sensitive photodetector. The incident angle necessary to centre the diffracted light beam on to the photodetector is related to sarcomere length. The instrument was tested by comparison with measurements obtained with a compound microscope. Discrepancies of several nanometers per half sarcomere were observed between these two methods. When the incident angle of the laser beam was varied sinusoidally about its mean position the magnitude of the discrepancy was reduced. During steady passive shortening of the muscle fibres the output of the diffraction instrument often displayed pauses and brief periods of rapid shortening. These irregularities were eliminated by averaging the sarcomere length output over a range of illumination angles by oscillating the incident angle of the laser beam. The results suggest that at the spatial resolution of several nanometers per half sarcomere, volume diffraction effects can cause the apparent sarcomere length measured from the angle of coherent light diffraction to differ from the mean striation spacing. With incident-angle oscillation the time and spatial resolution of the equipment were satisfactory for the sarcomere length signal to be fed back to a length controller for a 'sarcomere length clamp'. In active contractions, stiffness was closely related to steady developed tension at sub-saturating calcium concentrations. Skinned fibres are less stiff than intact fibres at a given level of developed tension.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Chock P. B., Eisenberg E. Mechanism of action of troponin . tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem. 1981 Jan 25;256(2):575–578. [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. A diffraction system for measuring muscle sarcomere length [proceedings]. J Physiol. 1979 Jul;292:5P–6P. [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P. J., Kuhn H. J., Güth K., Rüegg J. C. Rate of isometric tension development in relation to calcium binding of skinned muscle fibres. Pflugers Arch. 1979 Nov;382(2):165–170. doi: 10.1007/BF00584218. [DOI] [PubMed] [Google Scholar]
- Güth K., Kuhn H. J. Stiffness and tension during and after sudden length changes of glycerinated rabbit psoas muscle fibres. Biophys Struct Mech. 1978 Jul 12;4(3):223–236. doi: 10.1007/BF02426087. [DOI] [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Two rigor states in skinned crayfish single muscle fibers. J Gen Physiol. 1976 Sep;68(3):267–280. doi: 10.1085/jgp.68.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., Teichholz L. E. The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol. 1970 Nov;211(1):19–35. doi: 10.1113/jphysiol.1970.sp009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack G. H., Iwazumi T., ter Keurs H. E., Shibata E. F. Sarcomere shortening in striated muscle occurs in stepwise fashion. Nature. 1977 Aug 25;268(5622):757–759. doi: 10.1038/268757a0. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Zite-Ferenczy F. Interpretation of light diffraction by cross-striated muscle as Bragg reflexion of light by the lattice of contractile proteins. J Physiol. 1979 May;290(2):317–330. doi: 10.1113/jphysiol.1979.sp012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. P., Hartt J. E., Podolsky R. J. Equatorial x-ray intensities and isometric force levels in frog sartorius muscle. J Mol Biol. 1979 Jul 25;132(1):53–67. doi: 10.1016/0022-2836(79)90495-9. [DOI] [PubMed] [Google Scholar]



