Abstract
Stuttering is a common and sometimes severe communication disorder, of unknown primary etiology, that exists in populations worldwide. Many types of evidence suggest a genetic contribution to stuttering; however, the complex inheritance of this disorder has hindered identification of these factors. We have employed highly inbred families to increase the power of linkage analysis of this disorder. Forty-four Pakistani families with documented or probable consanguinity, from the city of Lahore and surrounding areas, were included. Each family contained multiple cases of stuttering, which were diagnosed using the Stuttering Severity Instrument. Using the Marshfield Weber 9 marker panel, we performed a genomewide linkage scan focused on affected individuals and their parents. The analysis included 199 genotyped individuals, 144 affected and 55 unaffected. The Pedigree Relationship Statistical Test (PREST) was used to identify pedigrees that required additional specification of inbreeding. Initial nonparametric analysis gave evidence of linkage on chromosomes 1, 5, 7, and 12. Additional genotyping was performed on chromosome 12 to a 5-cM level of resolution, and 16 additional individuals were then included, bringing the number of families to 46. Analysis of the enlarged data set provided consistent evidence of linkage on chromosome 12: the Shomoz scoring function gave a nonparametric LOD score of 4.61, and a LOD score of 3.51 was obtained using the Sall scoring function. These results suggest that a locus on chromosome 12q may contain a gene with a large effect in this sample.
Stuttering is a speech disorder characterized by the presence of syllable repetitions, syllable prolongations, and interruptions in the smooth flow of speech known as blocks. The disorder typically arises in children, who often spontaneously recover. However, in a minority of cases, it persists into adulthood, with the result that up to 1% of the population worldwide displays significant symptoms (Bloodstein 1995). Approximately half of stutterers have a family history of the disorder, and twin and adoption studies support a genetic contribution (Howie 1981; Yairi et al. 1996; Felsenfeld and Plomin 1997; Drayna et al. 1999; Shugart et al. 2004). However, Mendelian inheritance is typically not observed, supporting the view of stuttering as a complex trait.
Because of this, we sought a specialized population to perform linkage studies. We have collected these families from in the city of Lahore, Pakistan, and surrounding areas. These families are highly inbred, with stuttering occurring in two or more generations, chosen with preference for a high number of affected individuals. We made video and audio recordings of speech samples by use of the Stuttering Severity Instrument (Riley 1980), and diagnosis was performed independently by two different clinicians specializing in stuttering, according to standard criteria, with 4% dysfluent words or syllables as the minimum for diagnosis as affected. We initially ascertained a group of 100 families, and we selected 56 for collection of blood and diagnostic speech samples and subsequent genotyping.
By use of the Marshfield Weber 9 marker panel (see the Center for Medical Genetics Web site), a total of 162 affected and 62 unaffected individuals were genotyped from these 56 Pakistani families affected with stuttering. For allele-frequency estimation, 27 population-matched unaffected controls were also genotyped. Multiplex PCR was done under standard conditions and analysis was carried out on an ABI377 using GeneScan 3.1 software, with allele sizes assigned using Genotyper v. 2.0.
Different numbers of families were informative for the various linkage analyses. In table 1, the number of families and the number of genotyped individuals—affected and unaffected—used in each analysis are shown. At most, 44 families, including 199 genotyped individuals (155 affected and 44 unaffected), were informative for the genomewide scan. A maximum of 46 families, including 220 genotyped individuals (160 affected and 60 unaffected), were informative for linkage performed using additional markers on chromosome 12. The supplementary tables (tables A1 and A2 [online only]) present information on the structure of the families used for the analyses. Here, we note that, in the maximum set of families informative for linkage, 61% (28/46) had at least one known consanguineous marriage. Known consanguineous marriages were always between cousins, with ∼60% being between first cousins. The power to detect linkage in this sample was not explicitly calculated, because of the unknown effects of the reduction in genetic heterogeneity caused by the use of pedigrees from this relatively isolated and inbred population.
Table 1.
Results of Genomewide Scan and Additional Genotyping on Chromosome 12[Note]
|
No. of Families Used |
No. of Subjects Genotyped |
LOD Score at Markera |
||||||
| Scoring Function and Data Set | As OriginallyPresented | After Split | Affected | Unaffected | D1S1677 | D5S408 | D7S559 | PAH |
| Shomoz, with overrelated individuals | 44 | 47 | 144 | 55 | 1.59 | 1.15 | 1.67 | 3.11 |
| Shomoz, without overrelated individuals | 41 | 43 | 130 | 48 | 1.40 | .92 | 2.20 | 3.45 |
| Sall, with overrelated individuals | 40 | 43 | 140 | 51 | 2.93 | 2.80 | .09 | 3.11 |
| Sall, without overrelated individuals | 37 | 39 | 126 | 44 | 2.77 | 2.41 | .09 | 3.28 |
| Shomoz, with overrelated individuals, with additional genotyping | 46 | 49 | 160 | 60 | … | … | … | 4.61 |
| Shomoz, without overrelated individuals, with additional genotyping | 43 | 45 | 143 | 53 | … | … | … | 5.20 |
| Sall, with overrelated individuals, with additional genotyping | 43 | 46 | 157 | 56 | … | … | … | 3.51 |
| Sall, without overrelated individuals, with additional genotyping | 40 | 42 | 140 | 49 | … | … | … | 3.64 |
Note.— Regions achieving LOD scores ⩾2 in at least one analysis. Shown are results with the Shomoz or Sall scoring function, including or excluding the individuals “corrected” for overrelatedness, and, for chromosome 12, including additional genotyping.
The scores presented are exactly at the marker listed for D1S1677, D5S408, and D7S559. In the case of PAH, the score shown is exactly at the marker when Shomoz is used; when Sall is used, however, the score shown is the largest in the region, which was found between D12S1041 (104.12 cM) and PAH (109.47 cM) in the genomewide scan and between PAH and D12S78 (111.87 cM) when extra markers were added to this region.
Mendelian inconsistencies were identified with PedCheck (O’Connell and Weeks 1998). Genotypes creating incompatibilities were re-examined and, if possible, corrected (but not removed if they could not be corrected). Next, both the Pedigree Relationship Statistical Test (PREST) (McPeek and Sun 2000) and the graphical method described by Sun et al. (2001) were employed to detect relationship misspecification. Four instances in which individuals appeared less closely related to other family members than specified (“underrelated”) and four in which individuals appeared to be more closely related to other family members than specified (“overrelated”) were identified. To remedy the cases of underrelatedness, the genotypes of three problematic individuals were deleted and a pair of full sibs was recoded as half sibs. (After this recoding, a rerun of PREST resulted in no detectable relationship incompatibilities in the pedigree.) The instances of overrelatedness appeared to be the result of unspecified inbreeding. Additional inbreeding loops were then added to these pedigrees. Because we could not be confident that these inbreeding loops were correctly specified—with existing tools, it is much more difficult to detect misspecification in inbred relationships than in outbred ones—all analyses were carried out two ways: (1) with these additional inbreeding loops included and (2) with all individuals who appeared to be overrelated removed. After relationships were respecified, all genotypes creating Mendelian inconsistencies were deleted.
Marker allele frequencies were estimated using the maximum likelihood method described by Boehnke (1991), as implemented in MENDEL (Lange et al. 2001). All genotyped individuals (224 persons from the families and 27 controls for the genomewide scan; 240 individuals from the families and 27 controls for the additional markers on chromosome 12) were used for allele-frequency estimation. With the exception of the markers TPO and F13A1, marker positions were taken from the sex-averaged Marshfield map (see the Center for Medical Genetics Web site). TPO and F13A1 were located with PYGMALION (Pluzhnikov et al. 2003), making use of information on their physical positions. Only genotyped affected individuals and the persons necessary to specify relationships among these individuals were included in the pedigrees used for linkage analysis. Three pedigrees had to be split (into two families each), and a distant inbreeding loop had to be removed from one pedigree to make linkage analysis computationally possible.
Multipoint allele-sharing methods (Kong and Cox 1997), as implemented in Allegro (Gudbjartsson et al. 2000a), were used to assess evidence for linkage. An exponential model (Kong and Cox 1997) and two scoring functions, Shomoz (Gudbjartsson et al. 2000b) and Sall (Whittemore and Halpern 1994), were used. Allegro was modified so that, for Shomoz only, scores from families containing a single genotyped affected whose parents were related could be included. Nonzero scores were possible with these families (when Shomoz was used), because Shomoz contains a term for identical by descent (IBD) sharing within a single individual. (Except for this term, Shomoz is identical to Spairs.) To take some account of the differing sizes of the pedigrees, families were weighted by the square roots of the standard deviations of their scores under the null hypothesis of no linkage.
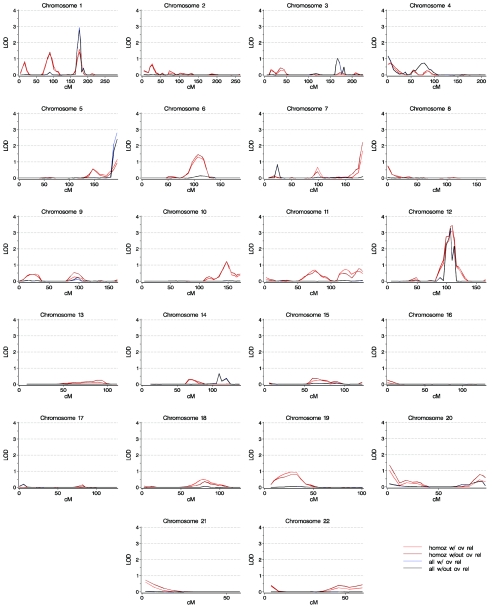
Figure 1 and table 1 demonstrate that the inclusion of individuals with evidence of overrelatedness—“corrected” for overrelatedness—did not greatly change the allele-sharing logarithm of the odds (LOD) scores. The use of different scoring functions, on the other hand, often led to dramatically altered LOD scores.
Figure 1.
Genomewide linkage scan for stuttering. Shown are results with Shomoz (“homoz”) and Sall (“all”) scoring functions and with the “corrected” overrelated individuals included (“w/ ov rel”) and excluded (“w/out ov rel”). The horizontal axis is distance, in cM, from the p-terminus.
LOD scores ⩾2 were achieved at four positions: on chromosome 1 at D1S1677 (175.62 cM), on chromosome 5 at D5S408 (195.49 cM), on chromosome 7 at D7S559 (181.97 cM), and on chromosome 12 close to PAH (109.47 cM) (table 1). On chromosomes 5 and 7, the largest scores were found at the most telomeric marker on the chromosome arms. Since LOD scores at the terminal marker of a chromosome estimate evidence for linkage less precisely than scores at markers located between two other markers—because scores at the final marker on a chromosome are multipoint on one side only—the large scores at the ends of chromosomes 5 and 7 should be interpreted with caution. In only one position, on chromosome 12 near PAH, were scores ⩾2 achieved in all four analyses. This region of chromosome 12 was also the location of the largest score in each of the analyses (table 1).
Because the PAH region on chromosome 12 appeared to be most promising, eight supplementary markers in this area were genotyped. Additional individuals were also genotyped for these markers, resulting in a maximum of 46 families with 220 individuals genotyped (160 affected) that were informative in this region. The additional genotyping led to higher LOD scores in each of the four analyses, with LOD scores increasing modestly with Sall, and by at least 1.5 LOD units with Shomoz (table 1).
To assess the significance of the genome-scan results, we compared the results from the framework scan with the “corrected” pedigree set to those from linkage analyses of 100 simulated data sets generated by gene dropping in Merlin (Abecasis et al. 2002), using identical pedigrees, allele frequencies, marker maps, and patterns of missing data. In the observed data, LOD scores >3 were achieved with both scoring functions in the same region of chromosome 12; in the simulated data sets, no region (here defined as 40 cM) was identified that gave scores >3 with both scoring functions. The maximum LOD score observed with either scoring function was 3.11 when the “corrected” pedigree set was used; scores of this magnitude or higher (with either scoring statistic) were achieved only twice in the simulated data set.
The contribution of individual families to the chromosome 12 peak was also examined. With either scoring statistic, regardless of the inclusion of the families that showed evidence of overrelatedness, the same three families (two of which were actually parts of a large family that had to be split) gave the largest contributions at PAH. By use of Spairs and the pedigrees, including the overrelated “corrected,” the LOD scores at PAH, before the fine-mapping markers were added, were 1.81 and 0.85 in the two families created by splitting a single family and 1.07 in the third high-scoring family. By use of Sall with the same pedigrees and markers, the LOD scores at the peak were 2.38 and 0.84 in the two parts of the split family and 0.25 in the third high-scoring family. Interestingly, in these families, the same allele of PAH exhibited excess sharing. Because this allele is relatively uncommon in the Pakistani population (estimated frequency .08) and both families are from the same geographical area, the observations are compatible with the hypothesis that there is a common mutational origin for stuttering in these families. Although the lack of a clearly identifiable mode of inheritance and the high degree of inbreeding in these families preclude confident assessment of disease-associated haplotypes, these families will provide a powerful substrate for follow-up studies. Given the complex genetics of stuttering, it is possible that alleles at numerous loci contribute to this phenotype, even in this relatively homogeneous sample. In the only previously published genomewide linkage survey for stuttering, a locus on chromosome 18 was identified (Shugart et al. 2004). That result was obtained in a collection of outbred North American families, and much of the score was due to a single family within the data set. Our results in the Pakistani population provide no significant evidence of linkage on chromosome 18, consistent with the view that stuttering, like numerous Mendelian and non-Mendelian genetic disorders, can be caused by mutations at many different loci.
Acknowledgments
We thank Dr. Bushra Raza and Ms. Jennifer Mundorff for stuttering diagnosis, Drs. Thomas Friedman and Andrew Griffith for helpful comments on the manuscript, and the members of the Pakistani families affected with stuttering for their participation in this research. This research was supported by the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (grants Z01–000046–04 [to D.D.] and DC-04415 [to N.J.C.]).
Appendix A: Supplementary Tables
Table A1.
Within-Family Genotyping Rates for Individuals and Affected Individuals[Note]
|
No. of Families (No. after Removal of Overrelated Individuals) for |
||
| No. of Individuals Genotyped | Markers from Whole-Genome Scan | Additional Markers on Chromosome 12 |
| All: | ||
| 1 | 2 (2) | 0 (0) |
| 2 | 6 (6) | 7 (7) |
| 3 | 13 (12) | 11 (11) |
| 4 | 7 (6) | 10 (9) |
| 5 | 8 (8) | 10 (9) |
| 6 | 4 (3) | 2 (2) |
| 7 | 5 (5) | 6 (5) |
| 8 | 1 (0) | 1 (0) |
| 9 | 0 (0) | 1 (1) |
| 13 | 1 (1) | 1 (1) |
| Affected: | ||
| 1 | 5 (5) | 2 (2) |
| 2 | 13 (11) | 14 (13) |
| 3 | 15 (15) | 18 (18) |
| 4 | 6 (6) | 6 (5) |
| 5 | 5 (3) | 4 (3) |
| 6 | 1 (1) | 3 (2) |
| 7 | 1 (1) | 1 (1) |
| 8 | 1 (1) | 1 (1) |
Note.— Counts of families by amount of genotyping and amount of affected-individual genotyping are given for the markers from the whole-genome scan and for the additional markers on chromosome 12. Families are included in the form that they were used for linkage analysis (see text).
Table A2.
Number of Various Types of Genotyped Affected Pairs[Note]
|
No. of Pairs (No. after Removal of Overrelated Individuals) |
||
| Pair Type | Outbred | Inbred |
| Genomewide scan: | ||
| Parent-child, related parents | NA | 16 |
| Full-sibling | 20 | 31 (23) |
| Half-sibling | 1 | 0 |
| Avuncular | 12 | 15 (14) |
| Grandparent-grandchild | 1 | 11 |
| Grand-avuncular | 1 | 2 |
| Double first cousin | 0 | 8 |
| First cousin + more distant cousin | 0 | 3 |
| First cousin | 5 | 24 (11) |
| First cousin once-removed | 2 | 10 |
| Second cousin | 1 | 4 |
| More distant cousin | 3 | 2 |
| For additional chromosome 12 markers: | ||
| Parent-child, related parents | NA | 17 (16) |
| Full-sibling | 21 | 34 (26) |
| Half-sibling | 1 | 0 |
| Avuncular | 12 | 21 (20) |
| Grandparent-grandchild | 1 | 13 (11) |
| Grandavuncular | 1 | 5 |
| Double first cousin | 0 | 9 |
| First cousin + more distant cousin | 0 | 3 |
| First cousin | 5 | 28 (14) |
| First cousin once removed | 2 | 10 |
| Second cousin | 1 | 4 |
| More distant cousin | 3 | 2 |
Note.— Numbers of informative, affected, genotyped pairs in the family structures that were split for linkage analysis and “corrected” for overrelatedness. Also, in parentheses, the numbers of informative, affected, genotyped pairs in the family structures that were split for linkage analysis and had the overrelated individuals removed (only when they were different). Affected pairs with more than one relationship were classified by their closest relationship, except in the case of first cousins. First-cousin pairs were subdivided by the relationship (if any) induced through the two parents not involved in the initially identified first-cousin relationship. Inbred pairs included at least one member with related parents or grandparents. For the whole genome-scan, 56 individuals with related parents (54 after overrelated individuals were removed) were also available for analysis; for additional markers on chromosome 12, 59 individuals with related parents (57 after overrelated individuals were removed) were also available for analysis. NA = not applicable.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Center for Medical Genetics Web site, http://research.marshfieldclinic.org/genetics/Map_Markers/maps/IndexMapFrames.html (for sex-averaged Marshfield map)
References
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101 10.1038/ng786 [DOI] [PubMed] [Google Scholar]
- Bloodstein O (1995) A handbook of stuttering. 5th ed. Singular Press, San Diego [Google Scholar]
- Boehnke M (1991) Allele frequency estimation from data on relatives. Am J Hum Genet 48:22–25 [PMC free article] [PubMed] [Google Scholar]
- Drayna D, Kilshaw J, Kelly J (1999) The sex ratio in familial persistent stuttering. Am J Hum Genet 65:1473–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld S, Plomin R (1997) Epidemiological and offspring analyses of developmental speech disorders using data from the Colorado Adoption Project. J Speech Lang Hear Res 40:778–791 [DOI] [PubMed] [Google Scholar]
- Gudbjartsson DF, Jonasson K, Frigge ML, Kong A (2000a) Allegro, a new computer program for multipoint linkage analysis. Nat Genet 25:12–13 10.1038/75514 [DOI] [PubMed] [Google Scholar]
- Gudbjartsson DF, Jonasson K, Frigge ML, Kong A (2000b) Fast multipoint linkage analysis and the program Allegro. Technical report, deCODE Genetics, Reykjavik [DOI] [PubMed] [Google Scholar]
- Howie PM (1981) Concordance for stuttering in monozygotic and dizygotic twin pairs. J Speech Hear Res 24:317–321 [DOI] [PubMed] [Google Scholar]
- Kong A, Cox NJ (1997) Allele-sharing models: LOD scores and accurate linkage tests. Am J Hum Genet 61:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K, Cantor R, Horvath S, Perola M, Sabatti C, Sinsheimer J, Sobel E (2001) MENDEL version 4.0: a complete package for the exact genetic analysis of discrete traits in pedigree and population data sets. Am J Hum Genetics Suppl 69:A1886 [Google Scholar]
- McPeek MS, Sun L (2000) Statistical tests for detection of misspecified relationships by use of genome-screen data. Am J Hum Genet 66:1076–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluzhnikov A, Wen W, Luban S, Chen Y, Cox NJ (2003) Efficient construction of genetic maps based on publicly available physical and genetic map data. Am J Hum Genet Suppl 73:A438 10.1086/377008 [DOI] [Google Scholar]
- Riley GD (1980) SSI-3, stuttering severity instrument for children and adults, 3rd ed. Western Psychological Services, Los Angeles [Google Scholar]
- Shugart YY, Mundorff J, Kilshaw J, Doheny K, Doan B, Wanyee J, Green ED, Drayna D (2004) Results of a genome-wide linkage scan for stuttering. Am J Med Genet 124:133–135 10.1002/ajmg.a.20347 [DOI] [PubMed] [Google Scholar]
- Sun L, Abney M, McPeek MS (2001) Detection of misspecified relationships in inbred and outbred pedigrees. Genet Epidemiol 21:S36–S41 [DOI] [PubMed] [Google Scholar]
- Whittemore AS, Halpern J (1994) A class of tests for linkage using affected pedigree members. Biometrics 50:118–127 [PubMed] [Google Scholar]
- Yairi E, Ambrose N, Cox N (1996) Genetics of stuttering: a critical review. J Speech Hear Res 39:771–784 [DOI] [PubMed] [Google Scholar]