Abstract
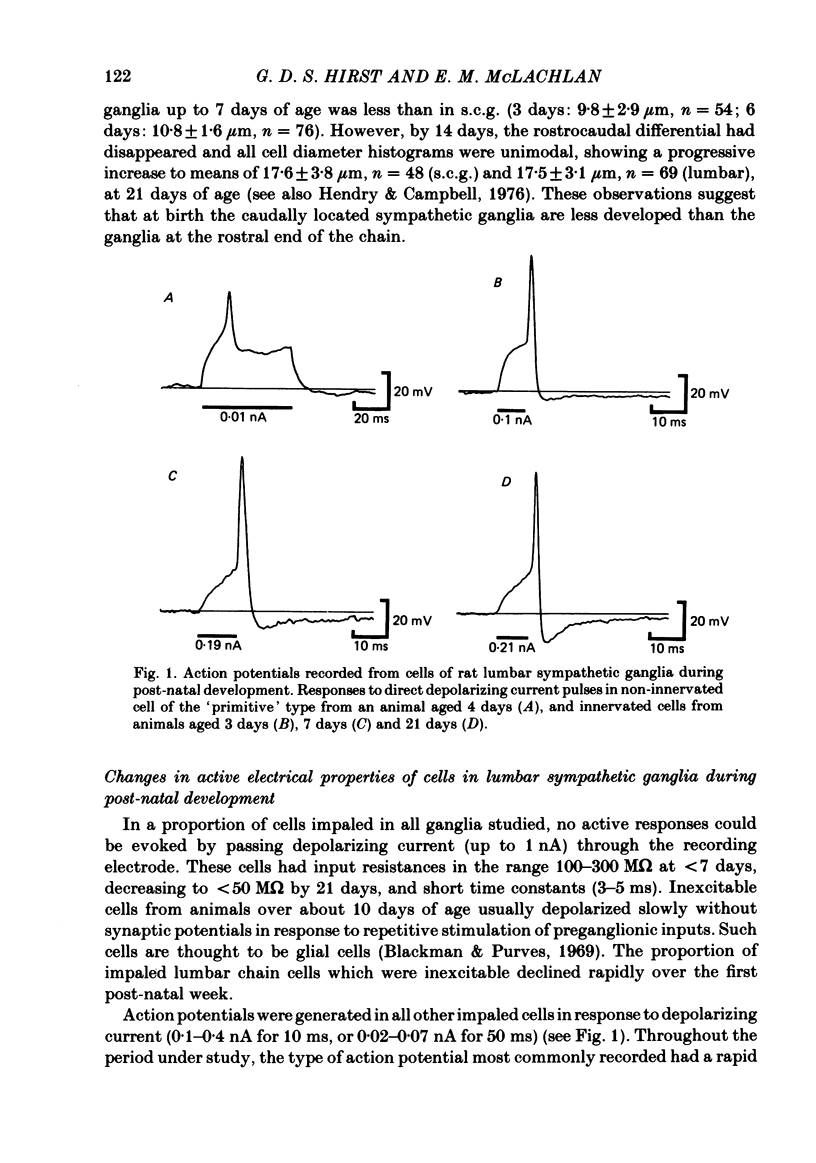
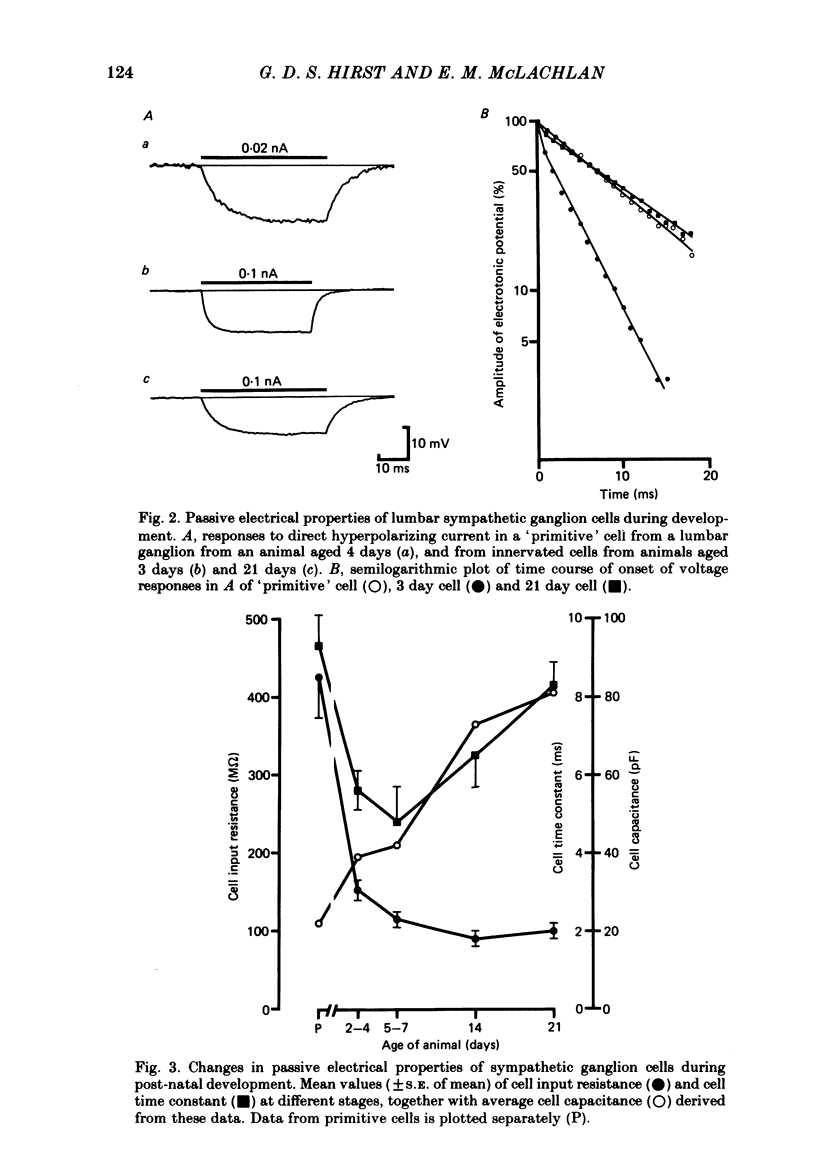
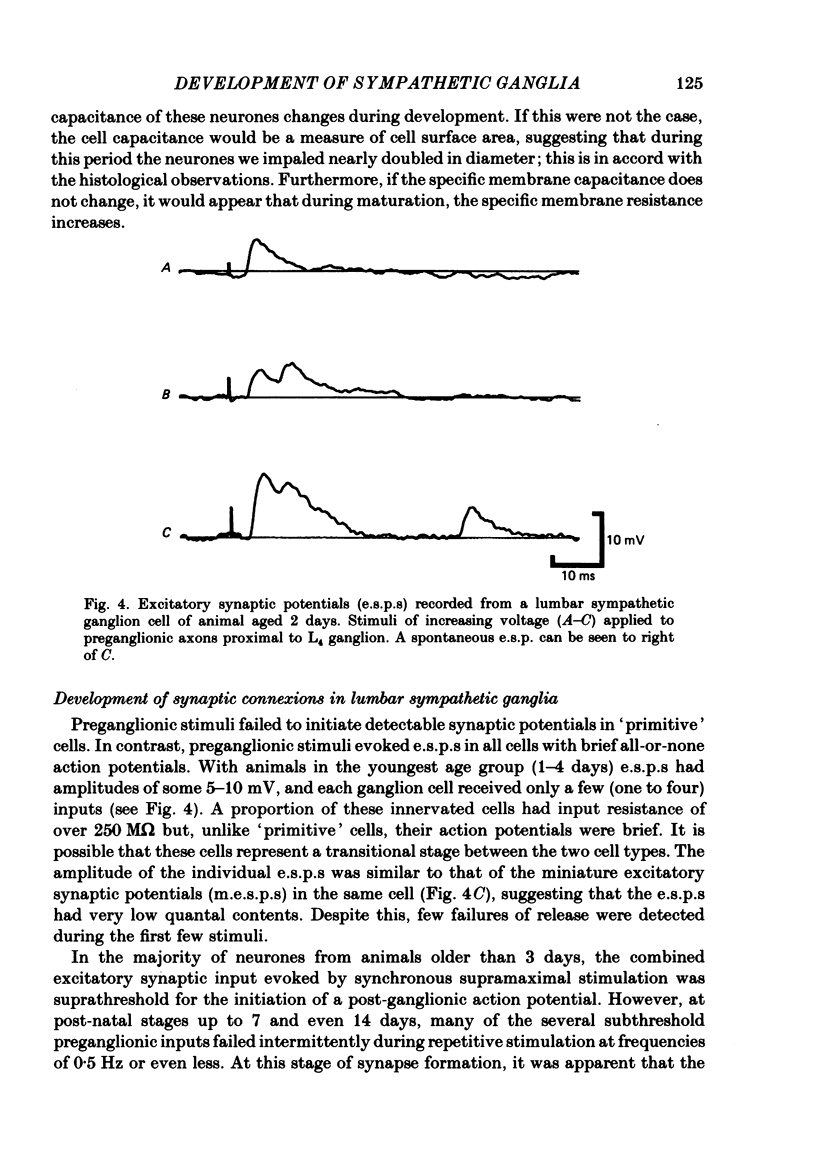
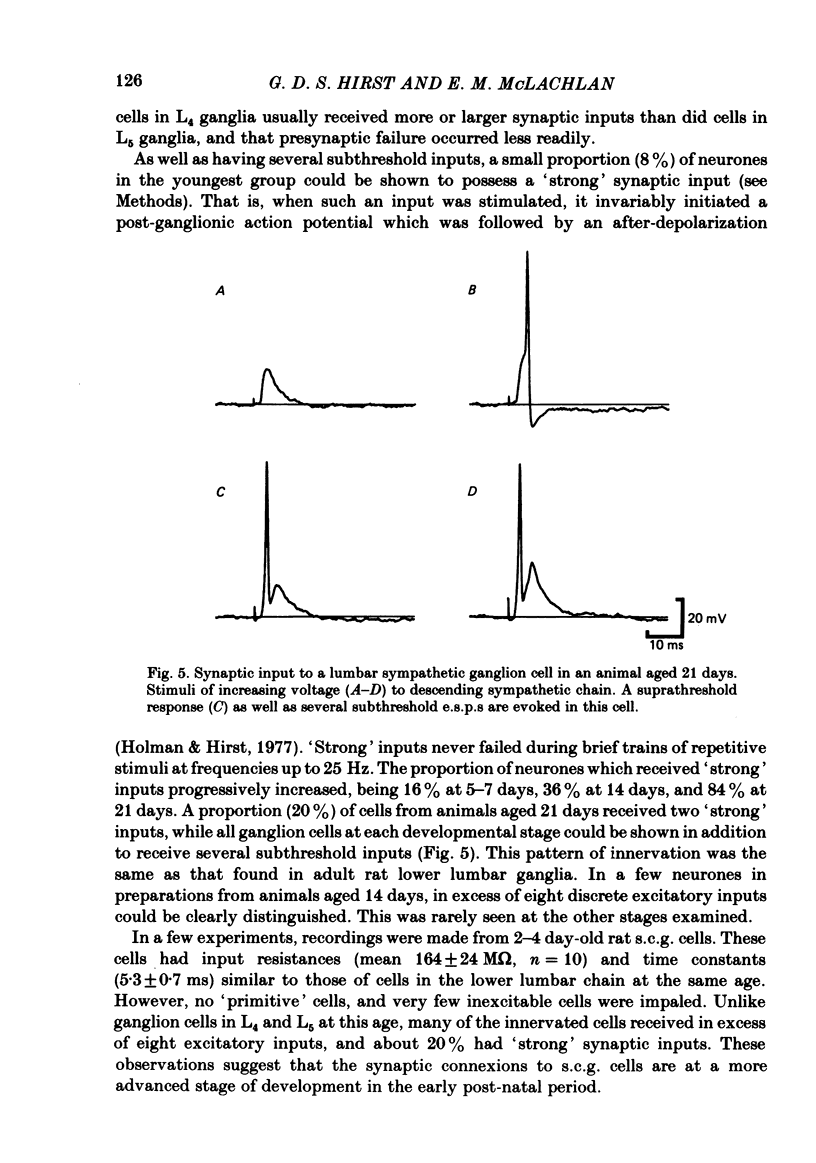
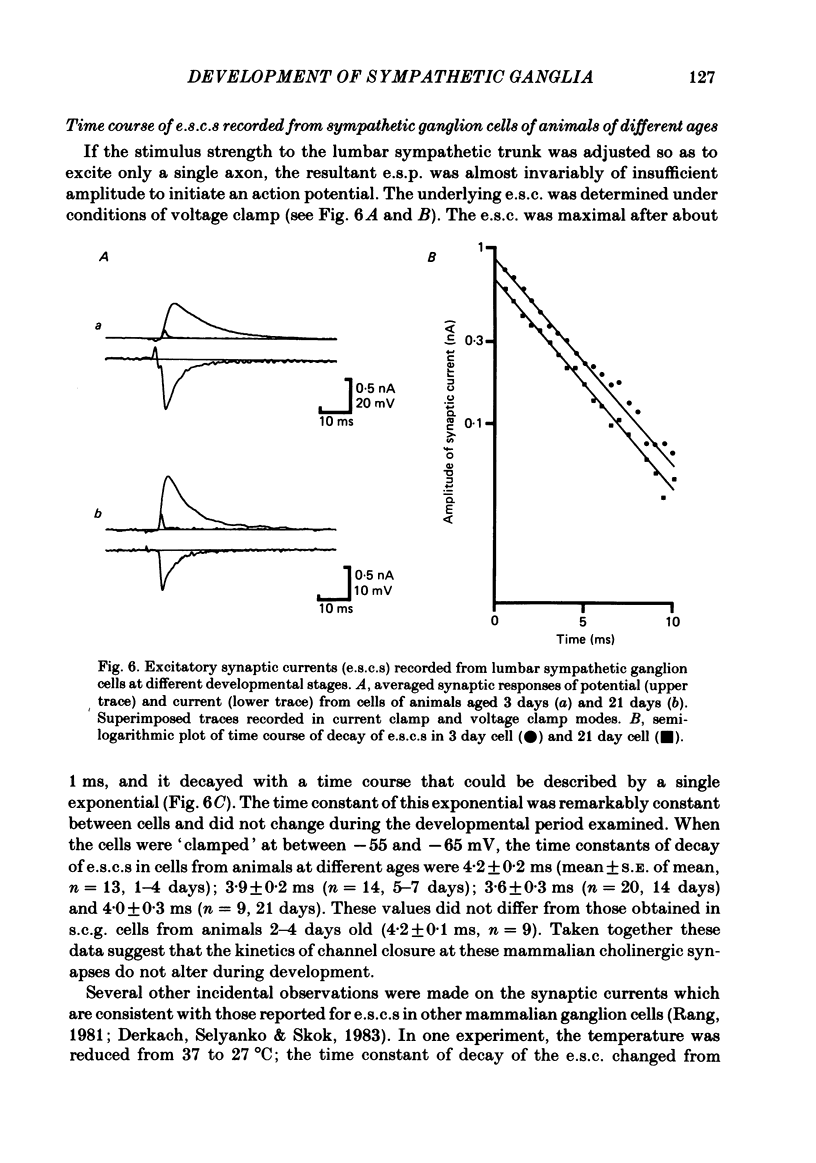
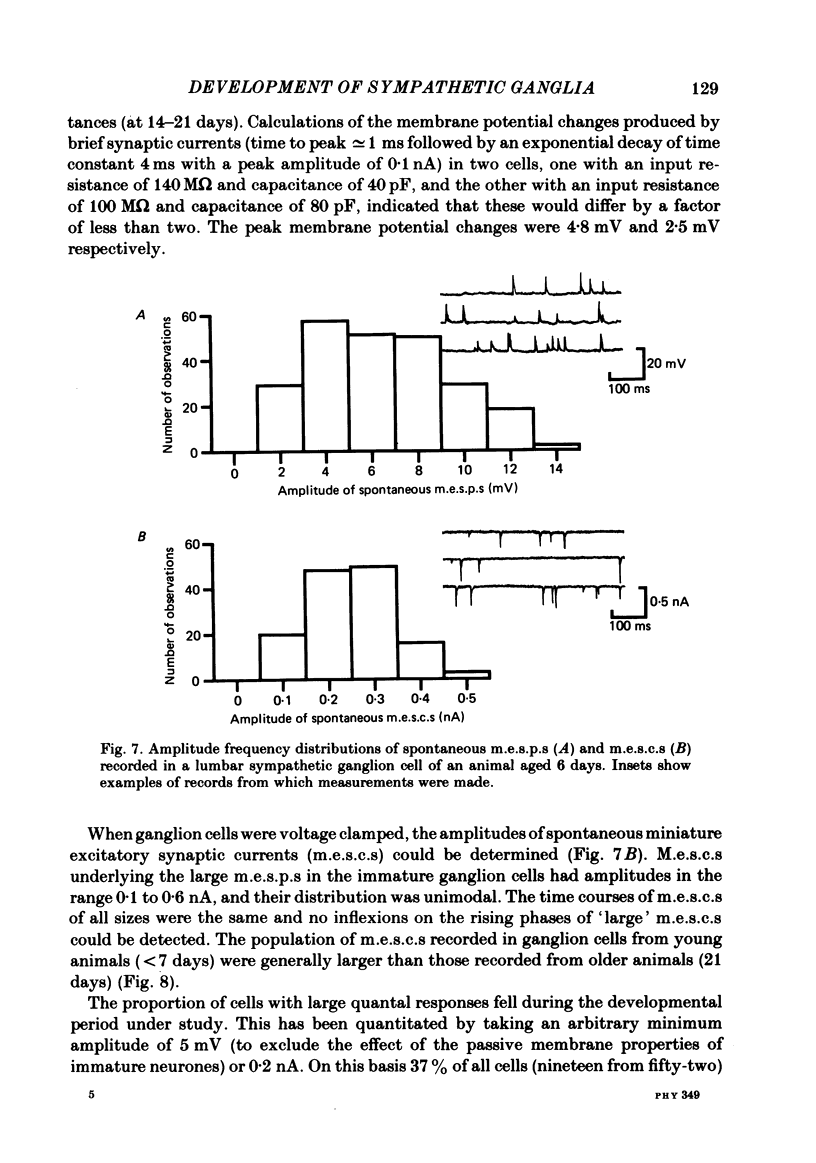
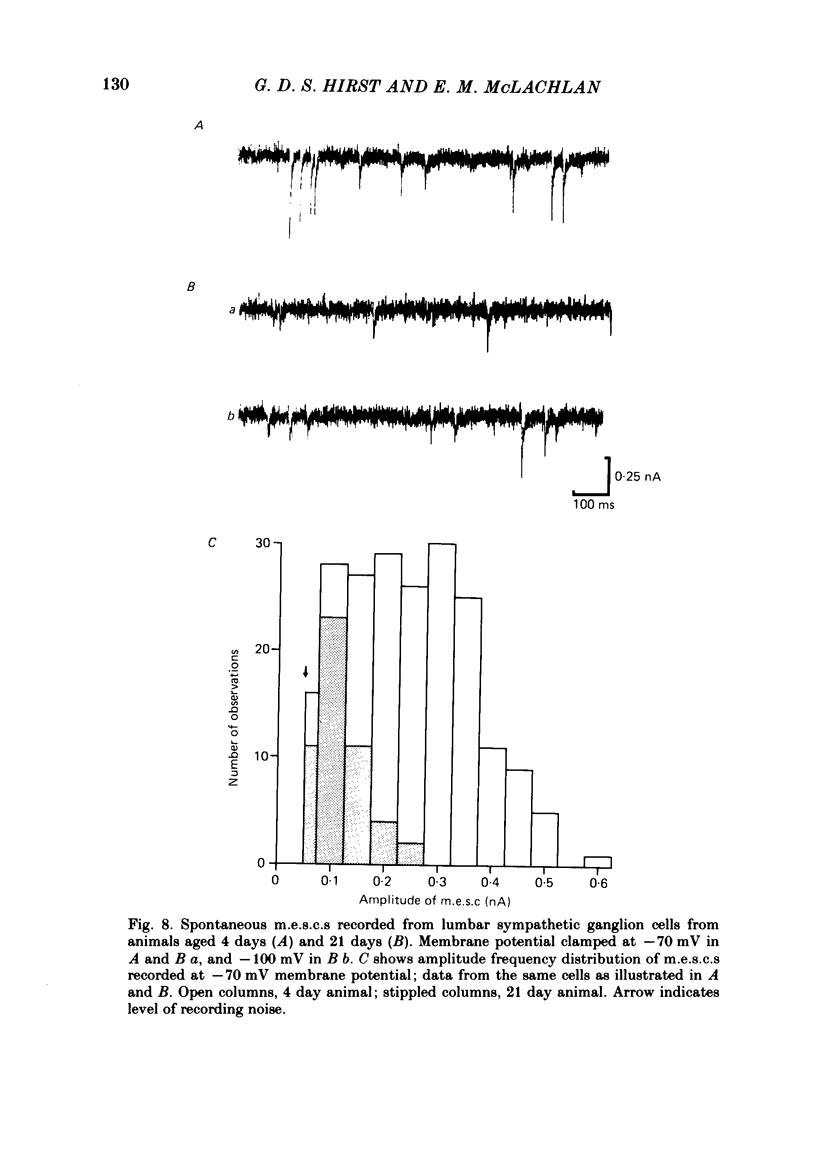
The initial stages in the development of functional synapses have been examined in ganglia of the lower lumbar sympathetic chain of the rat using intracellular recording techniques. In animals of age up to 7 days post-natal, many impaled cells were inexcitable and possessed no synaptic input. The proportion of excitable cells impaled increased with the age of the animal. Two types of action potential could be identified. Initially the synaptic input consisted of one or a few subthreshold synaptic potentials. The number of preganglionic inputs to each cell increased over the first 1-2 weeks after birth. The quantal content of each input was initially very low. At least some inputs showed an increase in quantal content during development; eventually one or occasionally two inputs became suprathreshold. Voltage-clamp studies indicated that the time course of excitatory synaptic currents did not change during development. The amplitudes of miniature excitatory synaptic currents in animals aged less than 10 days were some three to five times the size of those recorded from mature animals.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccaglini P. I., Spitzer N. C. Developmental changes in the inward current of the action potential of Rohon-Beard neurones. J Physiol. 1977 Sep;271(1):93–117. doi: 10.1113/jphysiol.1977.sp011992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the storage of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):657–668. doi: 10.1113/jphysiol.1972.sp009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Derkach V. A., Selyanko A. A., Skok V. I. Acetylcholine-induced current fluctuations and fast excitatory post-synaptic currents in rabbit sympathetic neurones. J Physiol. 1983 Mar;336:511–526. doi: 10.1113/jphysiol.1983.sp014595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Statistical fluctuations in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):665–688. doi: 10.1113/jphysiol.1976.sp011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock M. B., Peveto C. A. A preganglionic autonomic nucleus in the dorsal gray commissure of the lumbar spinal cord of the rat. J Comp Neurol. 1979 Jan 1;183(1):65–72. doi: 10.1002/cne.901830106. [DOI] [PubMed] [Google Scholar]
- Harvey A. L. Actions of drugs on developing skeletal muscle. Pharmacol Ther. 1980;11(1):1–41. doi: 10.1016/0163-7258(80)90067-4. [DOI] [PubMed] [Google Scholar]
- Harvey A. L., van Helden D. Acetylcholine receptors in singly and multiply innervated skeletal muscle fibres of the chicken during development. J Physiol. 1981 Aug;317:397–411. doi: 10.1113/jphysiol.1981.sp013832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry I. A., Campbell J. Morphometric analysis of rat superior cervical ganglion after axotomy and nerve growth factor treatment. J Neurocytol. 1976 Jun;5(3):351–360. doi: 10.1007/BF01175120. [DOI] [PubMed] [Google Scholar]
- Hill C. E., Hirst G. D., van Helden D. F. Development of sympathetic innervation to proximal and distal arteries of the rat mesentery. J Physiol. 1983 May;338:129–147. doi: 10.1113/jphysiol.1983.sp014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Redman S. J., Wong K. Post-tetanic potentiation and facilitation of synaptic potentials evoked in cat spinal motoneurones. J Physiol. 1981 Dec;321:97–109. doi: 10.1113/jphysiol.1981.sp013973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruschak K. A., Friedrich V. L., Jr, Giacobini E. Synaptogenesis in chick paravertebral sympathetic ganglia: a morphometric analysis. Brain Res. 1982 Jun;256(2):229–240. doi: 10.1016/0165-3806(82)90045-1. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. Modifications to synaptic transmission at group Ia synapses on cat spinal motoneurones by 4-aminopyridine. J Physiol. 1981 Dec;321:111–126. doi: 10.1113/jphysiol.1981.sp013974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Purves D. Post-natal reduction of neural unit size in the rabbit ciliary ganglion. J Physiol. 1981 Sep;318:143–159. doi: 10.1113/jphysiol.1981.sp013855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G., Orkand R. K. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Landmesser L. Pharmacological properties, cholinesterase activity and anatomy of nerve-muscle junctions in vagus-innervated frog sartorius. J Physiol. 1972 Jan;220(1):243–256. doi: 10.1113/jphysiol.1972.sp009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D. The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J Physiol. 1980 Apr;301:213–228. doi: 10.1113/jphysiol.1980.sp013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. An analysis of the release of acetylcholine from preganglionic nerve terminals. J Physiol. 1975 Feb;245(2):447–466. doi: 10.1113/jphysiol.1975.sp010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Jänig W. The cell bodies of origin of sympathetic and sensory axons in some skin and muscle nerves of the cat hindlimb. J Comp Neurol. 1983 Feb 20;214(2):115–130. doi: 10.1002/cne.902140202. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M. The formation of synapses in mammalian sympathetic ganglia reinnervated with preganglionic or somatic nerves. J Physiol. 1974 Feb;237(1):217–242. doi: 10.1113/jphysiol.1974.sp010479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J Physiol. 1981 Feb;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Brenner H. R. Change in synaptic channel gating during neuromuscular development. Nature. 1978 Nov 23;276(5686):401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M. The acetylcholine channel open time in chick muscle is not decreased following innervation. J Physiol. 1980 Jun;303:111–124. doi: 10.1113/jphysiol.1980.sp013274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C. Voltage- and stage-dependent uncoupling of Rohon-Beard neurones during embryonic development of Xenopus tadpoles. J Physiol. 1982 Sep;330:145–162. doi: 10.1113/jphysiol.1982.sp014334. [DOI] [PMC free article] [PubMed] [Google Scholar]


