Abstract
Danish nationwide surveillance data on invasive pneumococcal disease from the 5-year period from 1995 to 1999, including 5,452 isolates, are presented and described. Annual overall incidence rates, serotype distribution, and antimicrobial susceptibility patterns of the isolates were monitored. Major changes in the total annual incidence rate from 27/100,000 in 1996 to 17/100,000 in 1999 and a significant change in the proportion of invasive isolates belonging to types 1 and 12F were observed. The serotype coverage rate by the 23-valent polysaccharide vaccine among the elderly was 92.9%, and the serotype coverage rate by the 7-, 9-, and 11-valent pneumococcal conjugate vaccines among children less than 2 years old were 71.7, 75.2, and 81.4%, respectively. Invasive isolates with reduced susceptibility to penicillin or erythromycin increased from 1995 to 1999, with a high proportion of the penicillin-nonsusceptible invasive isolates originating from people 60 years old or older (57.0%). These observations underline the importance of adequate surveillance systems of invasive pneumococcal disease to introduce and maintain national vaccine strategies and adequate antibiotic policy.
Streptococcus pneumoniae (pneumococcus) remains an important cause of morbidity and mortality in both developing and industrialized countries. Pneumococcal infections range from less-serious infections, such as upper respiratory tract infections and otitis media, to life-threatening infections, such as pneumonia, bacteremia, and meningitis. Infections are most common among the very young, the elderly, and people belonging to various risk groups due to underlying illness (6, 13).
Although 90 different capsular types of pneumococci have been described, only a few of these are responsible for most infections in the industrialized countries. In children, fewer than 11 capsular types cause most infections (9). Hence, new protein-conjugated pneumococcal vaccines, including 7, 9, or 11 capsular types, are being developed for the prevention of invasive pneumococcal infections in children, and a 7-valent vaccine was introduced in United States at the beginning of 2000 (1).
Since its introduction in 1983, a 23-valent capsular polysaccharide vaccine has been used in many countries. It is recommended for the prevention of invasive infections in patients older than 2 years with increased risk of obtaining pneumococcal infections (22). It is documented that the serotype distribution among the pneumococcal isolates causing invasive infections varies according to time and geography (9, 20, 25, 27). Thus, surveillance of the type distribution in the various countries is increasingly important. In several countries, there was an increase in the incidence of invasive pneumococcal infection in the late 1990s (5; J. Giesecke and H. Fredlund, Letter, Lancet 349:699, 1997 [Erratum, 349:1106]) and, in some countries, a few types were dominant among the invasive strains (4, 10, 20). It has previously been discussed whether there was a real increase in the number of invasive cases or whether it was just the result of more sensitive and more frequently used blood culture systems (24, 26). In recent years there has been an increase in the frequency of pneumococcal isolates with reduced sensitivity to various antibiotics in many countries, and this is of major concern. Penicillin-resistant and multiresistant pneumococcal strains are speading both within and between countries (8, 16). Detailed surveillance of antibiotic resistance among pneumococci has therefore been implemented in many countries, and a restrictive antibiotic policy is recommended in order to cope with this problem (2, 12).
In Denmark, a complete nationwide surveillance of invasive pneumococcal infections with regard to serotype distribution and antibiotic susceptibility was established in the 1960s. Data from this surveillance have been published (18, 19). Since 1996, national surveillance of antibiotic resistance among both invasive and noninvasive isolates of pneumococci has been carried out. Before 1994, antibiotic resistance among invasive pneumococcal isolates in Denmark was almost nonexistent, whereas countries close to Denmark had reported increasing problems in this area.
Since 1996 in Denmark, the 23-valent pneumococcal vaccine is offered to people above the age of 64 years and also to people belonging to certain risk groups. A growing but still small number of elderly people have been vaccinated in order to prevent invasive pneumococcal infections.
The objectives of the present study were to describe the overall epidemiology of invasive pneumococcal disease in Denmark during the 5-year study period (1995 to 1999), and to evaluate whether changes in serotype distribution could explain changes in the incidence of invasive pneumococcal disease observed during the study period. We also investigated whether changes in serotype distribution could influence the serotype coverage rate offered by the 23-valent pneumococcal vaccine, the new 7-valent vaccine, and the future 9- or 11-valent pneumococcal conjugate vaccines. Finally, we wanted to determine whether the number of invasive pneumococcal isolates with reduced sensitivity to various antibiotics had remained insignificant in Denmark during this period.
MATERIALS AND METHODS
For decades, the vast majority of pneumococci isolated from patients with invasive pneumococcal infections (bacteraemia and meningitis) in Danish hospitals have been sent routinely to the Streptococcus Unit, Statens Serum Institut (SSI), for serotyping and antibiotic susceptibility testing. Since 1996, regardless of the isolation site, all pneumococci with reduced sensitivity to penicillin (MIC >0.064 μg/ml) have also been sent to the Streptococcus Unit for typing and antibiotic susceptibility testing. Besides being the National Pneumococcal Reference Centre, the Streptococcus Unit serves as a WHO Collaborating Centre for Reference and Research on Pneumococci, and we type ca. 3,000 pneumococcal strains per year.
In this study, all invasive pneumococci from patients in Danish hospitals received from 1995 to 1999 were monitored. Each isolate was characterized by age and gender of the patient, isolation site, and date of isolation.
All isolates were serotyped by the Quellung reaction by using type-specific pneumococcal rabbit antisera, which are commercially available at the SSI, Copenhagen, Denmark. Antimicrobial susceptibility testing was performed first as a screening with the agar disk diffusion test using oxacillin (1-μg disk; AB Biodisk, Solna, Sweden) and erythromycin (78 μg, Neo-Sensitabs; Rosco, Copenhagen, Denmark) on 10% horse blood agar plates (SSI). Isolates with reduced sensitivity against oxacillin or erythromycin was further characterized by determining the MICs of penicillin, erythromycin, ceftriaxone, and ciprofloxacin by using the E-test (AB Biodisk) on resistance plates (4.6 mm; SSI). Susceptibility to tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole was tested by agar disk diffusion test with chloramphenicol (10 μg, Neo-Sensitabs; Rosco), tetracycline (80 μg, Neo-sensitabs), and trimethoprim-sulfa (5.2 plus 240 μg, Neo-Sensitabs) on resistance plates (4.6 mm; SSI). Pneumococci were incubated overnight at an elevated CO2 level (5%) at 35°C. The results of antimicrobial susceptibility testing were interpreted according to the NCCLS guidelines (17).
In case of multiple isolates from the same patient, only one was included in the study. Strains isolated from both blood and cerebrospinal fluid (CSF) from the same patient in 1 month was included as a CSF isolate only.
The age-specific incidences of invasive infections were calculated as the number of cases per 100,000 inhabitants per year for each age group. The number of people per age group was obtained from Statistics Denmark. For statistical evaluation, a chi-squared test was used.
RESULTS
In the 5-year period under discussion, we received 5,457 invasive pneumococcal isolates from patients in Danish hospitals. Five isolates (0.09%; one from CSF in 1996, three from blood in 1996, and one from blood in 1997) were not included due to lack of information regarding age of the patients. Of the remaining 5,452 isolates, 2,701 (49.5%) came from male patients and 2,748 (50.5%) were from female patients. The gender of the patient was unknown for three isolates. As shown in Table 1, 4,963 (91%) of the isolates came from blood, and 489 (9%) were from CSF. The total number of isolates rose from 1995 and 1996 to 1,410 and fell during the rest of the period to 877 isolates in 1999. This fall was due mainly to a decrease in the number of blood isolates since the number of CSF isolates remained relatively constant. However, since 1996 we found a tendency toward an increase in the proportion of CSF isolates from 7.3% of all invasive isolates in 1996 to 11.6% in 1999. During the whole study period, 478 (8.8%) of the isolates came from children younger than 15 years old and, among these, 65.2% were isolated from children young than 2 years old. The remaining 91.2% of isolates came from people more than 14 years of age. The percentage of strains isolated from children varied from 7.2 to 10.2% during the study period, with no general trend of increase or decrease. The annual rates of invasive pneumococcal infections among 100,000 inhabitants per year in Denmark are shown in Table 1. The overall incidence increased in the period from 1995 to 1996 to 27/100,000/year and decreased to 17/100,000/year in 1999, i.e., a fall of 37%.
TABLE 1.
Number of isolates from blood and CSF obtained from subjects in three age groups (<2, 2 to 14, and >14 years of age) in Denmark from 1995 to 1999
| Yr | No. of isolates (%)
|
No. of isolates (%) in subjects of age:
|
Incidence/100,000 inhabitants | ||||
|---|---|---|---|---|---|---|---|
| Total | Blood | CSFa | <2 yr | 2-14 yr | >14 yr | ||
| 1995 | 1,072 | 978 (91.2) | 94 (8.8) | 70 (6.5) | 28 (2.7) | 974 (90.8) | 21 |
| 1996 | 1,410 | 1,307 (92.7) | 103 (7.3) | 79 (5.6) | 52 (3.7) | 1,279 (90.7) | 27 |
| 1997 | 1,146 | 1,041 (90.9) | 105 (9.1) | 56 (4.9) | 36 (3.1) | 1,054 (92.0) | 22 |
| 1998 | 947 | 861 (90.8) | 86 (9.2) | 45 (4.8) | 23 (2.4) | 879 (92.8) | 18 |
| 1999 | 877 | 776 (88.4) | 101 (11.6) | 61 (6.9) | 28 (3.3) | 788 (89.8) | 17 |
| Total | 5,452 | 4,963 (91.0) | 489 (9.0) | 311 (5.7) | 167 (3.1) | 4,974 (91.2) | |
If there was both a blood and a CSF isolate from the same patient obtained within 1 month, this was registered only as a CSF isolate.
Figure 1 presents the age-specific incidences and gender of patients with invasive pneumococcal infections in Denmark from 1995 to 1999. The age-specific incidence is highest among children younger than 2 years and among elderly people. Below the age of 60 years, the age distribution demonstrates a higher proportion of cases among males. A higher proportion of females is found among patients older than 60 years.
FIG. 1.
Age-specific incidences of invasive pneumococcal infections in Denmark from 1995 to 1999. For each pair of columns, the first column reflects the number of cases from male patients and the second column shows the number of cases from female patients.
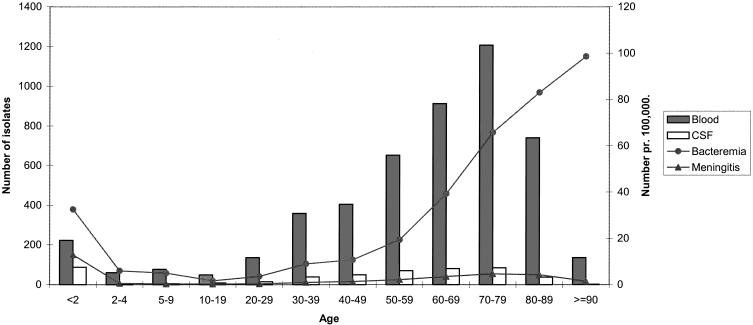
Figure 2 shows the number of blood and CSF isolates in the different age groups during the 5-year period. According to the findings shown in Fig. 1, most blood and CSF isolates were found among children below 2 years of age and among elderly people. The age-specific incidences of bacteraemia and meningitis are also included. The incidence of meningitis was highest among children below the age of 2 years, whereas the highest incidence of bacteremia was found among the elderly.
FIG. 2.
Age-specific incidences of pneumococcal bacteremia and meningitis in Denmark from 1995 to 1999. A total of 5,452 invasive pneumococcal isolates are shown distributed by site of isolation.
Table 2 shows the 23 most common serotypes isolated from all patients and from three age groups: children younger than 2 years old, 2- to 59-year-old people, and people older than 60 years. The types marked with an asterisk are the types included in the 23-valent vaccine, and two asterisks mark the types also included in the 7-valent vaccine.
TABLE 2.
The 23 most frequent serotypes found among invasive pneumococcal isolates from three age groups in Denmark from 1995 to 1999a
| Order of frequency | <2 yr
|
2-59 yr
|
≥60 yr
|
All age groups
|
Cum. %b | ||||
|---|---|---|---|---|---|---|---|---|---|
| Type | No. | Type | No. | Type | No. | Type | No. | ||
| 1 | 14** | 56 | 1* | 509 | 1* | 421 | 1* | 939 | 17.2 |
| 2 | 6B** | 55 | 4** | 166 | 4** | 328 | 4** | 507 | 26.5 |
| 3 | 19F** | 33 | 12F* | 164 | 14** | 325 | 14** | 468 | 35.1 |
| 4 | 23F** | 21 | 7F* | 102 | 12F* | 234 | 12F* | 410 | 42.6 |
| 5 | 18C** | 19 | 9V** | 91 | 3* | 196 | 7F* | 275 | 47.6 |
| 6 | 7F* | 17 | 14** | 87 | 9V** | 167 | 9V** | 271 | 52.6 |
| 7 | 4** | 13 | 8* | 86 | 8* | 167 | 3* | 268 | 57.5 |
| 8 | 6A | 13 | 3* | 70 | 7F* | 156 | 8* | 256 | 62.2 |
| 9 | 9V** | 13 | 5* | 67 | 23F** | 127 | 6B** | 200 | 65.9 |
| 10 | 12F* | 12 | 6B** | 55 | 9N* | 120 | 23F** | 194 | 69.4 |
| 11 | 1* | 9 | 18C** | 54 | 6A | 110 | 9N* | 171 | 72.6 |
| 12 | 24F | 6 | 9N* | 47 | 22F* | 99 | 19F** | 168 | 75.7 |
| 13 | 38 | 5 | 19F** | 46 | 19F** | 89 | 6A | 161 | 78.6 |
| 14 | 22F* | 5 | 22F* | 46 | 6B** | 90 | 22F* | 150 | 81.4 |
| 15 | 9N* | 4 | 23F** | 46 | 19A* | 70 | 5* | 115 | 83.5 |
| 16 | 15C | 3 | 6A | 38 | 38 | 54 | 18C** | 112 | 85.5 |
| 17 | 3* | 2 | 11A* | 32 | 20* | 49 | 19A* | 101 | 87.4 |
| 18 | 5* | 2 | 20* | 30 | 5* | 46 | 20* | 81 | 88.9 |
| 19 | 20* | 2 | 19A* | 25 | 33F* | 45 | 11A* | 73 | 90.2 |
| 20 | 33F* | 2 | 10A* | 15 | 11A* | 40 | 38 | 72 | 91.5 |
| 21 | 34 | 2 | 24F | 15 | 18C** | 39 | 24F | 60 | 92.6 |
| 22 | ROUGH | 2 | 35F | 15 | 24F | 39 | 33F* | 60 | 93.7 |
| 23 | 10A* | 1 | 16F | 14 | 16F | 37 | 16F | 51 | 94.7 |
| Total | 297 | 1,820 | 3,048 | 5,163 | 94.7 | ||||
| No. of types not in 23-valent vaccine (no. of isolates) | 4 (16) | 3 (44) | 3 (130) | 4 (190) | |||||
| No. of types included in the 23-valent vaccine and not among the 23 most frequent types (no. of isolates) | 0 | 3 (28) | 4 (57) | 4 (85) | |||||
| Total no. of invasive isolates: | 311 | 1,938 | 3,203 | 5,452 | |||||
*, Type included in the 23-valent vaccine; **, type included in the 23-valent and 7-valent vaccines.
Cumulative relative frequency (percentage) of all (5,452) invasive isolates.
The age-specific serotype coverage rates of the 23-valent pneumococcal vaccine for the three age groups were 92.9, 93.7, and 92.9%. The overall serotype coverage rate was 92.9%. These calculations include cases caused by type 6A due to the cross-protection offered by antibodies against type 6B. The types not included in the 23-valent vaccine but among the 23 most common types are types 15C, 16F, 24F, 34, and 38. These constituted 3.5% of all of the invasive infections in the study period. Types included in the vaccine but which are not among the 23 most common types in the population are types 2, 10A, 15B, and 17F. These are the cause of 1.6% of all cases.
The serotype coverage rates of the 7-valent conjugate vaccine in the three age-groups were 71.7, 30.1, and 39.8% (cases caused by type 6A were included in the calculations). Adding types 1 and 5 to the 9-valent vaccine would increase the serotype coverage rates in the three age groups to 75.2, 59.8, and 54.4%, respectively, and adding types 3 and 7F to the 11-valent vaccine would raise the coverage rates to 81.4, 68.7, and 65.4%, respectively.
Table 3 shows both the 23 most prevalent pneumococcal types that caused invasive disease in Denmark from 1995 to 1999 and also the percentage of cases of invasive disease caused by each type. Major changes have occurred during the period for types 1 and 12F. Type 1 caused 26.5% of all invasive pneumococcal infections in Denmark in 1996, whereas in 1999 it was responsible for only 5.4% of the cases. Type 12F was responsible for 10.6% of all invasive cases in 1995 but for only 4.8% in 1999. For the remaining types, a small rise in frequency was observed for types 6A, 8, 9N, 9V, 14, 22F, and 23F, and a small decrease was found for types 4, 5, 16F, and 38. Finally, no change was observed for types 3, 6B, 7F, 11A, 18C, 19A, 19F, 20, 24F, and 33F. The decline in the number of cases caused by type 1 corresponds to the decline of the total number of invasive cases.
TABLE 3.
Distribution by year of the 23 most frequent serotypes among invasive pneumococcal isolates in Denmark from 1995 to 1999
| Order of frequencya | Type | 1995
|
1996
|
1997
|
1998
|
1999
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % of total | No. | % of total | No. | % of total | No. | % of total | No. | % of total | ||
| 1 | 1 | 146 | 13.61 | 374 | 26.49 | 253 | 22.08 | 119 | 12.57 | 47 | 5.35 |
| 2 | 4 | 116 | 11.00 | 132 | 9.35 | 93 | 8.12 | 86 | 9.08 | 78 | 8.87 |
| 3 | 14 | 89 | 8.29 | 108 | 7.65 | 83 | 7.24 | 101 | 10.67 | 88 | 10.01 |
| 4 | 12F | 114 | 10.62 | 112 | 7.93 | 83 | 7.24 | 59 | 6.23 | 42 | 4.78 |
| 5 | 7F | 65 | 6.06 | 51 | 3.61 | 57 | 4.97 | 38 | 4.01 | 64 | 7.28 |
| 6 | 9V | 41 | 3.82 | 60 | 4.25 | 54 | 4.71 | 61 | 6.44 | 55 | 6.26 |
| 7 | 3 | 56 | 5.22 | 58 | 4.11 | 57 | 4.97 | 45 | 4.75 | 52 | 5.92 |
| 8 | 8 | 35 | 3.26 | 51 | 3.61 | 60 | 5.24 | 57 | 6.02 | 53 | 6.03 |
| 9 | 6B | 52 | 4.85 | 44 | 3.12 | 38 | 3.32 | 30 | 3.17 | 36 | 4.10 |
| 10 | 23F | 24 | 2.24 | 39 | 2.76 | 49 | 4.28 | 46 | 4.86 | 37 | 4.21 |
| 11 | 9N | 28 | 2.61 | 35 | 2.48 | 27 | 2.36 | 47 | 4.96 | 34 | 3.87 |
| 12 | 19F | 28 | 2.61 | 46 | 3.26 | 41 | 3.58 | 22 | 2.32 | 32 | 3.64 |
| 13 | 6A | 23 | 2.14 | 38 | 2.69 | 29 | 2.53 | 39 | 4.12 | 32 | 3.64 |
| 14 | 22F | 29 | 2.70 | 26 | 1.84 | 29 | 2.53 | 26 | 2.75 | 40 | 4.55 |
| 15 | 5 | 36 | 3.36 | 38 | 2.69 | 18 | 1.57 | 6 | 0.63 | 17 | 1.93 |
| 16 | 18C | 22 | 2.05 | 27 | 1.91 | 22 | 1.92 | 15 | 1.58 | 26 | 2.96 |
| 17 | 19A | 18 | 1.68 | 25 | 1.77 | 20 | 1.75 | 21 | 2.22 | 17 | 1.93 |
| 18 | 20 | 15 | 1.40 | 20 | 1.42 | 15 | 1.31 | 19 | 2.01 | 12 | 1.37 |
| 19 | 11A | 13 | 1.21 | 16 | 1.13 | 18 | 1.57 | 15 | 1.58 | 11 | 1.25 |
| 20 | 38 | 26 | 2.42 | 21 | 1.49 | 7 | 0.61 | 5 | 0.53 | 13 | 1.48 |
| 21 | 24F | 10 | 0.93 | 12 | 0.85 | 16 | 1.40 | 8 | 0.84 | 14 | 1.59 |
| 22 | 33F | 10 | 0.93 | 14 | 0.99 | 7 | 0.61 | 19 | 2.01 | 10 | 1.14 |
| 23 | 16F | 17 | 1.58 | 10 | 0.71 | 12 | 1.05 | 8 | 0.84 | 4 | 0.46 |
| Subtotal | 1.015 | 94.68 | 1.357 | 96.24 | 1.088 | 94.94 | 892 | 94.19 | 814 | 92.82 | |
| Total | 1.072 | 1.410 | 1.146 | 947 | 877 | ||||||
Total, total number of invasive strains per year.
Table 4 shows the order of frequency of the 23 most common pneumococcal serotypes isolated from blood and/or CSF from patients of all ages from 1995 to 1999 in Denmark. Serotype 1 was found significantly more often in blood isolates than in CSF isolates, and types 6B, 12F,19F, and 24F were found more frequently among CSF isolates. Type 1 was represented in 9.7% of all CSF isolates in 1997 and in 1% in 1999; it was found in 27.9% of all blood isolates in 1996 compared to 5.9% in 1999.
TABLE 4.
Pneumococcal types isolated from blood and/or CSF from patients of all ages in Denmark from 1995 to 1999
| Order of frequency | Type | CSF
|
Blood and CSF
|
||
|---|---|---|---|---|---|
| No. | % Totala | No. | % Total | ||
| 1 | 1 | 19 | 3.9 | 939 | 17.2a |
| 2 | 4 | 29 | 5.9d | 507 | 9.3 |
| 3 | 14 | 33 | 6.7c | 468 | 8.6 |
| 4 | 12F | 51 | 10.4bd | 410 | 7.5 |
| 5 | 7F | 32 | 6.5 | 275 | 5.0 |
| 6 | 9V | 26 | 5.3 | 271 | 5.0 |
| 7 | 3 | 26 | 5.3d | 268 | 4.9 |
| 8 | 8 | 22 | 4.5d | 256 | 4.7 |
| 9 | 6B | 30 | 6.1bc | 200 | 3.7 |
| 10 | 23F | 14 | 2.9 | 194 | 3.6 |
| 11 | 9N | 14 | 2.9d | 171 | 3.1 |
| 12 | 19F | 33 | 6.7bc | 168 | 3.1 |
| 13 | 6A | 16 | 3.3 | 161 | 3.0 |
| 14 | 22F | 16 | 3.3 | 150 | 2.8 |
| 15 | 5 | 4 | 0.8 | 115 | 2.1 |
| 16 | 18C | 19 | 3.9c | 112 | 2.1 |
| 17 | 19A | 6 | 1.2 | 101 | 1.9 |
| 18 | 20 | 8 | 1.6 | 81 | 1.5 |
| 19 | 11A | 8 | 1.6 | 73 | 1.3 |
| 20 | 38 | 4 | 0.8 | 72 | 1.3 |
| 21 | 24F | 12 | 2.5b | 60 | 1.1 |
| 22 | 33F | 6 | 1.2 | 60 | 1.1 |
| 23 | 16F | 1 | 0.2 | 51 | 0.9 |
| Subtotal | 429 | 5,163 | |||
| Other types | 60 | 289 | |||
| Total | 489 | 5,452 | |||
Superscript letters indicate significance as follows: a, found significantly more often in blood isolates than in CSF (P < 0.001); b, found significantly more often in CSF than in blood isolates (P < 0.001); c, found significantly more often in children ≤14 years old (P < 0.001); d, found significantly more often in adults >14 years old (P < 0.001).
Among children ≤14 years of age, types 1, 14, 6B, 19F, 18C, and 23F were found significantly more often (P < 0.001) than the other types; among adults >14 years, types 1, 4, 14, 12F, 3, and 19F were the most common. Some types were found significantly more frequently (P < 0.001) in children than in adults. These were types 6B, 14, 18C, and 19F. Types 3, 4, 8, 9N, and 12F were significantly more common among adults (P < 0.001).
Table 5 shows antibiotic susceptibility data of the invasive pneumococcal strains to penicillin and erythromycin for the 5-year period. The number of isolates with an MIC of penicillin of >0.064 and <2 μg/ml increased from 5 (0.5%) isolates in 1995 to 28 (3.2%) in 1999. The number of strains for which the MIC of penicillin was ≥2 μg/ml (MICs of 1.5 μg/ml or higher measured by E-test are included in this group) remained almost constant at ca. 0.5%. The MICs of penicillin were 4 μg/ml for one isolate, 3 μg/ml for one isolate, and 2 μg/ml for the remaining isolates. Among the isolates with reduced sensitivity to penicillin, only very few strains (two to three per year) also had reduced sensitivity to erythromycin. In contrast, among the penicillin-susceptible isolates, the number of isolates with reduced sensitivity to erythromycin rose from 6 (0.6%) to 31 (3.5%) in 1999. All of these isolates were resistant to erythromycin (MIC of ≥4 μg/ml). Only a few strains were multiresistant, i.e., had reduced sensitivity to three or more groups of antibiotics, namely, between one and eight isolates (0.1 to 0.9%) per year. Most of these isolates are intermediately resistant to penicillin (MIC of >0.064 and <2 μg/ml). Only few strains were resistant to tetracyclin, chloramphenicol, and ceftriaxone, i.e., <0.5% in each group, and there was no increase of this number. The number of isolates with reduced sensitivity to trimethoprim-sulfamethoxazole increased from 0.3% in 1995 to 2.3% in 1999.
TABLE 5.
Invasive pneumococcal isolates with reduced sensitivity to penicillin and/or erythromycin in Denmark from 1995 to 1999
| Yr | No. of isolates (%)a
|
||||
|---|---|---|---|---|---|
| Penicillin (MIC >0.064 and <2 μg/ml) | Penicillin (MIC ≥2 μg/ml) | Penicillin nonsusceptible and erythromycin nonsusceptible (MIC >0.5 μg/ml) (%) | Penicillin susceptible and erythromycin nonsusceptible (MIC >0.5 μg/ml) (%) | Multiply resistantb and penicillin nonsusceptible (MIC >0.064 μg/ml) (%) | |
| 1995 | 5 (0.5) | 5 (0.5) | 2 (0.2) | 6 (0.6) | 2 (0.2) |
| 1996 | 17 (1.2) | 11 (0.8) | 2 (0.1) | 8 (0.6) | 1 (0.1) |
| 1997 | 23 (2.0) | 4 (0.3) | 2 (0.2) | 11 (1.0) | 8 (0.7) |
| 1998 | 17 (1.8) | 1 (0.1) | 2 (0.2) | 24 (2.5) | 1 (0.1) |
| 1999 | 28 (3.2) | 3 (0.3) | 3 (0.3) | 31 (3.5) | 8 (0.9) |
The percentage of all invasive isolates is given in parentheses.
That is, showing reduced susceptibility to three or more groups of antibiotics.
Table 6 shows the order of frequency of the serotypes of the 114 invasive pneumococcal isolates with reduced susceptibility to penicillin from the three age groups in Denmark from 1995 to 1999.
TABLE 6.
Order of frequency of serotypes among the 114 penicillin-nonsusceptible (MIC >0.064 μg/ml) invasive pneumococcal isolates from three age groups in Denmark from 1995 to 1999a
| Order of frequency | <2 yr
|
2-59 yr
|
≥60 yr
|
|||
|---|---|---|---|---|---|---|
| Type | No. (%) | Type | No. (%) | Type | No. (%) | |
| 1 | 19F | 3 | 9V | 10 | 9V | 17 |
| 2 | 6B | 2 | 23F | 5 | 14 | 12 |
| 3 | 9V | 2 | 14 | 5 | 19F | 9 |
| 4 | 19A | 2 | 19F | 4 | 19A | 8 |
| 5 | 14 | 1 | 5 | 3 | 15B | 4 |
| 6 | 34 | 1 | 15B | 3 | 6A | 4 |
| 7 | Rough | 1 | 6B | 2 | 35F | 2 |
| 8 | 15C | 2 | 5 | 2 | ||
| 9 | 19A | 2 | 10A | 1 | ||
| 10 | 35F | 1 | 23F | 1 | ||
| 11 | 6B | 1 | ||||
| 12 | 15C | 1 | ||||
| 13 | 23B | 1 | ||||
| 14 | 9N | 1 | ||||
| 15 | Rough | 1 | ||||
| Total | 12 (10.5) | 37 (32.5) | 65 (57.0) | |||
Valves in parentheses give the percentage of the 114 penicillin-nonsusceptible (MIC >0.064 μg/ml) invasive pneumococcal isolates.
Nonsusceptible isolates found in children younger than 2 years contained only six different serotypes and a nonencapsulated (rough) strain. These isolates represent 10.5% of all of the nonsusceptible isolates. Among the 2- to 59-year-old people, nonsusceptible isolates represented 10 serotypes and constituted 32.5% of all nonsusceptible isolates. In people older than 60 years, nonsusceptible isolates represented 14 different serotypes and one rough pneumococcal strain and constituted 57.0% of the nonsusceptible isolates. The penicillin-nonsusceptible types 6B, 9V, 14,19A, and 19F were found in all three age groups.
DISCUSSION
Denmark has a population of 5.5 million people, and a nationwide surveillance of invasive pneumococccal infections has been carried out for decades. This surveillance makes it possible to monitor changes in the incidences of invasive disease, serotype distribution, and antibiotic susceptibility among the pneumococcal isolates.
Since the 1960s and until 1996, there were a constant rise of the total incidence of invasive pneumococcal infections to 27 cases per 100,000 per year. After 1996 there was a marked decrease to 17 cases per 100,000/year in 1999. The number of blood isolates fell significantly from 1,307 isolates in 1996 to 776 in 1999. This decline paralleled a decline in the percentage of invasive strains with serotype 1 from 26.5% in 1996 to 5.4% of all cases in 1999. A decrease was found in the proportion of both blood and CSF isolates representing type 1. Type 1 is a common type, but its frequency among invasive isolates has varied significantly. In 1939, type 1 caused 23.8% of all invasive pneumococcal infections in Denmark. This rate fell dramatically to only 5.3% from 1955 to 1970 (14, 15), increased to 11 to 12% from 1983 to 1994 (18, 19), and finally rose to 17.2% from 1995 to 1999. The reason for the increase of the percentage of CSF isolates during the period from 1996 to 1999 may be at least partially explained by the fact that type 1 was more frequently found in blood than in CSF, and the marked decline in the frequency of type 1 since 1996 is therefore most evident among the blood isolates. Whether the rising number of invasive cases of pneumococcal infection in the 1990s that has been observed in a number of countries, including Denmark, is due to better and more frequently used blood culturing systems has been discussed previously (24, 26). Whether the introduction of new automatic blood culturing systems in the late 1990s, some of which we found to disfavor the detection of pneumococci, could explain this observed decrease has also been considered. Arguing against the latter hypothesis are the facts that the number of CSF isolates containing type 1 also declined and that a certain number of pneumococcal types were more prevalent during the same period. Based on the results from previous studies and on those of the present study, it seems that biologic variations of the immunity in the population and/or in the pneumococcal isolates are a contributory cause of the observed changing incidences of invasive pneumococcal infections. Moreover, the introduction of new serotypes or certain clones of a serotype against which the population has low immunity may increase the number of cases with that particular clone for a period of time (20, 28).
This was recently shown in Sweden, where an increase of the overall incidence of pneumococcal bacteremia from 1987 to 1997 was ascribed to the introduction and spread of two new pneumococcal clones of types 1 and 14 (20).
The frequency of serotype 12F also fell markedly from 10.6% in 1995 to 4.8% in 1999, whereas we observed only small changes in the frequencies of the remaining 34 types. The changing serotype distribution highlights the importance of surveillance of serotypes with regard to the introduction of new conjugate pneumococcal vaccines and to the recommendations for use of the 23-valent polysaccharide vaccine. We found a high serotype coverage of the 23-valent pneumococcal vaccine in three age groups—children less than 2 years, people aged 2 to 59 years, and elderly people above 60 years—namely, 92.9, 93.7, and 92.9%. The 23-valent pneumococcal vaccine was introduced in Denmark in 1983 for use in special risk groups. Since 1996, it has been suggested for general use for elderly people, but the vaccine uptake by the elderly during the study period was relatively low, i.e., between 10 to 30,000 doses/year, which accounts for <3% of the elderly, so this finding cannot alone explain the decline in the incidence of invasive pneumococcal disease in this age group. However, according to the serotype coverage rate, the impact of vaccination against pneumococcal disease among the elderly could be significant if it was more widely used. As new pneumococcal conjugate vaccines are introduced, the 23-valent vaccine will remain relevant for older children, adults, and elderly people at risk. The 7-valent pneumococcal conjugate vaccine, which has already been introduced in the United States, would cover 71.7% of invasive pneumococcal infections in children younger than 2 years of age. Based on a 19-year (1981 to 1999) nationwide surveillance study of invasive pneumococcal infections in children in Denmark (11), we calculated the coverage rate in children ≤6 years of age to be 65.9%. This coverage rate is lower than the rate found in other countries such as the United States (3, 23).
Among the 5,452 pneumococcal isolates received by our unit, 9% were isolated from CSF. This percentage remained almost constant and was comparable to what was found in the previous period from 1989 to 1994 (19). Some serotypes are more frequent in CSF than in blood, namely, types 6B, 12F, 18C, 19F, and 24F. Types 1 and 5 are more frequently isolated from blood. Compared to the 5-year period from 1989 to 1994, the distribution of site of isolation of the particular serotypes has not changed in Denmark.
For many years, antibiotic resistance among pneumococcal isolates has been almost nonexistent in Denmark. In the 5-year period from 1989 to 1994, <1% of all invasive isolates showed reduced susceptibility to penicillin. This very low percentage of resistance among pneumococci is in contrast to the vast problems described in countries both far from and close to Denmark (7, 21, 29). In Denmark, there is a restrictive policy on the use of antibiotics, and this has been put forth as the explanation for the generally low occurrence of resistance among various microorganisms in Denmark. Probably, resistant clones are frequently introduced into Denmark. This does not seem to be significant as long as the antibiotic pressure in the population is low. However, the results of the present study show an increasing occurrence of antibiotic resistance among pneumococcal isolates. The percentage of isolates with reduced susceptibility to penicillin has increased four times from 0.9% in 1995 to 3.5% in 1999, and the percentage of isolates with reduced susceptibility to erythromycin among the penicillin susceptible strains has grown from 0.6% in 1995 to 3.5% in 1999. The number of strains with reduced susceptibility to trimethoprim-sulfa has also increased, whereas the percentage of strains with reduced susceptibility to the other antibiotics tested was constant ca. 0.5%. Thus, although the number of pneumococcal isolates with reduced susceptibility to antibiotics is still low in Denmark, an increase has been observed, and it seems more important than ever to keep a restrictive antibiotic policy.
Several studies have shown that the highest proportion of resistant pneumococcal isolates was found among children and especially among children attending day care centers. This raises the question of whether there is, among children in daycare centers, an important reservoir of resistant pneumococci that could spread to the rest of the population. However, we found that only 10.5% of the invasive isolates with reduced susceptibility to penicillin was isolated from children younger than 2 years of age, whereas 57.0% came from persons older than 60 years. Seven different serotypes were detected in the nonsusceptible isolates from children, whereas fifteen serotypes were detected in the nonsusceptible isolates from the elderly. The serotypes detected correspond to the serotypes of penicillin-nonsusceptible pneumococci described internationally. Preliminary results from an ongoing study in Denmark have shown that, although there seems to be a high carriage rate of pneumococci in day care centers for children, only a few percent of these are penicillin nonsusceptible (M. S. Kaltoft and H. B. Konradsen, Abstr. 19th Annu. Meet. Eur. Soc. Pediatr. Infect. Dis., abstr. 65, 2001). This means that most often it is elderly people who are infected with penicillin-nonsusceptible pneumococcal isolates in Denmark. Since the majority of the penicillin-nonsusceptible serotypes are included in the 23-valent pneumococcal vaccine, it seems relevant and important to offer vaccination with the 23-valent pneumococcal vaccine to this risk group.
REFERENCES
- 1.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety, and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 2.Butler, J. C., J. Hofmann, M. S. Cetron, J. A. Elliott, R. R. Facklam, and R. F. Breiman. 1996. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance. Syst. J. Infect. Dis. 174:986-993. [DOI] [PubMed]
- 3.Butler, J. C., R. F. Breiman, H. B. Lipman, J. Hofmann, and R. R. Facklam. 1995. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978-1994: implications for development of a conjugate vaccine. J. Infect. Dis. 171:885-889. [DOI] [PubMed] [Google Scholar]
- 4.Colman, G., E. M. Cooke, B. D. Cookson, P. G. Cooper, A. Efstratiou, and R. C. George. 1998. Pneumococci causing invasive disease in Britain 1982-1990. J. Med. Microbiol. 47:17-27. [DOI] [PubMed] [Google Scholar]
- 5.de Neeling, A. J., W. van Pelt, C. Hol, E. E. Ligtvoet, L. J. Sabbe, A. Bartelds, and J. D. van Embden. 1999. Temporary increase in incidence of invasive infection due to Streptococcus pneumoniae in The Netherlands. Clin. Infect. Dis. 29:1579-1580. [DOI] [PubMed] [Google Scholar]
- 6.Fedson, D. S., J. Anthony, and G. Scott. 1999. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine 17(Suppl. 1):S11-S18. [DOI] [PubMed] [Google Scholar]
- 7.Fenoll, A., I. Jado, D. Vicioso, A. Perez, and J. Casal. 1998. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990 to 1996). J. Clin. Microbiol. 36:3447-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakenbeck, R., P. Reichmann, and C. Sibold. 1994. Evolution and spread of beta-lactam resistant Streptococcus pneumoniae. Klin. Lab. 3:230-235.
- 9.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use. Part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 10.Hedlund, J., S. B. Svenson, M. Kalin, J. Henrichsen, B. Olsson-Liljequist, G. Mollerberg, and G. Kallenius. 1995. Incidence, capsular types, and antibiotic susceptibility of invasive Streptococcus pneumoniae in Sweden. Clin. Infect. Dis. 21:948-953. [DOI] [PubMed] [Google Scholar]
- 11.Kaltoft, M. S., N. Zeuthen, and H. B. Konradsen. 2001. Epidemiology of invasive pneumococcal infections in children aged 0 to 6 years in Denmark: a 19-year nationwide surveillance study. Acta Paediatr. Suppl. 89:3-10. [DOI] [PubMed] [Google Scholar]
- 12.Kamme, C., K. Ekdahl, and S. Molstad. 1999. Penicillin-resistant pneumococci in southern Sweden, 1993-1997. Microb. Drug Resist. 5:31-36. [DOI] [PubMed] [Google Scholar]
- 13.Klein, J. O. 1981. The epidemiology of pneumococcal disease in infants and children. Rev. Infect. Dis. 3:246-253. [DOI] [PubMed] [Google Scholar]
- 14.Lund, E. 1970. Types of pneumococci found in blood, spinal fluid, and pleural exudate during a period of 15 years (1954-1969). Acta Pathol. Microbiol. Scand. B Microbiol. Immunol. 78:333-336. [DOI] [PubMed] [Google Scholar]
- 15.Lund, E., and J. Henrichsen. 1978. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae, p. 241-262. In T. Bergan and J. Norris (ed.), Methods in microbiology. Academic Press, Inc., New York, N.Y.
- 16.McGee, L., K. Klugman, and A. Tomasz. 2000. Serotypes and clones of antibiotic-resistant pneumococci, p. 375-379. In A. Tomasz (ed.), Streptococcus pneumoniae: molecular biology and mechanisms of disease. Liebert, New York, N.Y.
- 17.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial susceptibility testing. Ninth informational supplement. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Nielsen, S. V., and J. Henrichsen. 1993. Capsular types and susceptibility to penicillin of pneumococci isolated from cerebrospinal fluid or blood in Denmark,1983-1988. Scand. J. Infect. Dis. 25:165-170. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen, S. V., and J. Henrichsen. 1996. Incidence of invasive pneumococcal disease and distribution of capsular types of pneumococci in Denmark, 1989-94. Epidemiol. Infect. 117:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordmark, B. H., M. Kalin, A. Örtqvist, et al. 2001. Dynamics of penicillin-susceptible clones in invasive pneumococcal disease. J. Infect. Dis. 184:861-869. [DOI] [PubMed] [Google Scholar]
- 21.Pantosti, A., F. D'Ambrosio, A. Tarasi, S. Recchia, G. Orefici, and P. Mastrantonio. 2001. Antibiotic susceptibility and serotype distribution of Streptococcus pneumoniae causing meningitis in Italy, 1997-1999. Clin. Infect. Dis. 31:1373-1379. [DOI] [PubMed] [Google Scholar]
- 22.Robbins, J. B., R. Austrian, C. J. Lee, S. C. Rastogi, G. Schiffman, J. Henrichsen, P. H. Makela, C. V. Broome, R. R. Facklam, R. H. Tiesjema, et al. 1983. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J. Infect. Dis. 148:1136-1159. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph, K. M., A. J. Parkinson, A. L. Reasonover, L. R. Bulkow, D. J. Parks, and J. C. Butler. 2001. Serotype distribution and antimicrobial resistance patterns of invasive isolates of Streptococcus pneumoniae: Alaska, 1991-1998. J. Infect. Dis. 182:490-496. [DOI] [PubMed] [Google Scholar]
- 24.Schonheyder, H. C., H. T. Sorensen, B. Kristensen, and B. Korsager. 1997. Reasons for increase in pneumococcal bacteraemia. Lancet 349:1554.. [DOI] [PubMed] [Google Scholar]
- 25.Scott, J. A., A. J. Hall, R. Dagan, J. M. Dixon, S. J. Eykyn, A. Fenoll, M. Hortal, L. P. Jette, J. H. Jorgensen, F. Lamothe, C. Latorre, J. T. Macfarlane, D. M. Shlaes, L. E. Smart, and A. Taunay. 1996. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin. Infect. Dis. 22:973-981. [DOI] [PubMed] [Google Scholar]
- 26.Smith, M. D., J. Stuart, N. J. Andrews, W. A. Telfer-Brunton, and K. A. Cartwright. 1998. Invasive pneumococcal infection in South and West England. Epidemiol. Infect. 120:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sniadack, D. H., B. Schwartz, H. Lipman, J. Bogaerts, J. C. Butler, R. Dagan, G. Echaniz-Aviles, N. Lloyd Evans, A. Fenoll, N. I. Girgis, et al. 1995. Potential interventions for the prevention of childhood pneumonia: geographic and temporal differences in serotype and serogroup distribution of sterile site pneumococcal isolates from children--implications for vaccine strategies. Pediatr. Infect. Dis. J. 14:503-510. [PubMed] [Google Scholar]
- 28.Vilhelmsson, S. E., A. Tomasz, and K. G. Kristinsson. 2001. Molecular evolution in a multidrug-resistant lineage of Streptococcus pneumoniae: emergence of strains belonging to the serotype 6B Icelandic clone that lost antibiotic resistance traits. J. Clin. Microbiol. 38:1375-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, and A. Schuchat. 2001. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]