Abstract
Autosomal dominant parkinsonism has been attributed to pathogenic amino acid substitutions in leucine-rich repeat kinase 2 (LRRK2). By sequencing multiplex families consistent with a PARK8 assignment, we identified a novel heterozygous LRRK2 mutation. A referral sample of 248 affected probands from families with autosomal dominant parkinsonism was subsequently assessed; 7 (2.8%) were found to carry a heterozygous LRRK2 6055G→A transition (G2019S). These seven patients originate from the United States, Norway, Ireland, and Poland. In samples of patients with idiopathic Parkinson disease (PD) from the same populations, further screening identified six more patients with LRRK2 G2019S; no mutations were found in matched control individuals. Subsequently, 42 family members of the 13 probands were examined; 22 have an LRRK2 G2019S substitution, 7 with a diagnosis of PD. Of note, all patients share an ancestral haplotype indicative of a common founder, and, within families, LRRK2 G2019S segregates with disease (multipoint LOD score 2.41). Penetrance is age dependent, increasing from 17% at age 50 years to 85% at age 70 years. In summary, our study demonstrates that LRRK2 G2019S accounts for parkinsonism in several families within Europe and North America. Our work highlights the fact that a proportion of clinically typical, late-onset PD cases have a genetic basis.
Parkinsonism is a clinical syndrome characterized by bradykinesia, resting tremor, muscle rigidity, and postural instability (Gelb et al. 1999). Parkinson disease (PD [(MIM 168600]) is the second most frequent neurodegenerative disorder, after Alzheimer disease; it affects >1% of the population aged >55 years and is the most common cause of parkinsonism (de Rijk et al. 1995). Neuropathological findings in PD are loss of pigmented neurons in the brain stem—substantia nigra and locus coeruleus—with intracellular Lewy body inclusions found within surviving neurons (Forno 1996).
Although PD is considered a sporadic disease, various hereditary forms of parkinsonism have been recognized (Vila and Przedborski 2004). A major breakthrough in recent years has been the mapping and cloning of a number of genes causing monogenic forms of parkinsonism. The role of genetics in sporadic late-onset PD has, however, remained controversial due, in part, to the different associated pathologies and the variable but overlapping spectrum of clinical signs and symptoms seen in monogenic familial forms of parkinsonism.
Genomic multiplication and missense mutations in the α-synuclein gene were initially identified in a small number of families with autosomal dominant parkinsonism (PARK1 [MIM 168601] and PARK4 [MIM 605543]) (Polymeropoulos et al. 1997; Kruger et al. 1998; Singleton et al. 2003; Chartier-Harlin et al. 2004; Farrer et al. 2004; Zarranz et al. 2004). Patients present with levodopa-responsive parkinsonism, although early-onset dementia is frequent (Spira et al. 2001). α-Synuclein antibodies robustly stain Lewy bodies and Lewy neurites in familial and sporadic PD (Spillantini et al. 1997), and common genetic variability in the α-synuclein promoter has been implicated in sporadic PD (Pals et al. 2004).
Autosomal recessive mutations in three genes—parkin, DJ-1, and PINK1—have been linked with early-onset (i.e., subjects aged <45 years at onset) parkinsonism (PARK2 [MIM 600116 and MIM 602544], PARK6 [MIM 605909], and PARK7 [MIM 606324]) (Kitada et al. 1998; Bonifati et al. 2003; Valente et al. 2004a). A large number of pathogenic mutations and rearrangements have been identified in the parkin gene (reviewed by Mata et al. [2004]). Mutations in the DJ-1 and PINK1 genes seem to be more rare (Abou-Sleiman et al. 2003; Valente et al. 2004b).
Our group recently identified pathogenic mutations in a novel gene, the leucine-rich repeat kinase 2 gene (LRRK2), in six families with autosomal dominant parkinsonism linked to the PARK8 locus (MIM 607060) (Zimprich et al. 2004a). Paisan-Ruiz and colleagues (2004) independently confirmed these findings in British and Basque families. The PARK8 locus was originally mapped in a large Japanese family, the Sagamihara family, presenting with autosomal dominant parkinsonism (Funayama et al. 2002). Across PARK8-linked families, the age at onset of symptoms is late (aged >50 years), albeit variable. Brain autopsy findings in four members of the Sagamihara family showed pure nigral neuronal degeneration without coexisting pathology (Funayama et al. 2002). In contrast, nigral neuronal loss, tauopathy, and Lewy body synucleinopathy have been described elsewhere for families A and D (Wszolek et al. 2004; Zimprich et al. 2004a).
Here, we describe a novel LRRK2 mutation in 13 families from diverse North American and European origins. Segregation analysis provided evidence of pathogenicity and an estimate of age-associated penetrance, whereas haplotype analysis suggested that the mutation originates from a common and ancient founder.
Patients and control individuals in this study were examined by neurologists specialized in movement disorders. A full history, including family history and neurological examination, was completed for each patient. A clinical diagnosis of PD required the presence of at least two of three cardinal signs (resting tremor, bradykinesia, and rigidity), improvement through adequate dopaminergic therapy, and the absence of atypical features or other causes of parkinsonism. Families with two or more members affected by PD in at least two consecutive generations were considered to be consistent with an autosomal dominant pattern of inheritance. The institutional review boards of all participating institutions approved our clinicogenetic protocols, and informed consent was obtained from all patients and control subjects.
Blood samples were taken and genomic DNA was extracted using standard techniques. Six small families (194, 281, 3081, 3082, 3083, and 3211) were identified elsewhere with positive LOD scores (range 0.29–0.38) for microsatellite markers within the PARK8 locus (Zimprich et al. 2004b). For each of these probands, all 51 exons of the LRRK2 gene were amplified by PCR and were sequenced. Electrophoresis was performed under standard conditions on an ABI 3730 automated sequencer, and data were analyzed with SeqScape software version 2.1.1 (Applied Biosystems) and were compared with the published sequence of LRRK2 (GenBank accession number AY792511). Primers are available on request.
On identification of a novel, heterozygous LRRK2 6055G→A transition (G2019S) in the proband and affected sibling of family 3211, we designed a probe employing TaqMan chemistry on ABI 7900 (Applied Biosystems) to screen for this specific mutation. We examined another 242 affected probands with familial parkinsonism that was consistent with autosomal dominant transmission of disease. These probands and their families were referred from many regions of North America, Asia, and Europe. Positive samples originated from centers in the United States, Norway, Ireland, and Poland. Hence, we subsequently assessed whether LRRK2 6055G→A (G2019S) was common in appropriate population samples. We screened 435 Norwegian, 271 Irish, and 100 Polish patients with idiopathic PD and 1,200 American, 550 Norwegian, 330 Irish, and 180 Polish control subjects (2,260 control individuals in total). All mutations discovered were confirmed by direct sequencing. Finally, all participating family members of LRRK2 G2019S–substitution carriers (affected and unaffected) were screened for the mutation.
In available family members, 17 microsatellite markers and four SNPs were genotyped for linkage analyses and to determine whether a single haplotype was associated with the LRRK2 mutation. Microsatellite markers were chosen to span the PARK8 region, including D12S87, D12S1648, D12S2080, D12S2194, D12S1048, D12S1301, and D12S1701. LRRK2 is located between D12S2194 and D12S1048. We also developed 10 novel microsatellite markers in this region (table 1) by searching for repeat polymorphisms by use of the RepeatMasker software (RepeatMasker Web site) with in silico BAC sequences covering the PARK8 locus (University of California–Santa Cruz Genome Bioinformatics). (The physical position of markers is from the National Center for Biotechnology Information [NCBI] build 34.) One primer of each pair was labeled with a fluorescent tag. PCR reactions were performed under standard conditions, and PCR products were run on an ABI 3100 genetic analyzer. Results were analyzed using Genescan 3.7 and Genotyper 3.7 software (Applied Biosystems). The four exonic LRRK2 SNPs included were rs7966550, rs1427263, rs11176013, and rs11564148. Marker-allele frequencies were estimated by genotyping unrelated individuals from North America (table 2).
Table 1.
Novel Chromosome 12 STR Markers[Note]
| Marker | Primer Sequence | Physical Positiona(bp) |
| D12S2514 | F: 5′-TTGCAGCTGTAAGGAATTTGGG-3′; R: 5′-GCATTCTTCAGCCTGAGACCC-3′ | 38873779 |
| D12S2515 | F: 5′-TGAAGGACACTGAACAAGATGG-3′; R: 5′-GCCATAGTCCTTCCATAGTTCC-3′ | 38974140 |
| D12S2516 | F: 5′-CGCAGCGAGCATTGTACC-3′; R: 5′-CTCGGAAAGTTTCCCAATTC-3′ | 38989214 |
| D12S2518 | F: 5′-CTGGTATTACCTCAACTGTGGCTC-3′; R: 5′-ACTGGTATGTTTAAGCCTGGCAC-3′ | 39034800 |
| D12S2519 | F: 5′-AGCAGCAGAGAAGATTTCAATAAC-3′; R: 5′-AATCATCTTTGAAAGAACCAGG-3′ | 39116816 |
| D12S2520 | F: 5′-CTGGCACCACTGTGATAGCTAAC-3′; R: 5′-TAAAGTTTCTGAGCAGGGAGGTG-3′ | 39119979 |
| D12S2521 | F: 5′-AAGTAAATGTTGCCTTCTGTGAAG-3′; R: 5′-TGGGCAACAAAGTGAGACC-3′ | 39128597 |
| D12S2522 | F: 5′-CCTTGATTTGATGAACTGCATAG-3′; R: 5′-CAGGGTAATCTACTTATGTTCTTCTG-3′ | 39132223 |
| D12S2523 | F: 5′-TAAACGAAGCTCCCTCACTGTAAG-3′; R: 5′-TCTTTGTAGCTGCGGTTGTTTC-3′ | 39147728 |
| D12S2517 | F: 5′-TCATGAAGATGTCTGTGATAGGGC-3′; R: 5′-CTCTATTGTGAGCAAACTGCATGG-3′ | 39282976 |
Note.— Assays for 10 novel markers within the PARK8 locus were developed.
The physical position on chromosome 12 (NCBI build 34) of the first base of the forward primer is given.
Table 2.
Allele Frequencies of PARK8 Markers
| Markera and Allele (bp) | Frequency (%)b |
| D12S87 (n=92): | |
| 150 | .5 |
| 154 | 1.1 |
| 156 | 27.2 |
| 158 | 33.2 |
| 160 | 11.4 |
| 162 | 2.7 |
| 164 | 6.0 |
| 166 | 17.4 |
| 168 | .5 |
| D12S1648 (n=91): | |
| 110 | 13.7 |
| 112 | 3.3 |
| 114 | 11.0 |
| 116 | 4.4 |
| 118 | 2.2 |
| 120 | 2.8 |
| 122 | 17.0 |
| 124 | 3.9 |
| 126 | 7.7 |
| 128 | 14.3 |
| 130 | 8.8 |
| 132 | 2.8 |
| 134 | 2.8 |
| 136 | 1.7 |
| 138 | .6 |
| 140 | 2.2 |
| 142 | 1.1 |
| D12S2080 (n=93): | |
| 176 | 1.6 |
| 180 | 20.2 |
| 184 | 44.7 |
| 188 | 22.9 |
| 192 | 10.6 |
| D12S2194 (n=87): | |
| 245 | .6 |
| 249 | 40.9 |
| 253 | 32.4 |
| 257 | 19.9 |
| 261 | 4.6 |
| 265 | 1.7 |
| D12S2514 (n=82): | |
| 285 | 11.0 |
| 291 | 53.1 |
| 294 | 32.3 |
| 297 | 1.2 |
| 300 | 2.4 |
| D12S2515 (n=93): | |
| 208 | 3.2 |
| 212 | 26.6 |
| 216 | 18.6 |
| 220 | 22.9 |
| 224 | 20.7 |
| 228 | 5.3 |
| 232 | 2.7 |
| rs7966550 (n=90): | |
| T | 90.6 |
| C | 9.4 |
| D12S2516 (n=78): | |
| 252 | 37.3 |
| 254 | 62.7 |
| rs1427263 (n=89): | |
| A | 63.5 |
| C | 36.5 |
| rs1116013 (n=88): | |
| A | 49.4 |
| G | 50.6 |
| rs11564148 (n=88): | |
| A | 26.1 |
| T | 73.9 |
| D12S2518 (n=90): | |
| 154 | 79.7 |
| 168 | 15.9 |
| 170 | 4.4 |
| D12S2519 (n=72): | |
| 132 | 29.5 |
| 134 | 22.6 |
| 138 | 22.6 |
| 140 | 25.3 |
| D12S2520 (n=85): | |
| 248 | 8.2 |
| 251 | 7.6 |
| 254 | 10.0 |
| 257 | 54.1 |
| 260 | 20.0 |
| D12S2521 (n=93): | |
| 311 | .5 |
| 315 | 10.8 |
| 319 | 20.4 |
| 323 | 8.1 |
| 327 | 7.0 |
| 331 | 4.8 |
| 335 | .5 |
| 355 | 1.1 |
| 359 | 7.5 |
| 363 | 13.4 |
| 367 | 7.0 |
| 371 | 7.0 |
| 375 | 6.5 |
| 379 | 3.8 |
| 383 | 1.1 |
| 387 | .5 |
| D12S2522 (n=93): | |
| 281 | 9.1 |
| 283 | 14.0 |
| 285 | .5 |
| 287 | 11.3 |
| 293 | .5 |
| 295 | 15.6 |
| 297 | 44.6 |
| 299 | 4.3 |
| D12S2523 (n=89): | |
| 305 | 18.9 |
| 314 | 41.1 |
| 317 | 8.9 |
| 320 | 30.0 |
| 323 | 1.1 |
| D12S2517 (n=93): | |
| 180 | 8.5 |
| 182 | 7.5 |
| 184 | 15.4 |
| 186 | 8.5 |
| 188 | 11.7 |
| 190 | 8.0 |
| 192 | 5.3 |
| 194 | 1.1 |
| 196 | 1.1 |
| 198 | 3.2 |
| 200 | .5 |
| 202 | 3.7 |
| 204 | 6.9 |
| 206 | 6.9 |
| 208 | 4.3 |
| 210 | 2.1 |
| 212 | 3.2 |
| 214 | 1.6 |
| 216 | .5 |
| D12S1048 (n=89): | |
| 211 | 37.2 |
| 214 | 21.1 |
| 217 | 17.8 |
| 220 | 2.2 |
| 223 | 6.7 |
| 226 | 11.7 |
| 229 | 3.3 |
| D12S1301 (n=93): | |
| 96 | .5 |
| 100 | 37.2 |
| 104 | 17.6 |
| 108 | 11.1 |
| 112 | 12.2 |
| 116 | 13.3 |
| 120 | 7.5 |
| 124 | .5 |
| D12S1701 (n=93): | |
| 89 | 4.3 |
| 91 | 4.8 |
| 93 | 10.8 |
| 95 | 40.0 |
| 97 | 16.0 |
| 99 | 12.4 |
| 101 | 11.8 |
| 103 | .5 |
The number of individuals genotyped is given for each marker (n).
Allele frequencies are for individual markers in U.S. control subjects.
Multipoint LOD scores for all families were calculated under the assumption of an autosomal dominant model by use of GENEHUNTER-PLUS (Kong and Cox 1997). The frequency of the deleterious allele was set at 0.0001; marker-allele frequencies were determined empirically. The map positions for each marker were taken from Rutgers combined linkage physical map, version 1.0 (MAP-O-MAT Web site). For tightly linked loci with no observed recombinants, intermarker genetic distances were assigned as 0.01 cM. PARK8 haplotypes were established for families with known phase; for those affected individuals or families for whom chromosomal phase could not be determined, both alleles are given (fig. 1). Haplotype frequencies in the general population were estimated from unrelated individuals by use of an estimation-maximization algorithm (Zaykin et al. 2002).
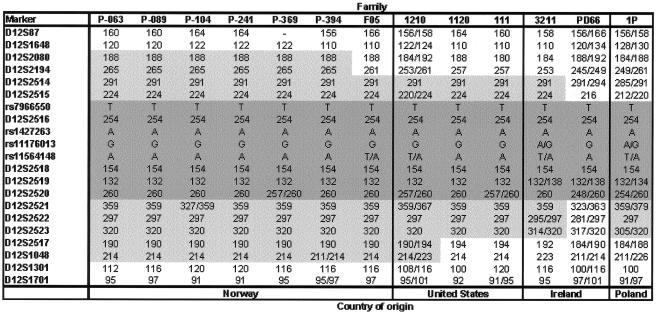
Figure 1.
Chromosome 12q12 markers on the disease haplotype (PARK8). Genotypes for mutation carriers from 13 families with LRRK2 G2019S are shown; those shared are highlighted in gray. For families whose phase could not be determined with certainty, both alleles are shown.
Age-dependent penetrance was estimated as the probability of a gene carrier becoming affected, at a given age, within the 13 families. The number of affected mutation carriers within each 5-year age group was divided by the total number of carriers (both affected and unaffected) within that group. For some affected family members, no DNA was available, and only historical data on the disease course was obtained. Those individuals were excluded from penetrance calculations.
We identified a heterozygous 6055G→A mutation in 7 (2.8%) of 248 probands from families presenting with autosomal dominant parkinsonism. Subsequently, six additional patients carrying the same mutation were identified through population-based screening in samples of European descent. In total, we identified 13 families: 7 originate from Norway, 3 from the United States, 2 from Ireland, and 1 from Poland. Of the U.S. families, one was reported to be of Russian/Romanian ancestry (family 1120), a second claimed to be of Italian descent (family 1210), and the ethnic origin was unknown for a third kindred (family 111). After genotyping 42 additional family members, 22 were found to carry the mutation, 7 with a diagnosis of PD (table 3).
Table 3.
Demographic and Clinical Information for 13 Families with LRRK2 G2019S
|
Findings for Family |
|||||||||||||
| Characteristic | P-063 | P-089 | P-104 | P-241 | P-369 | P-394 | F05 | 1210 | 111 | 1120 | PD66 | 3211 | 1P |
| Country of origin | Norway | Norway | Norway | Norway | Norway | Norway | Norway | United States | United States | United States | Ireland | Ireland | Poland |
| No. of generations | 3 | 4 | 3 | 3 | 3 | 4 | 4 | 2 | 2 | 3 | 1 | 2 | 1 |
| No. of affected individuals | 2 | 4 | 4 | 1 | 3 | 4 | 5 | 2 | 3 | 3 | 1 | 3 | 1 |
| No. of typed individuals affected (unaffected) | 1 (6) | 2 (8) | 1 (1) | 1 (4) | 2 (3) | 1 (1) | 3 (6) | 1 (0) | 2(0) | 3 (3) | 1 (0) | 2 (6) | 1 (0) |
| No. of typed generations | 2 | 3 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 1 |
| Agea at onset in years (range) | 59 (53–65) | 59 (43–70) | 58 | 60 | 50 (43–61) | 66 | 64 (61–70) | 65 | 58 (57–58) | 59 (39–78) | 41 | 46 (40–52) | 73 |
| Maximum mLOD score | 0 | .30 | 0 | 0 | .60 | 0 | .90 | 0 | .09 | .30 | 0 | .30 | 0 |
Average ages at onset are given when n⩾2 affected individuals.
Three of the six original patients identified through the population-based screening had no known family history of PD. In the 10 remaining kindreds, the LRRK2 G2019S substitution segregates with disease, consistent with autosomal dominant transmission with reduced, age-associated penetrance. Simplified versions of the family pedigrees are presented in figure 2. Of note, a recently deceased member of family P-089 was affected but was not an LRRK2 mutation carrier. He had akinetic rigid parkinsonism unresponsive to levodopa. Although his chromosome 12q12 haplotypes were consistent with those of his offspring and siblings, he did not carry the disease-associated PARK8 haplotype. Hence, for the purposes of this study, he was considered a phenocopy and was excluded from further analyses. We did not identify the mutation in any of 2,260 control individuals.
Figure 2.
Pedigrees of families with LRRK2 G2019S. Blackened symbols denote family members affected with parkinsonism. An asterisk (*) denotes a genotyped individual, with “m” for mutation carriers and “wt” for wild-type LRRK2. To protect confidentiality, the genotypes and sexes of some unaffected individuals are not shown, and some family members for whom no information was available have been removed from the pedigrees.
Evidence for linkage to the PARK8 locus was found across families, with a combined maximum multipoint LOD (mLOD) score of 2.41, corresponding to a P value of 4.3×10-4. Positive LOD scores were found in all families in which more than one affected subject was genotyped (table 3). Since only a defined chromosomal region was investigated, rather than a genomewide search being done, the mLOD score exceeds that required for significance, P=.01 (Lander and Kruglyak 1995).
All affected members from the different families, except the phenocopy in family P-089, share a chromosome 12q12 and LRRK2 haplotype (fig. 1). Phase can be established for most markers in nine of the families; LRRK2 6055G→A mutation carriers share alleles for eight adjacent markers, including four exonic SNPs and four microsatellites. For the remaining families, the number of available samples from relatives was not sufficient to determine phase. However, the genotypes in these cases are consistent with a common LRRK2 G2019S allele. D12S2516 is located in intron 30, and D12S2518 is located in intron 45, whereas the two other shared markers are positioned 3′ of the LRRK2 gene. SNP rs7966550 is located in exon 22, and the remaining three SNPs are in exon 34. With use of the physical position of the shared and nonshared markers, the size of the shared haplotype was determined to be between 145 kb and 154 kb. The frequency of this haplotype is estimated to be 6.0% in the U.S. population (not considering the frequency of the LRRK2 6055G→A mutation).
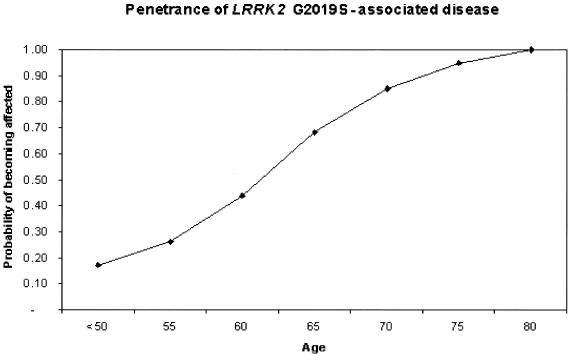
Age at onset of clinical symptoms was variable, even within the same family. Family 1120 had both the earliest and latest age at onset for a patient. The youngest affected patient had onset at age 39 years, whereas the oldest carrier presented with initial symptoms at age 78 years. Where recorded, most LRRK2 G2019S carriers have late-onset disease (aged >50 years at onset). The mean age at onset for affected mutation carriers (n=19) was 56.8 years (range 39–78 years). Unaffected carriers (n=15) have a mean age of 54.6 years (range 26–74 years). The penetrance of the mutation was found to be highly age dependent, increasing from 17% at age 50 years to 85% at age 70 years (fig. 3).
Figure 3.
Penetrance of LRRK2-associated disease, showing the probability of becoming affected by parkinsonism, in LRRK2 G2019S carriers, as a function of age.
We have identified a novel 6055G→A (G2019S) LRRK2 mutation in 13 white kindreds originating from several European and North American populations. In 10 families, the mutation segregates with autosomal dominant parkinsonism; three probands had no family history of PD. Positive mLOD scores were obtained in multiplex families and, combined, provide significant support for the PARK8 locus. The LRRK2 G2019S substitution was absent in a large number of control individuals of similar ethnicity. The physical size of the shared haplotype is small, between 145 kb and 154 kb. LRRK2 is located close to the centromere on chromosome 12, and there is generally a dearth of recombination at centromeres. LRRK2 spans several haplotype blocks, but local patterns of linkage disequilibrium are not available for the specific populations we have studied (International HapMap Project). The mutant allele is geographically widespread across European and American populations and is therefore likely to be ancient. The number of families linked to LRRK2 in this and previous studies now explains a proportion of genetically defined autosomal dominant parkinsonism greater than that explained by other genes.
Age is the single most consistent risk factor for development of PD and other neurodegenerative disorders (Lang and Lozano 1998), and our data also indicate that age is an important risk factor in LRRK2-associated parkinsonism. The mean age at onset, 56.8 years, of patients with the LRRK2 6055G→A mutation in this study is comparable to that of patients in other families linked to PARK8 (Funayama et al. 2002; Paisan-Ruiz et al. 2004; Zimprich et al. 2004a). The majority of patients in all families present with late-onset disease that is difficult to clinically distinguish from typical idiopathic PD. We found that the penetrance of of LRRK2 G2019S–associated disease was highly age dependent, increasing in a close-to-linear fashion from 17% at age 50 years to 85% at age 70 years. Interestingly, age at onset was variable in this study, both within and between different families, which suggests that other susceptibility factors, environmental or genetic, may influence the phenotype.
Although our findings clearly indicate that LRRK2 mutations account for a proportion of familial late-onset parkinsonism, historically, cross-sectional twin studies have not supported a genetic etiology for late-onset PD (Tanner et al. 1999; Wirdefeldt et al. 2004). The age-associated penetrance of LRRK2 mutations provides some explanation, since even large and well-designed twin studies are underpowered to detect incompletely penetrant mutations (Simon et al. 2002). LRRK2 mutations were also found in patients with apparently sporadic PD; three of the patients in the present study did not have any known affected first- or second-degree relatives. However, a caveat for the study of age-dependent penetrance is that carriers may die of other diseases before manifesting or being diagnosed with PD. Thus, it seems difficult to separate sporadic and familial PD and to hypothesize environmental causes as more important in one group and genetic causes as more prominent in the other. In light of these results, a family history of parkinsonism, previously considered an exclusion criterion for a diagnosis of PD, must be reconsidered (Hughes et al. 1992).
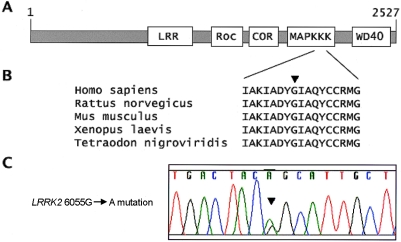
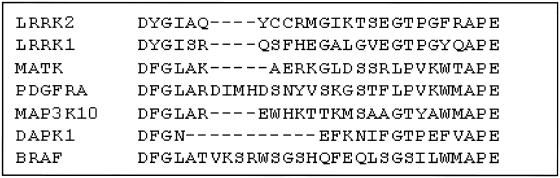
LRRK2 is a member of the recently defined ROCO protein family (Bosgraaf and Van Haastert 2003). In human, mouse, and rat, members of the ROCO protein family have five conserved domains (fig. 4). The LRRK2 kinase domain belongs to the MAPKKK subfamily of kinases. The active sites of all kinases are located in a cleft between an N-terminal and a C-terminal lobe, typically covered by an “activation segment,” in an inactive conformation. The activation segment must undergo crucial structural changes to allow access to peptide substrates and to orient key catalytic amino acids (Huse and Kuriyan 2002). In different kinases, the activation segment starts and ends with the conserved residues asp-phe-gly (DFG) and ala-pro-glu (APE), respectively (Nolen et al. 2004). The LRRK2 G2019S substitution changes the highly conserved glycine (G) at the start of this segment (fig. 5). In a German family we described elsewhere, an I2020T mutation is located in an adjacent codon (Zimprich et al. 2004a). In other kinases, oncogenic mutations in residues within the activation segment of the kinase domain have an activating effect (Davies et al. 2002). Thus, we postulate that LRRK2 G2019S and I2020T mutations may have an activating effect on the kinase activity of LRRK2. A mutation causing “gain of function” of the resulting protein would also be compatible with the dominant mode of disease transmission observed in the presented families.
Figure 4.
LRRK2 with the novel G2019S substitution. A, Schematic drawing of LRRK2 with predicted protein domains. LRR = leucine-rich repeat; Roc = Ras in complex proteins; COR = C-terminal domain of Roc; MAPKKK = mitogen-activated protein kinase kinase kinase; WD40 = WD40 repeats. B, The human LRRK2 protein sequence in the region of the G2019S mutation, aligned with orthologs from rat (GenBank accession number XP_235581), mouse (GenBank accession number AAH34074), frog (GenBank accession number AAH76853), and puffer fish (GenBank accession number CAG05593). The mutation is indicated by a blackened arrowhead. C, Chromatogram showing the 6055G→A transition (G2019S).
Figure 5.
Aligned amino acid sequences of the activation segment of different human kinases. In most kinases, the activation segment starts and ends with the conserved residues DFG and APE, respectively. In LRRK2 and LRRK1, phenylalanine is changed to tyrosine, an amino acid with a similar structure. LRRK2 = leucine-rich repeat kinase 2; LRRK1 = leucine-rich repeat kinase 1; MATK = megakaryocyte-associated tyrosine kinase; PDGFRA = platelet-derived growth factor receptor alpha; MAP3K10 = mitogen-activated protein kinase kinase kinase 10; DAPK1 = death-associated protein kinase 1; BRAF = v-raf murine sarcoma viral oncogene homolog B1.
The identification of LRRK2 mutations as a cause of parkinsonism in a large number of families from different populations heralds an exciting time in the field of neurogenetics. The elucidation of the functional effects of this gene and of the G2019S substitution will further our understanding of pathways that play a pivotal role in PD.
Acknowledgments
The authors gratefully thank the patients and families for their participation in this study. Mayo Clinic Jacksonville is an M. K. Udall Parkinson’s Disease Research Center of Excellence (National Institute of Neurological Disorders and Stroke grant P01 NS40256); the authors thank all collaborators from the Udall Center. This study was also supported by National Institute of Aging grant R01 AG022579, National Institutes of Health grant R01 NS36960, Research Council of Norway grant 153487/V50, the Unger-Vetlesen Medical Fund, the Sigurd K. Thoresen Foundation, and the Research and Development Office, Health and Personal Social Services, Northern Ireland.
Electronic-Database Information
The accession number and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nih.gov/Genbank/ (for LRRK2 [accession number AY792511], rat [accession number XP_235581], mouse [accession number AAH34074], frog [accession number AAH76853], and puffer fish [accession number CAG05593])
- International HapMap Project, http://www.hapmap.org/
- MAP-O-MAT, http://compgen.rutgers.edu/mapomat/ (for version 1.1)
- NCBI, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=nucleotide
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for PARK1, PARK4, PARK2,PARK6, PARK7, and PARK8)
- RepeatMasker, http://www.repeatmasker.org/
- University of California–Santa Cruz Genome Bioinformatics, http://genome.ucsc.edu/
References
- Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, Wood NW (2003) The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann Neurol 54:283–286 [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299:256–259 [DOI] [PubMed] [Google Scholar]
- Bosgraaf L, Van Haastert PJ (2003) Roc, a Ras/GTPase domain in complex proteins. Biochim Biophys Acta 1643:5–10 [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A (2004) α-Synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167–1169 [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, et al (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954 [DOI] [PubMed] [Google Scholar]
- de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meche FG, Hofman A (1995) Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology 45:2143–2146 [DOI] [PubMed] [Google Scholar]
- Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW (2004) Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann Neurol 55:174–179 [DOI] [PubMed] [Google Scholar]
- Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272 [DOI] [PubMed] [Google Scholar]
- Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F (2002) A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann Neurol 51:296–301 [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56:33–39 [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Kuriyan J (2002) The conformational plasticity of protein kinases. Cell 109:275–282 [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608 [DOI] [PubMed] [Google Scholar]
- Kong A, Cox NJ (1997) Allele-sharing models: LOD scores and accurate linkage tests. Am J Hum Genet 61:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O (1998) Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet 18:106–108 [DOI] [PubMed] [Google Scholar]
- Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11:241–247 [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM (1998) Parkinson’s disease: first of two parts. N Engl J Med 339:1044–1053 [DOI] [PubMed] [Google Scholar]
- Mata IF, Lockhart PJ, Farrer MJ (2004) Parkin genetics: one model for Parkinson’s disease. Hum Mol Genet 13:R127–R133 [DOI] [PubMed] [Google Scholar]
- Nolen B, Taylor S, Ghosh G (2004) Regulation of protein kinases; controlling activity through activation segment conformation. Mol Cell 15:661–675 [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, de Munain AL, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44:595–600 [DOI] [PubMed] [Google Scholar]
- Pals P, Lincoln S, Manning J, Heckman M, Skipper L, Hulihan M, Van den Broeck M, De Pooter T, Cras P, Crook J, Van Broeckhoven C, Farrer MJ (2004) α-Synuclein promoter confers susceptibility to Parkinson’s disease. Ann Neurol 56:591–595 [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047 [DOI] [PubMed] [Google Scholar]
- Simon DK, Lin MT, Pascual-Leone A (2002) “Nature versus nurture” and incompletely penetrant mutations. J Neurol Neurosurg Psychiatry 72:686–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K (2003) α-Synuclein locus triplication causes Parkinson’s disease. Science 302:841 [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) α-Synuclein in Lewy bodies. Nature 388:839–840 [DOI] [PubMed] [Google Scholar]
- Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA (2001) Clinical and pathological features of a parkinsonian syndrome in a family with an Ala53Thr α-synuclein mutation. Ann Neurol 49:313–319 [PubMed] [Google Scholar]
- Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW (1999) Parkinson disease in twins: an etiologic study. JAMA 281:341–346 [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW (2004a) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304:1158–1160 [DOI] [PubMed] [Google Scholar]
- Valente EM, Salvi S, Ialongo T, Marongiu R, Elia AE, Caputo V, Romito L, Albanese A, Dallapiccola B, Bentivoglio AR (2004b) PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol 56:336–341 [DOI] [PubMed] [Google Scholar]
- Vila M, Przedborski S (2004) Genetic clues to the pathogenesis of Parkinson’s disease. Nat Med Suppl 10:S58–S62 [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Gatz M, Schalling M, Pedersen NL (2004) No evidence for heritability of Parkinson disease in Swedish twins. Neurology 63:305–311 [DOI] [PubMed] [Google Scholar]
- Wszolek ZK, Pfeiffer RF, Tsuboi Y, Uitti RJ, McComb RD, Stoessl AJ, Strongosky AJ, Zimprich A, Müller-Myhsok B, Farrer MJ, Gasser T, Calne DB, Dickson DW (2004) Autosomal dominant parkinsonism associated with variable synuclein and tau pathology. Neurology 62:1619–1622 [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG (2004) The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55:164–173 [DOI] [PubMed] [Google Scholar]
- Zaykin DV, Westfall PH, Young SS, Karnoub MA, Wagner MJ, Ehm MG (2002) Testing association of statistically inferred haplotypes with discrete and continuous traits in samples of unrelated individuals. Hum Hered 53:79–91 [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Müller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T (2004a) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44:601–607 [DOI] [PubMed] [Google Scholar]
- Zimprich A, Müller-Myhsok B, Farrer M, Leitner P, Sharma M, Hulihan M, Lockhart P, Strongosky A, Kachergus J, Calne DB, Stoessl J, Uitti RJ, Pfeiffer RF, Trenkwalder C, Homann N, Ott E, Wenzel K, Asmus F, Hardy J, Wszolek Z, Gasser T (2004b) The PARK8 locus in autosomal dominant parkinsonism: confirmation of linkage and further delineation of the disease-containing interval. Am J Hum Genet 74:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]